Abstract
Peroxiredoxin 6 (Prdx6) is a 1-Cys member of the peroxiredoxin superfamily that plays an important role in antioxidant defense. Glutathionylation of recombinant Prdx6 mediated by π glutathione S-transferase (GST) is required for reduction of the oxidized Cys and completion of the peroxidatic catalytic cycle in vitro. This study investigated the requirement for πGST in intact cells. Transfection with a plasmid construct expressing πGST into MCF7, a cell line that lacks endogenous πGST, significantly increased phospholipid peroxidase activity as measured in cell lysates and protected intact cells against a peroxidative stress. siRNA knockdown indicated that this increased peroxidase activity was Prdx6 dependent. Interaction between πGST and Prdx6, evaluated by the Duolink Proximity Ligation Assay, was minimal under basal conditions but increased dramatically following treatment of cells with the oxidant, tert-butyl hydroperoxide. Interaction was abolished by mutation of C47, the active site for Prdx6 peroxidase activity. Depletion of cellular GSH by treatment of cells with buthionine sulfoximine had no effect on the interaction of Prdx6 and πGST. These data are consistent with the hypothesis that oxidation of the catalytic cysteine in Prdx6 is required for its interaction with πGST and that the interaction plays an important role in regenerating the peroxidase activity of Prdx6.
Keywords: peroxidase, glutathionylation, cysteinyl sulfenic acid, glutathione, oxidant stress
Introduction
Peroxiredoxin 6 (Prdx6) is a ‘moonlighting’ protein that has both phospholipase A2 (PLA2) and peroxidase activities (Chen et al., 2000). The PLA2 activity of Prdx6 is Ca2+ independent and maximal at acidic pH and plays an important role in lung surfactant phospholipid synthesis and turnover (Fisher et al., 2005; Fisher et al., 2006). Its peroxidase activity protects cells against oxidant stress through glutathione (GSH)-dependent reduction of various hydroperoxides including phospholipid hydroperoxides to the corresponding water and alcohols (Fisher et al., 1999; Wang et al., 2008). Unlike the other mammalian members of the family that contain two conserved cysteines in the catalytic site, Prdx6 has a single conserved cysteine and has been called 1-cys peroxiredoxin (Kang et al., 1998; Schremmer et al., 2007). Prdx6 is expressed in all tissues but at particularly high levels in brain, eye, testes, and lung (Kim et al., 1998; Rhee et al., 2001; Singh and Shichi, 1998).
We have observed that purified recombinant Prdx6 protein generated in E coli often lacks peroxidase activity. Further, partial purified native enzyme from bovine lung was enzymatically active, while purification to homogeneity yielded enzyme that inactivated with time, presumably due to the oxidation of its single conserved cysteine (Manevich et al., 2004). Evaluation of the partially purified preparation showed the presence of GSH S-transferase pi (πGST). In vitro incubation of purified inactive recombinant Prdx6 with GSH-loaded πGST resulted in the formation of a complex between the two proteins (Manevich et al., 2004; Ralat et al., 2006; Ralat et al., 2008). Further study indicated that GSH is dispensable for the formation of the protein complex but is required for reactivation of the enzyme from the sulfenic state (Ralat et al., 2006). These results led us to hypothesize that intracellular activation of Prdx6 requires its interaction with πGST followed by glutathionylation of the oxidized Cys47; subsequent dissociation of the heterodimer and reduction of glutathionylated Prdx6 by GSH, restores catalytic activity (Manevich et al., 2004; Ralat et al., 2006).
In preliminary studies, recombinant Prdx6 protein delivered to cells by a protein transporting reagent protected cells that expressed πGST (H441) against oxidant stress but was ineffective in cells (MCF7) lacking endogenous πGST (Manevich et al., 2004). Here, we examined the interaction of Prdx6 and πGST in intact cells by the Duolink Proximity Ligation Assay (DPLA) and the role of πGST in supporting the peroxidase activity of Prdx6 by siRNA knockdown of endogenous Prdx6 coupled with plasmid-driven expression of πGST in cells that do not normally express this protein. These results provide definitive evidence for the important role of πGST in the Prdx6 catalytic cycle.
Materials and Methods
Reagents
GSH, glutathione reductase, NADPH, 15-lipoxygenase, 1-chloro-2,4-dinitrobenzne (CDNB), tert-butylhydroperoxide (t-BOOH), and molecular mass standards were from Sigma (St. Louis, MO). πGST and a polyclonal antibody to this protein were from Oxford Biomedical Research (Rochester Hills, MI). Chloroform and methanol were obtained from Fisher Scientific (Pittsburgh, PA). L-buthionine-S, R-sulfoximine (BSO) and monochlorobimane (mCB) were purchased from Invitrogen. 1-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PLPC) came from Avanti-Polar Lipids (Birmingham, AL). PLPC hydroperoxide (PLPCOOH) was prepared by treatment of PLPC with 15-lipoxygenase and was purified as described previously (Fisher et al., 1999). The polyclonal anti-Prdx6 peptide antibody was purchased from Abfrontier (Seoul, South Korea), the monoclonal anti-Prdx6 antibody was from Chemicon (Billerica, MA). siRNA targeting Prdx6 and nontargeting control siRNA were purchased from Applied Biosystems/Ambion (Austin, TX). The plasmid construct expressing πGST was generated in either pIRES2-ZsGreen vector or pDsRed-Monomer-N1vector. Human πGST cDNA in the pUC120 plasmid (a kind gift of Dr. Roberta Colman) was PCR-amplified into both the pIRES-ZsGreen plasmid and the pDsRed-Monomer-N1 plasmid (Clontech, Mountain View, CA). The forward primer for PCR amplification and cloning into both plasmids was: 5′ ACGTCCTCGAGATGCCGCCCTACACCGTGGTC 3′. The XhoI site (underlined) was used for cloning. The reverse primer for the pIRES-ZsGreen was: 5′ ACGTGGATCCTCACTGTTTCCCGTTGCCATTC 3′ and the reverse primer for cloning into the pDsRed-Monomer-N1 was: 5′ ACGT GGATCCGACTGTTTCCCGTTGCCATTG 3′. The BamHI site (dashed underline) was used for cloning the 3′ end into each plasmid. Note the (inadvertent) substitution of a C for G as the terminal 3′ nucleotide in the ZsGreen reverse primer but the resultant single amino acid substitution did not cause any observed differences from the results obtained with Ds Red (see below). Plasmid constructs expressing wild type or mutant (C47S and S32A) mouse Prdx6 in pIRES2-ZsGreen vector were described previously (Chatterjee et al., 2011).
Cell culture
Pulmonary microvascular endothelial cells (PMVEC) were isolated from the lungs of wild-type and Prdx6 null mice using collagenase and adherence to magnetic beads coated with mAb to platelet endothelial cell adhesion molecule (PECAM; BD Biosciences Pharmingen, Palo Alto, CA.) as described previously (Milovanova et al., 2008; Milovanova et al., 2006). The endothelial phenotype of the preparation was routinely confirmed by evaluating cellular uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-acetylated low-density lipoprotein (DiI-Ac-LDL) and by immunostaining for PECAM. Cells at passage 4 or 5 were used for the experiments. The human lung carcinoma cell line A549 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The human breast adenocarcinoma cell line MCF7 was obtained from the University of Pennsylvania Cell Center. Depending on the cell line, cells were grown in MEM, DMEM or RPMI medium (GIBCO Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum plus 1% penicillin/streptomycin under 5% CO2 at 37°C. The protein content of cell preparations was determined with the Coomassie Blue assay (BioRad, Richmond, CA).
Transfection and siRNA knockdown
MCF7 cells were transfected with constructs expressing human πGST (either ZsGreen or DsRed vectors) using GenJet transfection reagent (SignaGen, Rockville, MD) according to the manufacturer’s protocol. Medium (1ml/well) with serum and antibiotics was added freshly 30–60 min before transfection. DNA and GenJet (3 μl) were diluted separately into 100 μl of serum-free DMEM with high glucose, vortexed gently, incubated at room temperature for 5 min, then the diluted GenJet reagent was added into the diluted DNA solution. The mixture was vortexed briefly, incubated at room temperature for 15 min, and the mixure was added to the cells dropwise. The medium was changed at 18 h after transfection. The cells were harvested at 48 h post-transfection and cell lysate was analyzed for protein by immunoblot and for enzymatic activity by the peroxidase assay. Increasing amounts (0–12 μg) of the πGST vector were used for transfection; empty vector was added so that the total amount of DNA remained constant.
For transient knockdown of Prdx6, MCF7 cells at 40–50% confluence in six-well plates were transfected with either specific Prdx6 siRNA or non-targeted (scrambled) control siRNA using the siRNA transfection reagent GenMute (SignaGen) according to the manufacturer’s protocol. Medium (1 ml per well) with serum and antibiotics was added 30–60 min before transfection. siRNA (5 pmole; final concentration 5 nM) was diluted into 100 μl GenMute transfection buffer and incubated at room temperature for 5 min. GenMute reagent (1 μl) was added to the siRNA solution, the mixture was vortexed briefly, incubated at room temperature for 15 min, and then added dropwise to the cells. The medium was changed at 18 h after transfection. For some experiments, cells were transfected with πGST constructs at 24 h after addition of siRNA.
Immunoblot analysis
Cell pellets were resuspended in PBS and then sonicated. SDS/PAGE (12% Tris-Glycine, 1 mm) used the Nu-Page system (Invitrogen); the sample was heated at 95°C for 10 min before loading. Western blot analysis was performed using the two-color Odyssey LI-COR (Lincoln, NE) technique as described previously (Milovanova et al., 2006). Rabbit polyclonal antibodies against Prdx6 and πGST were used to detect the proteins. The secondary antibody was IrDye 800 goat anti-rabbit (Rockland, Gilberstville, PA) for imaging on the green 800 nm channel.
Measurement of peroxidase and GST activities
Peroxidase activity of cell lysates was determined using the NADPH/GSH coupled assay (Fisher et al., 1999). The reaction buffer (3 ml) was 50 mM Tris–HC1 (pH 8.0), 2 mM NaN3, 0.1 mM EDTA, 0.3 mM NADPH, 0.36 mM GSH, and 0.23 units/ml GSH reductase. Cell lysate in buffer was preincubated for 5 min with continuous stirring. Fluorescence was continuously recorded at 460 nm (340nm excitation). After a steady baseline was achieved, the reaction was started by the addition of substrate (100 μM PLPCOOH plus 0.1% Triton X-100) and the linear change in fluorescence was recorded for 5–10 min. The change in fluorescence was corrected for the relatively small baseline of non-enzymatic oxidation of NADPH. Enzymatic activity was expressed as nmol NADPH oxidized/min/mg protein.
Peroxidase activity also was assayed using the end-point ferrous xylenol orange (FOX) assay to avoid the use of GSH in the reaction buffer (Jiang et al., 1992). The cell lysate (0.1 ml) was mixed with 0.9 ml FOX reagent (900 ml methanol and 100 ml 250 mM H2SO4 per L of reagent containing 880 mg buylated hydroxytoluene, 76 mg xylenol orange, and 98 mg (NH4)2Fe(SO4)2·6H2O) and incubated for 30 min at room temperature before measuring the absorbance at 560 nm. Activity was calculated by equating the absorbance value for wild type cells (without BSO) to their peroxidase activity measured by the NADPH/GSH coupled assay.
GST activity was measured with substrate 1-chloro-2,4-dinitrobenzene (CDNB) by monitoring formation of the conjugate of CDNB and GSH at 340 nm (Habig et al., 1974) using a Varian 50 Bio spectrophotometer. Cell lysate was added into the reaction buffer (1 ml) containing CDNB (final concentration 3 mM) and GSH (final concentration 2.5 mM) at 25 °C. The reaction buffer was 0.1 M potassium phosphate containing 1 mM EDTA and 2.5% ethanol (pH 6.5). Authentic πGST was used as a positive control. The negative control was the complete assay mixture without cell lysate or enzyme. A unit of activity is defined as the amount of enzyme that converts 1 μmol/min of CDNB to the conjugate.
Flow Cytometry Data Acquisition and Analysis
MCF7 cells were harvested and washed with PBS three times after transfection with either ZsGreen πGST or empty ZsGreen vector for 48 hours and then treated with 100 μM t-BOOH for 24 hours. Cells were analyzed for green color on a four-color, dual laser FACSCalibur (Becton Dickinson, San Jose, CA) at the Abramson Cancer Center Flow Cytometry and Cell Sorting Resource Laboratory of the University of Pennsylvania Medical Center. ZsGreen positive cells were measured in the FL1 channel.
Untransfected cells were used to set the gate (x axis) for identification of transfection. The cytometric data were analyzed using CellQuest™ acquisition/analysis software (Becton Dickinson, San Jose, CA).
Interaction of πGST and Prdx6 in intact cells
The Duolink in situ Proximity Ligation Assay (DPLA, Olink Bioscience, Uppsala, Sweden) was used according to the manufacturer’s protocol (www.olink.com/products-services/duolink) to detect the interaction between Prdx6 and πGST in intact cells. PMVEC from Prdx6 null mice were grown to 70% confluence on glass coverslips and transfected with constructs expressing wild type or mutated Prdx6 ZsGreen vector using the Amaxa Nucleofector system (program T-023) and the Basic Endothelial Nucleofector Kit (Lonza Group, Basel, Switzerland) according to the manufacturer’s protocol. MCF7 cells were transfected with human πGST using the GenJet transfection reagent. Cells were cultured for an additional 48 h after transfection.
For analysis of cell association by the Duolink technique, transfected MCF7 and A549 cells were exposed to 200 μM t-BOOH for 2h; non-exposed cells were used as control. Cells were rinsed with PBS and fixed with 3% paraformaldehyde for 30 min at room temperature, followed by a 10 min incubation with 0.2% Triton X-100 solution in PBS and 40 min blocking in a 5% solution of bovine serum albumin in PBS containing 0.2% Triton X-100. Cells were immunolabeled with mouse monoclonal antibody to Prdx6 and rabbit polyclonal antibody to πGST (1:100 dilution in 1% bovine serum albumin in PBS) for 1 h at room temperature. Anti-mouse and anti-rabbit secondary antibodies with attached DPLA probes are supplied in the Duolink kit which also includes 4′, 6 - diamidino – 2 – phenylindole (DAPI) for nuclear staining. Cells were observed by confocal microscopy at 60× magnification; a fluorescence signal indicates that two intracellular proteins are separated by <40 nm (Fredriksson et al., 2002).
GSH depletion and measurement of cellular GSH content
GSH was depleted from PMVEC by incubation with 0.1 mM buthionine sulfoximine (BSO) for 24 hours (Arrick et al., 1981; Hawkins et al., 2010). Cellular GSH content was determined by incubating the cells with 0.1 mM monochlorobimane (mCB) for 20 min and evaluating cellular fluorescence at 470 nm (excitation at 400 nm) by confocal microscopy (Hawkins et al., 2010; Kamencic et al., 2000).
Statistical analysis
Data are expressed as mean ± SE. Statistical significance was determined by analysis of variance followed by Pairwise Multiple Comparison Procedures using the Holm-Sidak method (Sigma Stat 3.1, Jandel Scientific, San Jose, CA). Differences between mean values were considered statistically significant at P < 0.05.
Results
Transfection with πGST construct increases peroxidase activity of Prdx6 and protects cells against peroxidative stress
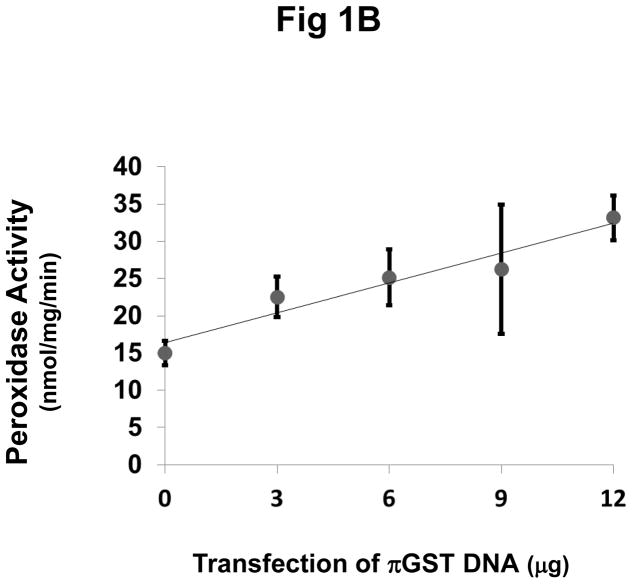
To investigate a role for πGST in the peroxidase activity of Prdx6 in intact cells, we transfected πGST DNA into MCF7 cells. These cells have endogenous Prdx6 and do not normally express πGST (Fig. 1A). Note that previously we reported the absence of endogenous Prdx6 in this cell line as evaluated with the antibodies available at that time (Manevich et al., 2004). Expression of πGST in MCF7 cells increased as a function of the amount of πGST DNA that was transfected (Fig. 1A). Peroxidase activity in the cell lysates was measured by the NADPH/GSH coupled assay with PLPCOOH as substrate. We consider this substrate as indicative although not specific for the peroxidase activity of Prdx6 (Fisher et al., 1999). The peroxidase activity increased linearly with the amount of πGST DNA transfected and was approximately double the control value after transfection with 12 μg of the πGST construct (Fig. 1B).
Figure 1. Effect of transfection with πGST expression plasmid on πGST expression and cellular peroxidase activity of Prdx6.
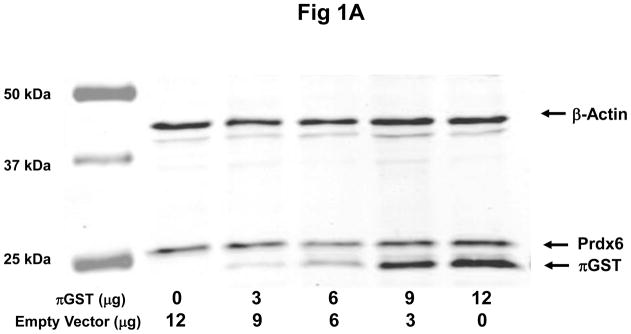
A: Expression of πGST protein after transfection detected by Western blotting. MCF7 cells were transfected with a construct expressing πGST ZsGreen vector and probed with polyclonal antibodies against πGST or Prdx6; β-actin was used as a loading control. Decreasing empty vector DNA was co-transfected with increasing πGST vector DNA in order to use consistent DNA for each transfection experiment. B: Peroxidase activity determined by NADPH/GSH coupled assay after transfection as described in A. Values are mean ± SE for 3 or more experiments. The line was drawn by least mean squares analysis.
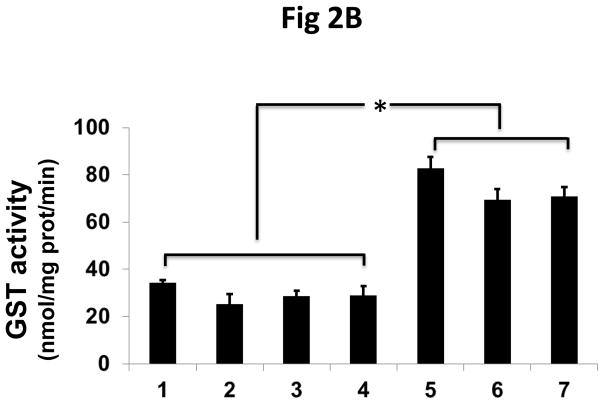
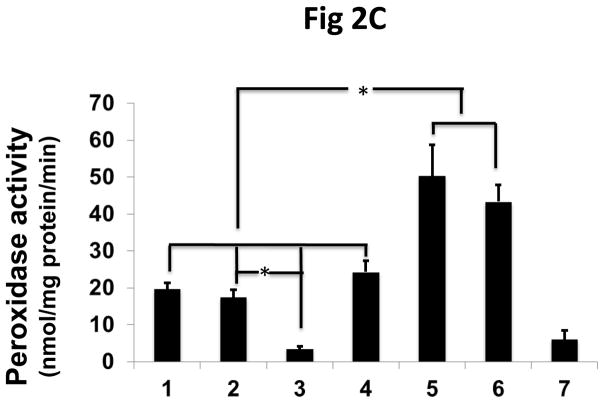
To confirm that the increased peroxidase activity of the transfected cells is due to the interaction of πGST and Prdx6, we used siRNA to knock down endogenous Prdx6 in MCF7 cells followed by transfection with πGST. The efficiency of knockdown was about 60%–80% (Fig 2A, lanes 3 and 7). The increase in πGST expression is reflected by increased GST activity (Fig. 2B). The GST activity of non-transfected cells presumably reflects the presence of GST isoforms other than pi. Knockdown of endogenous Prdx6 had no significant effect on basal GST activity following transfection but peroxidase activity of the cells decreased significantly (Fig. 2C). Further, transfection of cells with πGST did not increase the cellular peroxidase activity when endogenous Prdx6 was knocked down, (Fig 2C, lane 7). These experiments utilized the DsRed πGST plasmid but similar results were obtained for transfection with ZsGreen πGST plasmid (supplemental Fig. 1). These data indicate that transfection of intact cells with πGST increases their peroxidase activity (Fig. 1) and that this increase requires Prdx6 (Fig. 2). The physiological effect of transfection of MCF7 cells with the vector expressing ZsGreen ± πGST was evaluated by determining the cellular resistance to oxidative stress. The transfection efficiency as determined by flow cytometry for ZsGreen expression ranged from 16.8 to 34.6% (n=3), but was almost identical for the 2 different vectors (ZsGreen plasmid and ZsGreen πGST plasmid) in each of the 3 experiments (Supplemental Figure 2). The MCF7 cells were exposed to 100 μM t-BOOH for 24 h. The number of green cells per 105 total cells was determined before and after the oxidative stress in order to calculate the % of green cells that had been killed during the exposure. Cell killing was 27.9 ± 3.7 % for the empty vector transfected cells and 6.4 ± 1.4 % for πGST- transfected cells, a statistically significant difference (p < 0.05). Thus, transfection of πGST into cells that express Prdx6 protected against a peroxidative stress. These results are compatible with the increase in peroxidase activity of cell lysates in the presence of πGST (fig. 1B). It is unlikely that πGST itself was responsible for the anti-oxidant effect of transfection since this protein has minimal peroxidase activity in the absence of Prdx6 (Fig. 2C).
Figure 2. The effect of Prdx6 knockdown on peroxidase activity of MCF7 cells that express πGST.
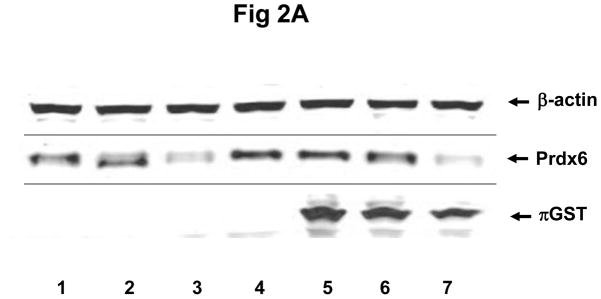
A: Prdx6 and πGST expression determined by Western blotting of MCF7 cells transfected with 12 μg πGST DNA (DsRed vector) and/or knock-down of Prdx6 with siRNA. Lane 1. untreated; lane 2. scrambled siRNA; lane 3. Prdx6 siRNA; lane 4. empty vector; lane 5. πGST; lane 6. scrambled siRNA plus πGST; lane 7. Prdx6 siRNA plus πGST. B: GST activity determined by monitoring the formation of the conjugate of CDNB and GSH for the cell preparations described in A. C: Peroxidase activity determined by NADPH/GSH coupled assay for the cells as described in A. Values in B and C are mean +SE for 3 or more experiments. *p<0.05 for the indicated comparisons.
Association of πGST and Prdx6 in intact cells
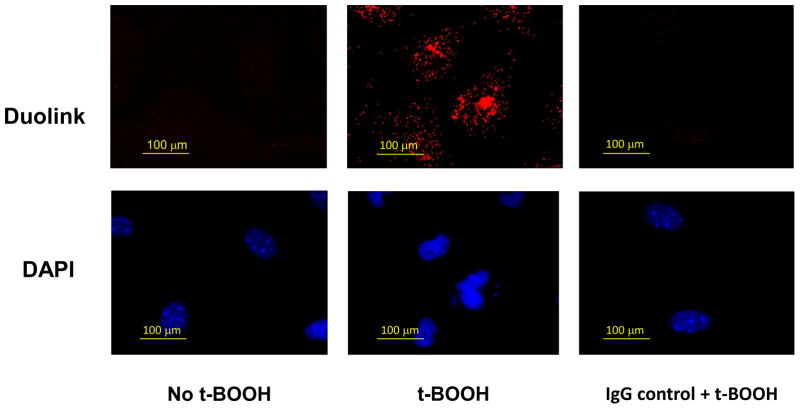
To investigate a possible interaction of Prdx6 and πGST in cells, we conducted the Duolink Proximity Ligation Assay (DPLA) to determine the proximity of the two proteins in fixed PMVEC, cells that endogenously express the 2 proteins. No fluorescence signal was demonstrated with cells in the basal state (Fig. 3). However, after a 2 h treatment of cells with t-BOOH (0.2 mM), there was a strong positive signal as shown by the red fluorescence (Fig. 3), indicating that πGST and Prdx6 are in proximity (< 40 nm apart). A similar result was obtained following 2h treatment of A549 cells with H2O2 (0.5 mM) (Supplemental Fig. 3A) or MCF7 cells that had been transfected with a plasmid containing πGST DNA and treated with t-BOOH (0.2 mM) (Supplemental Fig. 3B). Thus, DPLA revealed that the association of endogenous Prdx6 and transfected exogenous πGST occurred only in the presence of oxidative stress. These results are compatible with an interaction between πGST and the oxidized state of the Prdx6 protein although the DPLA test demonstrates only that the proteins are in close proximity, and not that they have actually interacted. Western blot of the MCF7 cell lysate following 2h t-BOOH treatment of cells indicated no change in total Prdx6 or πGST protein expression as compared with control (Supplemental Fig. 4). Based on the density of the Prdx6 standard, the Prdx6 content of MCF7 cells was 278 ± 8 ng/106 cells (mean ±SE, n=6) or 0.07 % of total cellular protein.
Figure 3. Interaction of endogenous Prdx6 and πGST in mPMVEC as detected by the Duolink Proximity Ligation Assay (DPLA).
Mouse pulmonary microvascular endothelial cells were incubated with or without t-BOOH, (200 μM) for 2 h. Cells were then fixed and probed with mouse monoclonal anti-Prdx6 and rabbit polyclonal anti-πGST antibodies using the DPLA protocol. The red fluorescence indicates proximity (<40 nm) of the two proteins. For IgG control + t-BOOH treated cells, the sample was probed with the corresponding non-specific IgG; IgG control was similar in the absence of t-BOOH (not shown). Cells were counter-stained with 4′, 6–diamidino-2-phenylindole (DAPI) to show nuclei.
Mutation of the peroxidase but not the PLA2 active site of Prdx6 enzymatic activity abolishes its association with πGST
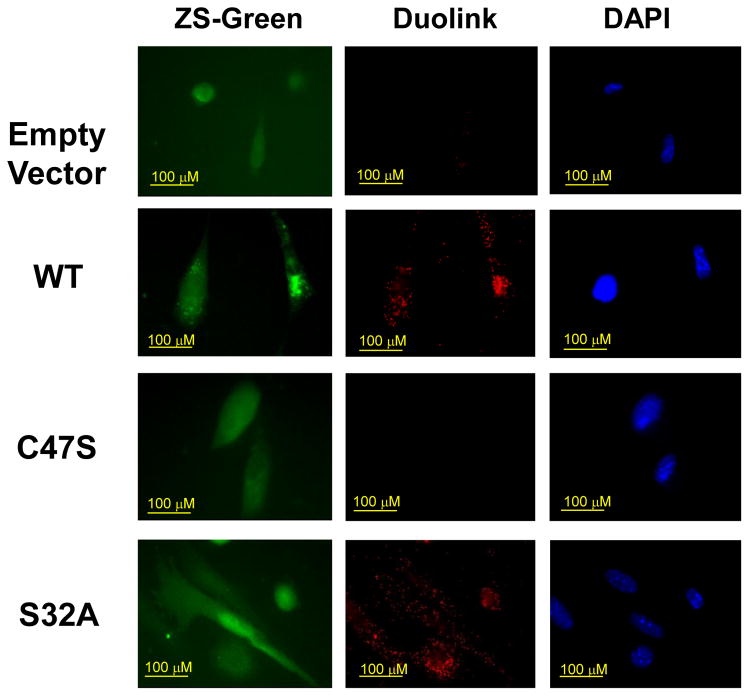
The above studies indicated that association of πGST with Prdx6 required the presence of an oxidant (t-BOOH or H2O2), presumably resulting in generation of the cysteinyl sulfenic acid at C47, the conserved cysteine of Prdx6 and the residue responsible for peroxidase activity. (Human and mouse Prdx6 have an additional Cys residue although at different positions in the protein). We thus examined the role of C47 in the interaction of Prdx6 and πGST by transfecting Prdx6 null PMVEC with the C47S construct leading to expression of the mutant proteins (Lien et al., 2012). Transfection with constructs for wild type Prdx6 and S32A Prdx6 were the controls. S32A represents the mutation of the active site for PLA2 activity. Previous studies have shown that transfection with wild type and S32A constructs rescues peroxidase activity while transfection with C47S does not (Lien et al., 2012). Interaction of πGST with Prdx6 as shown by DPLA occurred for the wild type and S32A mutant transfectants but was abolished by mutation of C47 (Fig 4).
Figure 4. Effect of mutation of the catalytically active sites of Prdx6 on the interaction of Prdx6 and πGST.
Pulmonary microvascular endothelial cells from Prdx6 null mice transfected with pIRES-ZsGreen empty vector or containing wild type or mutant Prdx6 were treated with t-BOOH (50 μM) for 2 h and then probed with mouse monoclonal anti-Prdx6 and rabbit polyclonal anti-πGST antibodies for the Duolink Proximity Ligation Asssay (middle column). ZsGreen images indicate transfection of cells (left column) and DAPI staining shows cell nuclei (right column). The conditions are: Empty vector-negative control (transfected with empty-pIRES-ZsGreen-vector); WT-positive control (transfected with wild type Prdx6); C47S-mutation at active site of peroxidase activity (transfected with C47S-pIRES-ZsGreen-Prdx6); S32A-mutation at active site of PLA2 activity (transfected with S32A-pIRES-ZsGreen-Prdx6).
Depletion of cellular GSH decreases the peroxidase activity of Prdx6 but does not affect the interaction of Prdx6 and πGST
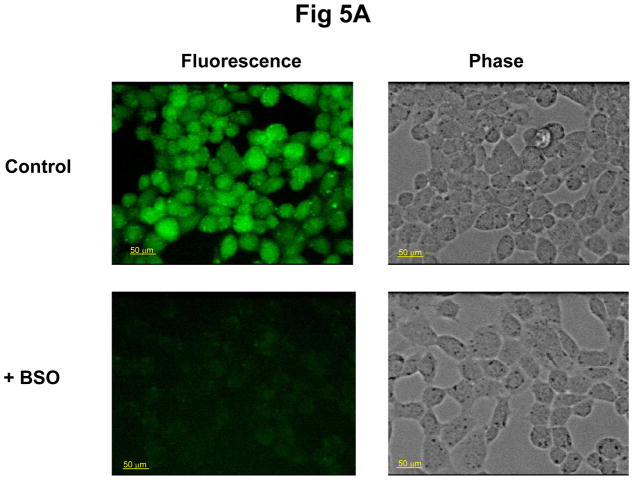
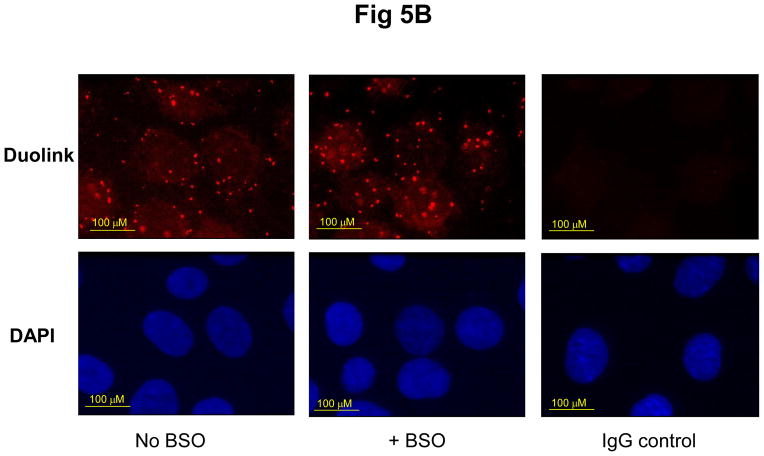
To examine the role of GSH in the interaction of Prdx6 and πGST, we depleted the GSH from wild type PMVEC by incubating cells for 24 hours with 0.1 mM BSO. The GSH content of the treated cells was almost completely depleted as determined with the GSH cross-linking fluorophore, mCB (Fig. 5A). Depletion of GSH did not affect the co-localization of Prdx6 and πGST as determined by DPLA assay (Fig.5B). However, the peroxidase activity (PLPCOOH substrate) in cell lysate as determined by the FOX assay was decreased markedly by GSH depletion (Table 1).
Figure 5. Effect of depletion of GSH on the interaction of Prdx6 and πGST.
Mouse pulmonary microvascular endothelial cells were incubated for 24 h without or with BSO 0.1 mM and then evaluated for GSH content (A) and by DPLA (B). A. Cells after BSO treatment were incubated with 0.1 mM mCB for 20 min and then were examined for specific fluorescence by confocal microscropy (ex 400 nm; em470 nm). The decrease in fluorescence reflects decreased GSH content of the cells; B: Cells after BSO treatment were treated with t-BOOH (50 μM) for 2 h, then probed with mouse monoclonal anti-Prdx6 and rabbit polyclonal anti-πGST antibodies for DPLA. The IgG control indicates the background fluorescence. There was no effect of GSH depletion with BSO on the interaction of the two proteins.
Table 1.
The effect of GSH depletion on peroxidase activity of mouse pulmonary microvascular endothelial cells
| Absorbance (% of WT) | Peroxidase activity (nmol/min/mg protein) | |
|---|---|---|
| WT | 100 | 15.6 ± 0.44 |
| KO | 33 ±5.7 | 5.15 ± 0.89 |
| WT+BSO | 36 ±4.7* | 5.62 ± 0.73* |
| KO+BSO | 28 ±2.3 | 4.37 ± 0.36 |
Wild type (WT) or Prdx6 null (KO) mouse pulmonary microvascular endothelial cells were treated with or without BSO (100 μM) for 24 h and the absorbance was determined by the FOX end point assay. Absorbance was converted to activity based on the activity in WT cells measured by the NADPH/GSH coupled assay. The substrate for assay was PLPCOOH.
P < 0.05 vs. same cell type without BSO.
Discussion
Prdx6 is a non-seleno GSH peroxidase that has an important role in anti-oxidant defense (Fisher, 2011; Manevich and Fisher, 2005). Previously we have shown that the peroxidase activity of recombinant Prdx6 protein in vitro required πGST as part of the catalytic cycle and that the 2 proteins formed a heterodimer (Manevich et al., 2004; Ralat et al., 2006; Ralat et al., 2008). We proposed that GSH transferase activity is required for glutathionylation of Prdx6 and reduction of the cysteinyl sulfenic acid that is generated by interaction of Prdx6 with an oxidant. In a previous experiment, delivery of recombinant Prdx6 protein into MCF7 cells that lack πGST did not increase the phospholipid hydroperoxide peroxidase activity unless πGST was co-delivered (Manevich et al., 2004). We have now confirmed this requirement for πGST with MCF7 cells by showing that transfection with a vector expressing πGST resulted in increased cellular peroxidase activity and also protected cells against a peroxidative stress. Knockdown of Prdx6 with siRNA reversed the increased cellular peroxidase activity indicating that this effect of πGST transfection only occurred in the presence of Prdx6. These results are compatible with our hypothesis that regeneration of the peroxidase activity of oxidized Prdx6 and completion of its catalytic cycle requires πGST.
Our previous studies provided direct evidence for the formation of a complex between Prdx6 and πGST in vitro (Ralat et al., 2006; Ralat et al., 2008) and provided the basis for proposing heterodimerization of the two proteins. In the present study, we show that association of endogenous Prdx6 with πGST (either endogenous in endothelial and A549 cells or transfected in MCF7 cells) only occurs with intact cells that have been subjected to oxidative stress. The DPLA test indicated that the two proteins (Prdx6 and πGST) in oxidatively stressed cells are separated by < 40 nm. The close proximity of the proteins in response to oxidant treatment is compatible with their heterodimerization subsequent to Prdx6 oxidation. Mutation of the peroxidase active site (Cys47) of Prdx6 abolished the interaction, thus supporting the requirement for Cys oxidation in order to promote interaction of the two proteins. An alternate possibility that the Duolink signal was due to enzyme induction is unlikely because of the relatively short duration of exposure to t-BOOH (2h), the requirement for the presence of C47, and the western blot indicating no change in protein expression.
Although the identification of the physiological reductant for peroxidase activity of Prdx6 has been controversial, our studies have shown clearly that GSH (in the presence of πGST) can result in reduction of oxidized Prdx6 as required for the catalytic cycle (Manevich et al., 2004). To determine whether GSH is required for the interaction of πGST with oxidized Prdx6, cells were depleted of GSH by treatment with BSO and the effect on the interaction of the two proteins was evaluated. GSH depletion resulted in decreased peroxidase activity, supporting our previous findings that GSH acts as an electron donor for Prdx6 (Fisher et al., 1999), but had no effect on the πGST: Prdx6 association. This result is consistent with our previous in vitro studies; thus, the GSH tripeptide is a required step for reduction of the oxidized Cys but is dispensable for the formation of complex between Prdx6 and πGST (Ralat et al., 2006). In summary, the present results obtained with intact cells indicate that πGST forms a complex with Prdx6 and is a required step for reduction of the peroxidatically active cysteine (C47) and completion of the catalytic cycle.
Supplementary Material
Acknowledgments
We thank Drs. Jonni Moore and Tatyana Milovanova for assistance with the cell sorting studies, Dr. Roberta Colman for the πGST plasmid, Dr. David Speicher for helpful advice, and Molly McGoey for typing the manuscript. Presented in part at Experimental Biology (EB) meetings in 2010 (Anaheim, Ca), 2011 (Washington, DC) and 2012 (San Diego, Ca). Supported by R-01-HL105509.
Abbreviations
- Prdx6
peroxiredoxin 6
- GSH
glutathione
- GST
glutathione S-transferase
- DPLA
Duolink Proximity Ligation Assay
- t-BOOH
tert-butylhydroperoxide
- BSO
L-buthionino-S, R-sulfoximine
- mCB
monochlorobromine
- PLPCOOH
1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine hydroperoxide
- PMVEC
pulmonary micro vascular endothelial cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrick BA, Griffith OW, Cerami A. Inhibition of glutathione synthesis as a chemotherapeutic strategy for trypanosomiasis. J Exp Med. 1981;153:720–725. doi: 10.1084/jem.153.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Feinstein SI, Dodia C, Sorokina E, Lien YC, Nguyen S, Debolt K, Speicher D, Fisher AB. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem. 2011;286:11696–11701. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem. 2000;275:28421–28427. doi: 10.1074/jbc.M005073200. [DOI] [PubMed] [Google Scholar]
- Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A(2) activities. Antioxid Redox Signal. 2011;15:831–844. doi: 10.1089/ars.2010.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AB, Dodia C, Feinstein SI, Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J Lipid Res. 2005;46:1248–1256. doi: 10.1194/jlr.M400499-JLR200. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Dodia C, Manevich Y, Chen JW, Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem. 1999;274:21326–21334. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Dodia C, Yu K, Manevich Y, Feinstein SI. Lung phospholipid metabolism in transgenic mice overexpressing peroxiredoxin 6. Biochim Biophys Acta. 2006;1761:785–792. doi: 10.1016/j.bbalip.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, Ostman A, Landegren U. Protein detection using proximity-dependent DNA ligation assays. Nature Biotechnology. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase A. A novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biochem. 1974;249:7140–7147. [PubMed] [Google Scholar]
- Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Analytical Biochemistry. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Kamencic H, Lyon A, Paterson PG, Juurlink BH. Monochlorobimane fluorometric method to measure tissue glutathione. Analytical Biochemistry. 2000;286:35–37. doi: 10.1006/abio.2000.4765. [DOI] [PubMed] [Google Scholar]
- Kang SW, I, Baines C, Rhee SG. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J Biol Chem. 1998;273:6303–6311. doi: 10.1074/jbc.273.11.6303. [DOI] [PubMed] [Google Scholar]
- Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, Fisher AB. Cloning and expression of rat lung acidic Ca(2+)-independent PLA2 and its organ distribution. Am J Physiol. 1998;274:L750–761. doi: 10.1152/ajplung.1998.274.5.L750. [DOI] [PubMed] [Google Scholar]
- Lien YC, Feinstein SI, Dodia C, Fisher AB. The roles of peroxidase and phospholipase A2 activities of peroxiredoxin 6 in protecting pulmonary microvascular endothelial cells against peroxidative stress. Antioxid Redox Signal. 2012;16:440–451. doi: 10.1089/ars.2011.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc Natl Acad Sci U S A. 2004;101:3780–3785. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta. 2008;1783:1866–1875. doi: 10.1016/j.bbamcr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanova T, Chatterjee S, Manevich Y, Kotelnikova I, Debolt K, Madesh M, Moore JS, Fisher AB. Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. Am J Physiol Cell Physiol. 2006;290:C66–76. doi: 10.1152/ajpcell.00094.2005. [DOI] [PubMed] [Google Scholar]
- Ralat LA, Manevich Y, Fisher AB, Colman RF. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry. 2006;45:360–372. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- Ralat LA, Misquitta SA, Manevich Y, Fisher AB, Colman RF. Characterization of the complex of glutathione S-transferase pi and 1-cysteine peroxiredoxin. Arch Biochem Biophys. 2008;474:109–118. doi: 10.1016/j.abb.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- Schremmer B, Manevich Y, Feinstein S, Fisher AB. Peroxiredoxins in the lung with emphasis on peroxiredoxin VI. In: Flohe L, Harris JR, editors. Peroxiredoxin Systems. Springer; 2007. pp. 317–344. [DOI] [PubMed] [Google Scholar]
- Singh AK, Shichi H. A novel glutathione peroxidase in bovine eye. Sequence analysis, mRNA level, and translation. J Biol Chem. 1998;273:26171–26178. doi: 10.1074/jbc.273.40.26171. [DOI] [PubMed] [Google Scholar]
- Wang Y, Feinstein SI, Fisher AB. Peroxiredoxin 6 as an antioxidant enzyme: protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. Journal of Cellular Biochemistry. 2008;104:1274–1285. doi: 10.1002/jcb.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.