Abstract
After iron, zinc is the most abundant essential trace metal. Intracellular zinc ([Zn]i) is maintained across a wide range of cells and species in a tight quota (100 to 500 μM) by a dynamic process of transport, intracellular vesicular storage, and binding to a large number of proteins (estimated at 3-10% of human proteome). As such, zinc is an integral component of numerous metalloenzymes, structural proteins, and transcription factors. It is generally assumed that a vanishingly small component of [Zn]i, referred to as free or labile zinc, and operationally defined as the pool sensitive to chelation (by agents such as N, N, N’, N’-tetrakis [2-pyridylmethyl] ethylenediamine [TPEN]) and capable of detection by a variety of chemical and genetic sensors, participates in signal transduction pathways. Zinc deficiencies, per se, can arise from acquired (malnutrition, alcoholism) or genetic (mutations in molecules affecting zinc homeostasis, the informative and first example being acrodermatitis enteropathica) factors or as a component of various diseases (e.g., sickle cell disease, cystic fibrosis, sepsis). Hypozincemia has profound effects on developing humans, and all facets of physiological function (neuronal, endocrine, immunological) are affected, although considerably less is known regarding cardiovascular pathophysiology. In this review, we provide an update on current knowledge of molecular and cellular aspects of zinc homeostasis and then focus on implications of zinc signaling in pulmonary endothelium as it relates to programmed cell death, altered contractility, and septic and aseptic injury to this segment of the lung.
Keywords: zinc homeostasis, pulmonary endothelium, apoptosis, SLC39A14 or ZIP14, metallothionien (MT), acute lung injury (ALI)
The role of zinc was first reported in 1869 when it was discovered to be important for the growth of Aspergillus niger.[1] Zinc was not recognized to be important for human life until 1963 when zinc deficiency was discovered as a major contributing factor in nutritional dwarfism syndrome and hypogonadism.[2] It is now well established that zinc is important for numerous cellular functions including cell differentiation[3] and division,[3,4] DNA synthesis,[4,5] RNA transcription,[4,5] and maintaining plasma membrane integrity.[6] Recent approaches using bioinformatics methods to mine existing protein databases indicate that approximately 10% of the human proteome is zinc dependent.[7] Zinc plays three major biological roles: a structural component of at least 3,000 proteins,[8] including transcription factors,[8] cytokines, and receptors;[8] a catalytic component of more than 300 enzymes[9] that regulate many cellular activities including DNA synthesis and maintaining membrane stability;[10–12] and a regulator of enzyme activity by acting as an activator or inhibitor ion.[10] Total intracellular zinc is maintained in a concentration range from 100 to 500 μM[13] across numerous cell types. Zinc is considered a trace metal; however, this is because more than 99% of intracellular zinc is protein bound. The concentration of labile [Zn]i is vanishingly small with estimates between 10-9 M[14,15] to 10-12 M[16] and it is this fraction that may act as a second messenger in cell signaling[17,18] in a fashion well supported for other divalent cations such as calcium.
Zinc has been referred to as a “double edged sword”[19] as both zinc deficiency and zinc excess are associated with adverse effects on cell physiology.[11,20–25] Zinc deficiency stimulates inter-nucleosomal DNA fragmentation and apoptosis in intestinal,[26] neural,[27] respiratory epithelium,[28] and in systemic endothelium,[29] and high levels of zinc (> 250 μM) are associated with concentration dependent increases in cell death in cultured pulmonary[30,31] and cerebral[32] endothelia. In contrast, lower zinc concentrations (10 μM)[33–35] have been shown to inhibit cadmium-,[35] , linoleic acid-,[33] and tumor necrosis factor-α (TNF-α)-[33] induced apoptosis in systemic endothelial cells. At the systemic level, the following occurred: labile [Zn]i levels were demonstrated to be affected by changes in fluid shear stress levels in mouse aorta and in human umbilical vein endothelial cells indicating that zinc dyshomeostasis in the systemic endothelium may contribute to the development and progression of cardiovascular diseases; and zinc supplementation was shown to reverse systemic inflammation and organ damage, with a positive effect on overall mortality in mouse model of sepsis.[36] Little is known about the signaling role of labile [Zn]i in the pulmonary endothelium in the context of lung diseases. In this review, we discuss the impact of zinc homeostasis and signaling, as well as its efficacy as a cyto-protectant in pathophysiological processes of pulmonary endothelial cell injury and death.
INTRACELLULAR ZINC HOMEOSTASIS
Intracellular zinc concentration is maintained by the coordinated activity of a large family of zinc transporters (ZnT and ZIP)[37] and zinc binding proteins such as metallothionein (MT)[37] (Fig. 1). Zinc transporters are encoded by one of two of the solute-linked carrier (SLC) gene families: SLC 30 (also known as zinc exporters or ZnT1-10);[38] and SLC39 (also known as zinc importers or ZIP1-14).[38] ZnT transporters reduce cytoplasmic zinc by promoting zinc efflux from cells or into intracellular vesicles, while ZIP transporters increase cytoplasmic zinc by promoting zinc influx from extracellular and, perhaps, from vesicular stores into cytoplasm.[39]
Figure 1.
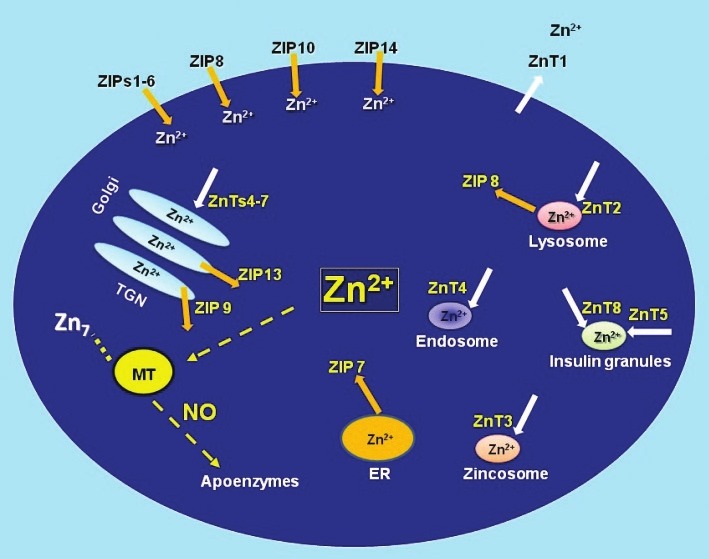
Regulators of intracellular zinc homeostasis. The labile pool of intracellular zinc is tightly controlled by zinc importers (ZIPs), zinc exporters (ZnTs), zinc storing vesicles, and zinc binding proteins such as metallothionein (MT). MT plays a critical role in zinc homeostasis acting as a buffer in the steady state while controlling the cellular distribution of transiently elevated zinc in response to perturbations and/or agonists such as nitric oxide (NO).[141] Modified figure from references.[74,142,143]
Metallothioneins
Metallothioneins (MT) are major zinc binding proteins that dynamically coordinate up to 7 mol Zn2+/mol MT via cysteine residues (approximately mol 30%).[40] MT is involved in the following: detoxification of heavy metals like mercury, cadmium, and alkylating cancer drugs;[41,42] scavenging free radicals;[41] and protection against DNA damage,[41] oxidative stress,[41] and apoptosis.[43] Mammals express at least four isoforms-MT-1, MT-2, MT-3, and MT-4. In humans, there are at least 16 MT genes located in chromosome 16 and most of them are associated with the MT-1 isoform.[44] MT-1 and MT-2 are expressed in many tissues and are particularly abundant in the liver, pancreas, intestine, and kidney.[45] MT-3 and MT-4 are minor isoforms with specific expression patterns in brain (MT-3) and stratified squamous epithelial cells (MT-4).[46] At the subcellular level, MT can be localized to a number of cellular compartments (i.e., mitochondria, cytosol, and nucleus)[47] as well as in the extracellular space.[48] The reduction potential of MT (less than -366mV[40]), makes it highly sensitive to physiological oxidants. We[49] and others[50–53] have shown that MT is sensitive to changes in cellular redox state and demonstrated that increases in reactive oxygen[54] or nitrogen[55,56] intermediates can oxidize or transnitrosate cysteines in its zinc sulfur clusters leading to liberation of zinc. As such, MT can be viewed as acting as a sensor and switch and connecting changes in cellular redox status with alterations in labile zinc (Fig. 1).
ZIPs
Fourteen ZIP family members have been reported in mammals.[57–59] The majority of ZIP family members are located on the plasma membrane[60–66] with the exception of ZIP7-8 and ZIP13 (Fig. 1) that are present in intracellular organelles . Gene knock-out technologies have provided valuable information regarding biological significance of the ZIP family members. Knockout (KO) mice lacking ZIP1, ZIP2, and ZIP3 are reported to have abnormal embryogenesis under zinc-limiting conditions.[62,67,68] ZIP4 KO mice embryos die during early development, whereas heterozygous mice exhibit a phenotype similar to acrodermatitis enteropathica (AE) secondary to impairment of intestinal absorption of zinc.[69–71] ZIP13 KO mice suffer from disorganization in hard connective tissue, including bone, teeth, skin, and eyes.[72] In humans, lack of ZIP13 is associated with spondylocheiro dysplasia, a form of Ehlers-Danlos syndrome.[72,73] Mice lacking ZIP14 have impaired G-protein coupled receptor (GPCR) signaling[74] and exhibit retarded growth and impaired gluconeogenesis. A summary of phenotype in genetically ablated or spontaneous mutants in various species (including humans) is provided in Table 1. Recent reports on association of single nucleotide polymorphisms of various zinc transporters with human disease are summarized in Table 2.
Table 1.
Phenotypical profile (in mouse, *human, **drosophila, ***sheep) of mutants of zinc transporters
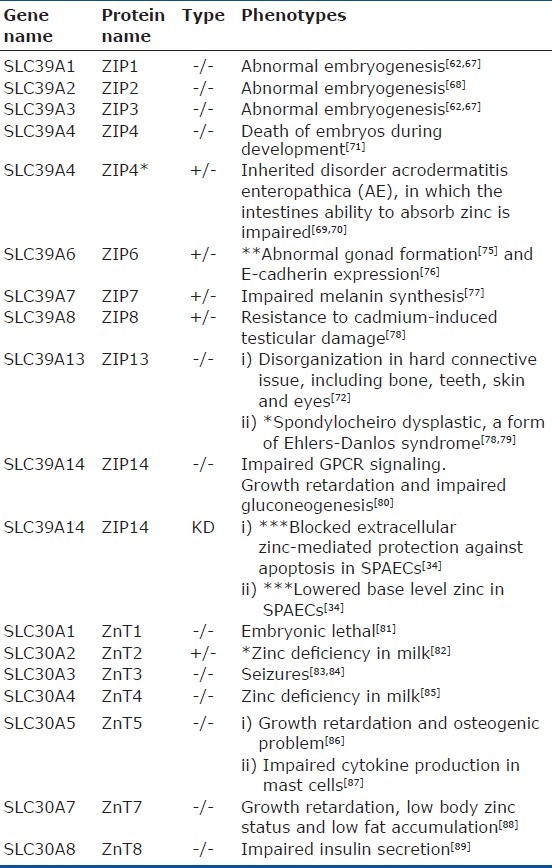
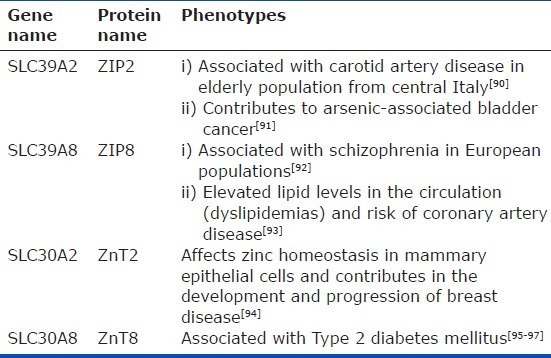
Table 2.
Phenotypical profile (in humans) of SNPs of zinc transporters
ZnTs
Ten ZnT family members have been reported in mammals.[59] Most ZnTs are located on intracellular organelles (i.e., golgi, endosomes, and endoplasmic reticulum)[98] (Fig. 1). ZnT 1 is the only ZnT exporter located at the plasma membrane, compatible with its role as the primary regulator of cellular zinc efflux.[99] ZnT1 knockout mice are embryonic lethal.[83] Disruption of the ZnT genes yields diverse phenotypes providing insight into the biologic function and specificity of the various family members. Mutations in ZnT2[82] and ZnT4[85] result in the production of zinc deficient milk in women and mice, respectively. ZnT3 knockout mice are prone to seizures.[83] Mice lacking in ZnT5 show growth retardation and osteogenic problems[86] and exhibit impaired cytokine production in mast cells.[87] Single-nucleotide polymorphism (SNPs) in ZnT8 are associated with type 2 diabetes in humans,[95] and deletion of the ZnT8 gene results in impaired insulin secretion in mice[89] (Table 1).
Manipulation of intracellular zinc levels have been shown to influence the expression and localization of zinc transporters[59] with reports of increased expression of members of ZIP family and decreased expression of ZnT family members in response to decreases in intracellular zinc.[100–104] Most of these studies have been performed in intestinal and respiratory epithelial or immune cells. While reported increases in ZIP1 and ZIP14 mRNA were shown to be normalized by dietary zinc supplementation in a mouse model of acute lung inflammation,[101] the mechanisms underlying the association between zinc homeostasis and lung disease remain largely unknown. ZIP6 was shown to play a role in blocking LPS-induced decreases in intracellular labile zinc and consequent maturation in mouse dendritic cells.[105] Zinc mediated cytoprotection against TNF-α-induced damage in human lung epithelial cells was shown to be dependent upon expression ZIP8.[38] We recently reported[34] the following in cultured sheep pulmonary artery endothelial cells (SPAECs): ZIP14 is sensitive to changes in intracellular labile zinc; and exogenous zinc mediated protection against LPS-induced apoptosis is dependent upon ZIP14.
ZINC HOMEOSTASIS IN THE PULMONARY ENDOTHELIUM
The Zalewski laboratory in Adelaide, Australia were the first to image labile zinc in the airway[12,106] and provide evidence that zinc chelation (via TPEN) enhanced hydrogen peroxide-induced caspase activation.[106] As reviewed by Troung Tran et al.,[4] intracellular zinc has also been shown to be important for ciliary function, wound healing (via re-epithelialization), and suppression of oxidative stress and apoptosis in the airway epithelium. Further evidence suggests that zinc deficiency sensitizes the lung to acute lung injury following alcohol induced epithelial dysfunction,[107] hyperoxia,[108] and polymicrobial sepsis.[36,109]
We have shown that zinc chelation (via TPEN) exacerbates LPS-induced apoptosis in pulmonary endothelium (SPAECs).[30] TPEN also reversed the protective effect of nitric oxide (NO) donors on LPS-induced apoptosis.[110] More recently, we reported that LPS induced time-dependent decreases in intracellular labile zinc (Fig. 2) in SPAECs using both live cell imaging and fluorescence-activated cell sorting (FACS) with the zinc-sensitive fluorophore, FluoZin-3 (Life Technologies, Grand Island, N.Y.).[111] We further verified the observed decrease in FluoZin-3 detectable zinc using a chimeric reporter encoding a zinc-sensitive metal-response element (MRE) fused to a luciferase gene.[111] The LPS-induced changes in labile zinc were accompanied by increases in ZIP14 mRNA. These effects were blocked by addition of exogenous zinc, as was LPS-induced apoptosis (increased caspase 3/7 activity and PS externalization).[111] In separate studies in SPAEC, siRNA knockdown of ZIP14 decreased basal levels of intracellular labile zinc and blocked zinc uptake (as determined by FluoZin-3), and abrogated zinc mediated protection against LPS-induced apoptosis (observed in WT and scrambled control).[34] Collectively, these data suggest that endogenous levels of labile zinc can modulate the sensitivity of pulmonary endothelium to the proapoptotic effects of LPS (Fig. 2) and implicate ZIP14 in affecting the ability of extracellular zinc to inhibit LPS-induced apoptosis in SPAEC (Fig. 3).
Figure 2.
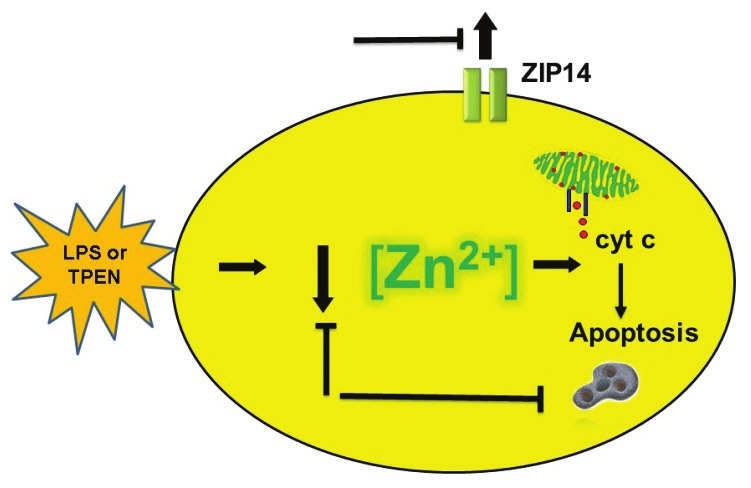
Functional role of labile zinc in LPS-induced apoptosis. LPS caused a decrease in labile zinc in SPAECs (as determined by zinc indicator, FluoZin-3, activity of zinc-sensitive MRE, and changes in steady-state mRNA of zinc importer, ZIP14). The contributory role of decreases in labile zinc in LPS-induced apoptosis (as determined by caspase-3/7 activation, cytochrome c release, and PS externalization) was verified by mimicking the effects of LPS with zinc chelator, TPEN. Blocking LPS- or TPEN- induced decreases in labile zinc inhibited consecutive increase in apoptosis and ZIP14 mRNA providing support for a signaling role of labile zinc in pulmonary endothelium.
Figure 3.
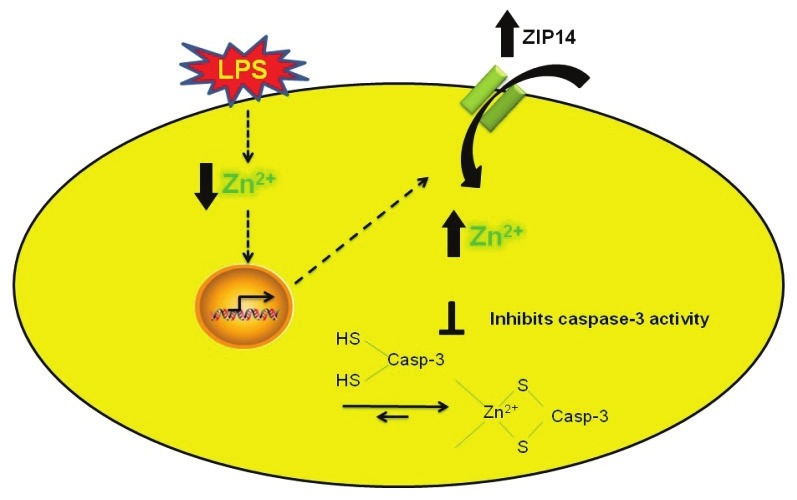
Cytoprotective effect of exogenous zinc is ZIP14 dependent. LPS induced decreases in labile zinc are associated with increases in capsase-3 activity and upregulation of zinc importer, ZIP14 to restore the loss of labile zinc mediated by LPS. Elevation in labile zinc via ZIP14 inhibits apoptosis by inhibiting caspase-3 activity. siRNA to ZIP14 blocked zinc uptake and abrogated zinc mediated protection against LPS-induced apoptosis.
The results we obtained in pulmonary arterial endothelial cells isolated from mature sheep are distinct from those obtained in SPAEC from fetal sheep. We initially noted that addition of large concentrations of zinc to the medium of SPAEC was associated with necrosis.[30] In contrast, elevations in intracellular labile zinc (via addition of exogenous zinc[31] or after exposure to large doses of H2O2[112] or NO[31]) were reported to induce apoptosis in fetal SPAEC. Alternatively, we have consistently noted that chelation of intracellular zinc with TPEN led to dose-dependent apoptosis in mature SPACE whereas a similar maneuver inhibited apoptosis in fetal SPAEC.[31,112]
NO-(MT)-Zn2+ SIGNALING IN PULMONARY ENDOTHELIUM
In aerobic conditions, NO (e.g., presumably via formation of nitrosonium ion intermediate) can S-nitrosate metallothionein[113] and cause the release of Zinquin detectable changes in labile zinc in intact cells.[114] We[49,115] have confirmed these observations and demonstrated the following: (1) S-nitrosation caused conformational changes of MT (via fluorescence resonance energy transfer techniques) in intact pulmonary endothelium consistent with zinc release;[49,116] (2) NO caused an increase in labile zinc in pulmonary artery endothelial cells;[115] and (3) MT was the requisite target for NO resulting in such changes in labile zinc.[115] Subsequent investigations supported the potential for MT to participate in intracellular signal transduction pathways in pulmonary endothelium.
Exposure of mouse lung endothelial cells (MLEC) to the NO donor, S-nitroso-N-acetylpenicillamine (SNAP, 200 μM), caused nuclear translocation of the zinc dependent transcription factor, MTF-1, and such activation was not apparent in MT null cells. Translocation of MTF-1 was associated with NO mediated increase in MT gene expression itself[117] suggested that S-nitrosation of zinc-thiolate clusters in MT and subsequent alterations in zinc homeostasis are participants in intracellular NO signaling pathways affecting gene expression.
We observed that zinc chelation (TPEN) abrogated hypoxic vasoconstriction in isolated perfused mouse lungs (IPL), and that IPL from MT null mice showed significantly less constriction than wild-type controls. Data obtained using NO-sensitive FRET reporters supported both enhanced NO production and S-nitrosation of MT during hypoxic exposure. These events were accompanied by NO-dependent increases in labile zinc (Fluo-Zin-3) in subpleural vessels of MT +/+, but not MT -/- mice. These data supported a role for zinc thiolate signaling in pulmonary vasoregulation. Subsequent studies in cell-based models revealed a link between hypoxia-induced elevations in labile zinc and changes in myosin light chain phosphatase (MLCP) activity, ultimately leading to stress fiber formation and endothelial cell contraction.[118]
Most recently, we showed that zinc chelation abrogates NO-mediated protection against LPS-induced apoptosis.[34] Relative changes in labile zinc after exposure to cytoprotective doses of the NO donor SNAP (250 μM) or exogenous zinc (10 μM) were assessed by Fluozin-3, and a comparable increase in intracellular labile zinc was noted in both conditions.[34] We, further showed that both NO-mediated increases in labile zinc, and NO-mediated protection against LPS-induced apoptosis, are dependent on MT via siRNA to sheep MT isoforms,[34] thus implicating NO-MT-Zn2+ signaling in apoptotic pathways in the pulmonary endothelium (Fig. 4).
Figure 4.
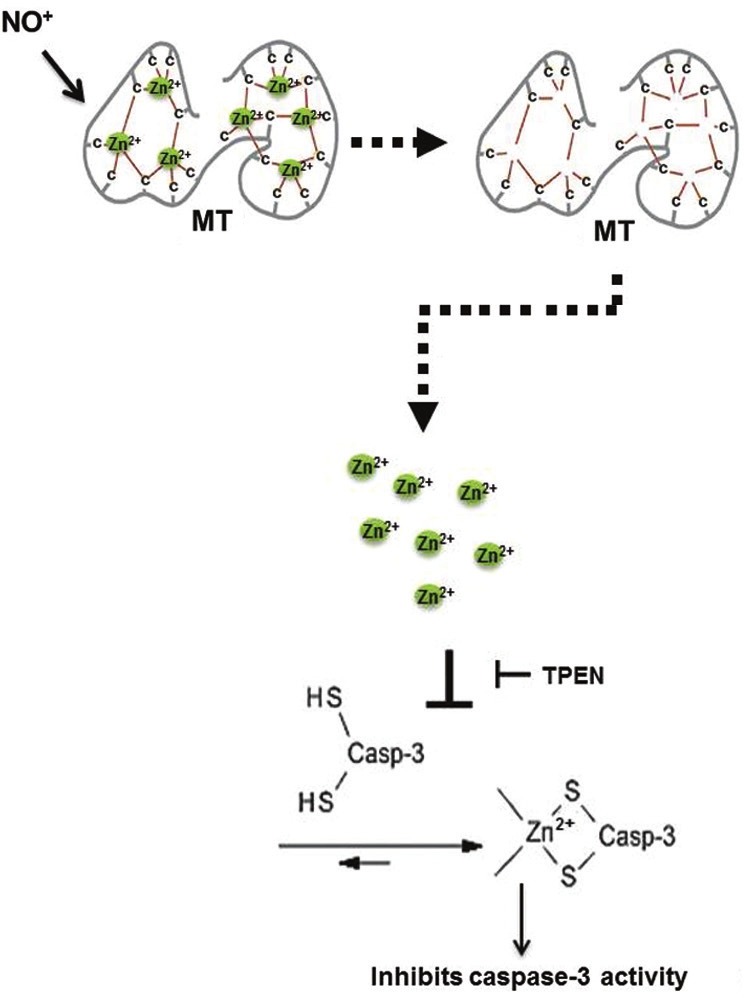
NO elevated zinc from MT inhibits apoptosis in pulmonary endothelial cells. Illustrates the link between apoptosis and elevation in labile zinc (via NO-MT signaling), which in turn inhibits capsase-3 activity in pulmonary endothelial cells. Modified figure from references.[34,40]
ZINC AS AN EFFECTOR MOLECULE IN PULMONARY ENDOTHELIUM
Decreases in labile zinc have been reported to precede the earliest detectable alterations in cell function,[119] morphology,[119] and apoptosis.[111,119] We and others have reported that chelation of zinc causes spontaneous apoptosis in pulmonary endothelia[30] and epithelia,[4] and elevations in labile zinc via ZIP14[34] or iNOS induced NO or NO donors[111,120–124] inhibits apoptosis in cultured pulmonary endothelial cells. An anti-apoptotic role of zinc has been reported in relation to a variety of stimuli including TNF-α,[125] cadmium,[126] cholesterol,[127] and linoleic acid[33] induced apoptosis. Although the molecular mechanism by which zinc inhibits apoptosis is not clear, several reports suggest that zinc inhibits the following: Ca2+/Mg2+-dependent endonucleases that are responsible for DNA fragmentation;[128] the activity of caspase-3, a critical protease in apoptosis;[129] the processing of caspase-3;[130,131] and bax activation, cytochrome c release, and apoptosome function.[132] Zinc also increases the ratio of Bcl-2 to Bax resulting in the inhibition of caspase activity.[133] We reported the following in SPAECs: decreases in labile zinc mediated by LPS cause casapse-3 activation;[104] LPS-induced caspase-3 activity is sensitive to pan caspase inhibitor;[104] and extracellular zinc inhibits LPS-induced caspase-3 activity.[104,106] We posed a question whether zinc directly binds capsase-3 and modulates its activity. Our results in vitro confirmed that zinc directly inhibits caspase-3 activity.[106] Although NO can S-nitrosate caspase-3 and inhibit its activity,[134] our results suggest that s-nitrosation of MT by NO leads to a release of zinc that is associated with a TPEN dependent cytoprotective caspase-3 inhibition, leading us to suggest that direct S-nitrosation of caspase-3 alone is not likely to account for these results. Collectively, our observation adds to the elegant studies in airway epithelium[12,106] that revealed the following: labile zinc proximity with procaspase-3 prevent the activation of procaspase-3; and zinc depletion activates procaspase-3.[106] These studies provide support for the antiapoptotic role of labile zinc in the lung.
ZINC HOMEOSTASIS AND ACUTE LUNG INJURY: COMPLEXITIES OF INTEGRATED RESPONSE
Several studies have demonstrated that zinc deficiency sensitizes the lung to acute injury. In particular, dietary restriction led to enhanced sensitivity to polymicrobial sepsis.[36,109] Hyperoxic[135,136] lung injury in mice and macrophage and epithelial cell dysfunction in alcohol fed rats was ascribed to zinc deficiency.[107] Zinc repletion reversed phenotype in all three conditions. Although pulmonary endothelial cell dysfunction may have been a component of all these models, any supportive insight into the cellular contributions of zinc dyshomeostasis to these observations largely relates to background information on zinc in respiratory epithelium.
Nonetheless, hypozincemia in septic or aseptic[59,98] conditions is a somewhat underappreciated phenomenon. Transmigration of zinc from tissues, including lung to liver, has been noted in hyperoxia,[137] bacterial sepsis,[138] and turpentine injury[139] and has been presumed to subserve the following: gluconeogenesis in liver; new protein synthesis in acute phase response; or host defense in an analogous fashion to hypoferremia in bacterial pneumonia.[139] Hepatic expression of ZIP14 appears critical in this phenomenon.[140] We recently (in unpublished observations) noted that hepatic expression of metallothionein was important in transmigration of zinc from lung to liver during hyperoxic lung injury apparently contributing to the unexpected observation that MT null mice were resistant to hyperoxia. Collectively, these observations suggest that additional insight into the mechanisms underlying such transmigration may provide new therapeutic targets and strategies and potentially support exogenous zinc as a rational therapeutic agent in acute lung injury.
In summary, compelling evidence is emerging in pulmonary endothelium to complement a larger and growing body of experience in extrapulmonary tissue that labile zinc is a key effector molecule. Critical aspects of the magnitude of labile pool of intracellular zinc accounting for these signaling pathways awaits more refined ratiometric or quantitative fluorescent indicators. Genetic and acquired aspects of zinc dyshomeostasis and deficiencies await further insight into the function and cellular distribution of large family of zinc transporters and metal binding proteins. Nonetheless, it is apparent that the facile and common nature of zinc and nitric oxide chemistry support a role for NO-MT-Zn2+ pathway and the uqibuitous nature of these molecules in sepsis and acute lung injury make them a rational novel therapeutic target.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Raulin J. Chemical Studies on vegetation. Ann Sci Nat. 1869;11:93–9. [Google Scholar]
- 2.Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961;31:532–46. doi: 10.1016/0002-9343(61)90137-1. [DOI] [PubMed] [Google Scholar]
- 3.Prasad AS. Zinc: An overview. Nutrition. 1995;11:93–9. [PubMed] [Google Scholar]
- 4.Truong-Tran AQ, Carter J, Ruffin R, Zalewski PD. New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol. 2001;79:170–7. doi: 10.1046/j.1440-1711.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 5.Prasad AS. Zinc in human health: Effect of zinc on immune cells. Mol Med. 2008;14:353–7. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 8.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem Rev. 2009;109:4682–707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 9.Gamsjaeger R, Liew CK, Loughlin FE, Crossley M, Mackay JP. Sticky fingers: Zinc-fingers as protein-recognition motifs. Trends Biochem Sci. 2007;32:63–70. doi: 10.1016/j.tibs.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Mocchegiani E, Muzzioli M, Giacconi R. Zinc and immunoresistance to infection in aging: New biological tools. Trends Pharmacol Sci. 2000;21:205–8. doi: 10.1016/s0165-6147(00)01476-0. [DOI] [PubMed] [Google Scholar]
- 11.Barceloux DG. Zinc. J Toxicol Clin Toxicol. 1999;37:279–92. doi: 10.1081/clt-100102426. [DOI] [PubMed] [Google Scholar]
- 12.Carter JE, Truong-Tran AQ, Grosser D, Ho L, Ruffin RE, Zalewski PD. Involvement of redox events in caspase activation in zinc-depleted airway epithelial cells. Biochem Biophys Res Commun. 2002;297:1062–70. doi: 10.1016/s0006-291x(02)02292-1. [DOI] [PubMed] [Google Scholar]
- 13.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 14.Colvin RA, Bush AI, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, et al. Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am J Physiol Cell Physiol. 2008;294:C726–42. doi: 10.1152/ajpcell.00541.2007. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Maret W. Transient fluctuations of intracellular zinc ions in cell proliferation. Exp Cell Res. 2009;315:2463–70. doi: 10.1016/j.yexcr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem Biol. 2006;1:103–11. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- 17.Williams RJ. Zinc: What is its role in biology? Endeavour. 1984;8:65–70. doi: 10.1016/0160-9327(84)90040-1. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, et al. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–45. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershfinkel M, Aizenman E, Andrews G, Sekler I. Zinc bells rang in Jerusalem! Sci Signal. 2010;3:mr2. doi: 10.1126/scisignal.3129mr2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–6. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 21.Sensi SL, Jeng JM. Rethinking the excitotoxic ionic milieu: The emerging role of Zn(2+) in ischemic neuronal injury. Curr Mol Med. 2004;4:87–111. doi: 10.2174/1566524043479211. [DOI] [PubMed] [Google Scholar]
- 22.Capasso M, Jeng JM, Malavolta M, Mocchegiani E, Sensi SL. Zinc dyshomeostasis: A key modulator of neuronal injury. J Alzheimers Dis. 2005;8:93–108. doi: 10.3233/jad-2005-8202. discussion 209-15. [DOI] [PubMed] [Google Scholar]
- 23.Mann JJ, Fraker PJ. Zinc pyrithione induces apoptosis and increases expression of Bim. Apoptosis. 2005;10:369–79. doi: 10.1007/s10495-005-0811-9. [DOI] [PubMed] [Google Scholar]
- 24.Cho E, Hwang JJ, Han SH, Chung SJ, Koh JY, Lee JY. Endogenous Zinc Mediates Apoptotic Programmed Cell Death in the Developing Brain. Neurotox Res. 2010;17:156–66. doi: 10.1007/s12640-009-9085-2. [DOI] [PubMed] [Google Scholar]
- 25.King JC, Cousins RJ. Modern Nutrition in Health and Disease. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 26.Elmes ME. Apoptosis in the small intestine of zinc-deficient and fasted rats. J Pathol. 1977;123:219–23. doi: 10.1002/path.1711230404. [DOI] [PubMed] [Google Scholar]
- 27.Harding AJ, Dreosti IE, Tulsi RS. Zinc deficiency in the 11 day rat embryo: A scanning and transmission electron microscope study. Life Sci. 1988;42:889–96. doi: 10.1016/0024-3205(88)90387-6. [DOI] [PubMed] [Google Scholar]
- 28.Zalewski PD, Forbes IJ, Betts WH. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II) Biochem J. 1993;296(Pt 2):403–8. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr. 1999;18:152–8. doi: 10.1080/07315724.1999.10718843. [DOI] [PubMed] [Google Scholar]
- 30.Tang ZL, Wasserloos K, St Croix CM, Pitt BR. Role of zinc in pulmonary endothelial cell response to oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2001;281:L243–9. doi: 10.1152/ajplung.2001.281.1.L243. [DOI] [PubMed] [Google Scholar]
- 31.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol. 2006;291:C555–68. doi: 10.1152/ajpcell.00509.2005. [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Kim JH, Xu J, Hsu CY, Ahn YS. Pyrrolidine dithiocarbamate induces bovine cerebral endothelial cell death by increasing the intracellular zinc level. J Neurochem. 1999;72:1586–92. doi: 10.1046/j.1471-4159.1999.721586.x. [DOI] [PubMed] [Google Scholar]
- 33.Meerarani P, Ramadass P, Toborek M, Bauer HC, Bauer H, Hennig B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor alpha. Am J Clin Nutr. 2000;71:81–7. doi: 10.1093/ajcn/71.1.81. [DOI] [PubMed] [Google Scholar]
- 34.Thambiayya K, Wasserloos KJ, Kagan VE, Stoyanovsky D, Pitt BR. A critical role for increased labile zinc in reducing sensitivity of cultured sheep pulmonary artery endothelial cells to LPS-induced apoptosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1287–95. doi: 10.1152/ajplung.00385.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szuster-Ciesielska A, Stachura A, Slotwinska M, Kamiñska T, Sniezko R, Paduch R, et al. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology. 2000;145:159–71. doi: 10.1016/s0300-483x(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 36.Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, et al. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2010;298:L744–54. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallee BL. The function of metallothionein. Neurochem Int. 1995;27:23–33. doi: 10.1016/0197-0186(94)00165-q. [DOI] [PubMed] [Google Scholar]
- 38.Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1127–36. doi: 10.1152/ajplung.00057.2008. [DOI] [PubMed] [Google Scholar]
- 39.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 40.Bell SG, Vallee BL. The metallothionein/thionein system: An oxidoreductive metabolic zinc link. Chembiochem. 2009;10:55–62. doi: 10.1002/cbic.200800511. [DOI] [PubMed] [Google Scholar]
- 41.Higashimoto M, Isoyama N, Ishibashi S, Inoue M, Takiguchi M, Suzuki S, et al. Tissue-dependent preventive effect of metallothionein against DNA damage in dyslipidemic mice under repeated stresses of fasting or restraint. Life Sci. 2009;84:569–75. doi: 10.1016/j.lfs.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Lazo JS, Pitt BR. Metallothioneins and cell death by anticancer drugs. Annu Rev Pharmacol Toxicol. 1995;35:635–53. doi: 10.1146/annurev.pa.35.040195.003223. [DOI] [PubMed] [Google Scholar]
- 43.Dutsch-Wicherek M, Tomaszewska R, Lazar A, Strek P, Wicherek Ł, Piekutowski K, et al. The evaluation of metallothionein expression in nasal polyps with respect to immune cell presence and activity. BMC Immunol. 2010;11:10. doi: 10.1186/1471-2172-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis SR, Cousins RJ. Metallothionein expression in animals: A physiological perspective on function. J Nutr. 2000;130:1085–8. doi: 10.1093/jn/130.5.1085. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen AE, Bohr A, Penkowa M. The Balance between Life and Death of Cells: Roles of Metallothioneins. Biomark Insights. 2007;1:99–111. [PMC free article] [PubMed] [Google Scholar]
- 46.Cherian MG, Jayasurya A, Bay BH. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat Res. 2003;533:201–9. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Ye B, Maret W, Vallee BL. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci U S A. 2001;98:2317–22. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao Q, Hong SH, Maret W. Lipid raft-dependent endocytosis of metallothionein in HepG2 cells. J Cell Physiol. 2007;210:428–35. doi: 10.1002/jcp.20874. [DOI] [PubMed] [Google Scholar]
- 49.Pearce LL, Gandley RE, Han W, Wasserloos K, Stitt M, Kanai AJ, et al. Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein. Proc Natl Acad Sci U S A. 2000;97:477–82. doi: 10.1073/pnas.97.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maret W. Cellular zinc and redox states converge in the metallothionein/thionein pair. J Nutr. 2003;133:1460S–2S. doi: 10.1093/jn/133.5.1460S. [DOI] [PubMed] [Google Scholar]
- 51.Maret W, Jacob C, Vallee BL, Fischer EH. Inhibitory sites in enzymes: Zinc removal and reactivation by thionein. Proc Natl Acad Sci U S A. 1999;96:1936–40. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krezel A, Hao Q, Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch Biochem Biophys. 2007;463:188–200. doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Krezel A, Maret W. Different redox states of metallothionein/thionein in biological tissue. Biochem J. 2007;402:551–8. doi: 10.1042/BJ20061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maret W, Vallee BL. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci U S A. 1998;95:3478–82. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu SX, Fabisiak JP, Tyurin VA, Borisenko GG, Pitt BR, Lazo JS, et al. Reconstitution of apo-superoxide dismutase by nitric oxide-induced copper transfer from metallothioneins. Chem Res Toxicol. 2000;13:922–31. doi: 10.1021/tx0000623. [DOI] [PubMed] [Google Scholar]
- 56.Zangger K, Oz G, Haslinger E, Kunert O, Armitage IM. Nitric oxide selectively releases metals from the amino-terminal domain of metallothioneins: Potential role at inflammatory sites. FASEB J. 2001;15:1303–5. doi: 10.1096/fj.00-0641fje. [DOI] [PubMed] [Google Scholar]
- 57.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 59.Lichten LA, Cousins RJ. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–76. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 60.Gaither LA, Eide DJ. Functional expression of the human hZIP2 zinc transporter. J Biol Chem. 2000;275:5560–4. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 61.Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003;278:33474–81. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 62.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Generation and characterization of mice lacking the zinc uptake transporter ZIP3. Mol Cell Biol. 2005;25:5607–15. doi: 10.1128/MCB.25.13.5607-5615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biol Chem. 2007;388:1301–12. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J. 2003;375:51–9. doi: 10.1042/BJ20030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tominaga K, Kagata T, Johmura Y, Hishida T, Nishizuka M, Imagawa M. SLC39A14, a LZT protein, is induced in adipogenesis and transports zinc. FEBS J. 2005;272:1590–9. doi: 10.1111/j.1742-4658.2005.04580.x. [DOI] [PubMed] [Google Scholar]
- 66.Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005;579:427–32. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis. 2006;44:239–51. doi: 10.1002/dvg.20211. [DOI] [PubMed] [Google Scholar]
- 68.Peters JL, Dufner-Beattie J, Xu W, Geiser J, Lahner B, Salt DE, et al. Targeting of the mouse Slc39a2 (Zip2) gene reveals highly cell-specific patterns of expression, and unique functions in zinc, iron, and calcium homeostasis. Genesis. 2007;45:339–52. doi: 10.1002/dvg.20297. [DOI] [PubMed] [Google Scholar]
- 69.Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–40. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 70.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dufner-Beattie J, Weaver BP, Geiser J, Bilgen M, Larson M, Xu W, et al. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum Mol Genet. 2007;16:1391–9. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- 72.Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, Higashiyama H, et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS One. 2008;3:e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giunta C, Elcioglu NH, Albrecht B, Eich G, Chambaz C, Janecke AR, et al. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome--an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am J Hum Genet. 2008;82:1290–305. doi: 10.1016/j.ajhg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem. 2011;16:1123–34. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathews WR, Ong D, Milutinovich AB, Van Doren M. Zinc transport activity of Fear of Intimacy is essential for proper gonad morphogenesis and DE-cadherin expression. Development. 2006;133:1143–53. doi: 10.1242/dev.02256. [DOI] [PubMed] [Google Scholar]
- 76.Van Doren M, Mathews WR, Samuels M, Moore LA, Broihier HT, Lehmann R. fear of intimacy encodes a novel transmembrane protein required for gonad morphogenesis in Drosophila. Development. 2003;130:2355–64. doi: 10.1242/dev.00454. [DOI] [PubMed] [Google Scholar]
- 77.Stathakis DG, Burton DY, McIvor WE, Krishnakumar S, Wright TR, O’Donnell JM. The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics. 1999;153:361–82. doi: 10.1093/genetics/153.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, et al. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci U S A. 2005;102:3401–6. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bin BH, Fukada T, Hosaka T, Yamasaki S, Ohashi W, Hojyo S, et al. Biochemical characterization of human ZIP13 protein: A homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J Biol Chem. 2011;286:40255–65. doi: 10.1074/jbc.M111.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin BH, Koseki H, et al. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS One. 2011;6:e18059. doi: 10.1371/journal.pone.0018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andrews GK, Wang H, Dey SK, Palmiter RD. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis. 2004;40:74–81. doi: 10.1002/gene.20067. [DOI] [PubMed] [Google Scholar]
- 82.Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem. 2006;281:39699–707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 83.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: An update. Arch Toxicol. 2012;86:521–34. doi: 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- 84.Cole TB, Robbins CA, Wenzel HJ, Schwartzkroin PA, Palmiter RD. Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res. 2000;39:153–69. doi: 10.1016/s0920-1211(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 85.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997;17:292–7. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 86.Inoue K, Matsuda K, Itoh M, Kawaguchi H, Tomoike H, Aoyagi T, et al. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum Mol Genet. 2002;11:1775–84. doi: 10.1093/hmg/11.15.1775. [DOI] [PubMed] [Google Scholar]
- 87.Nishida K, Hasegawa A, Nakae S, Oboki K, Saito H, Yamasaki S, et al. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J Exp Med. 2009;206:1351–64. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang L, Yu YY, Kirschke CP, Gertz ER, Lloyd KK. Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J Biol Chem. 2007;282:37053–63. doi: 10.1074/jbc.M706631200. [DOI] [PubMed] [Google Scholar]
- 89.Pound LD, Sarkar SA, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J. 2009;421:371–6. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giacconi R, Muti E, Malavolta M, Cardelli M, Pierpaoli S, Cipriano C, et al. A novel Zip2 Gln/Arg/Leu codon 2 polymorphism is associated with carotid artery disease in aging. Rejuvenation Res. 2008;11:297–300. doi: 10.1089/rej.2008.0671. [DOI] [PubMed] [Google Scholar]
- 91.Karagas MR, Andrew AS, Nelson HH, Li Z, Punshon T, Schned A, et al. SLC39A2 and FSIP1 polymorphisms as potential modifiers of arsenic-related bladder cancer. Hum Genet. 2012;131:453–61. doi: 10.1007/s00439-011-1090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carrera N, Arrojo M, Sanjuan J, Ramos-Ríos R, Paz E, Suárez-Rama JJ, et al. Association study of nonsynonymous single nucleotide polymorphisms in schizophrenia. Biol Psychiatry. 2012;71:169–77. doi: 10.1016/j.biopsych.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 93.Waterworth DM, Ricketts SL, Song K, Chen L, Zhao JH, Ripatti S, et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010;30:2264–76. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seo YA, Kelleher SL. Functional analysis of two single nucleotide polymorphisms in SLC30A2 (ZnT2): Implications for mammary gland function and breast disease in women. Physiol Genomics. 2010;42A:219–27. doi: 10.1152/physiolgenomics.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 96.Kifagi C, Makni K, Boudawara M, Mnif F, Hamza N, Abid M, et al. Association of genetic variations in TCF7L2, SLC30A8, HHEX, LOC387761, and EXT2 with Type 2 diabetes mellitus in Tunisia. Genet Test Mol Biomarkers. 2011;15:399–405. doi: 10.1089/gtmb.2010.0199. [DOI] [PubMed] [Google Scholar]
- 97.Xu J, Wang J, Chen B. SLC30A8 (ZnT8) variations and type 2 diabetes in the Chinese Han population. Genet Mol Res: GMR. 2012;11:1592–8. doi: 10.4238/2012.May.24.1. [DOI] [PubMed] [Google Scholar]
- 98.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 99.Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–49. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao J, Bobo JA, Liuzzi JP, Cousins RJ. Effects of intracellular zinc depletion on metallothionein and ZIP2 transporter expression and apoptosis. J Leukoc Biol. 2001;70:559–66. [PubMed] [Google Scholar]
- 101.Lang C, Murgia C, Leong M, Tan LW, Perozzi G, Knight D, et al. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L577–84. doi: 10.1152/ajplung.00280.2006. [DOI] [PubMed] [Google Scholar]
- 102.Truong-Tran AQ, Ruffin RE, Foster PS, Koskinen AM, Coyle P, Philcox JC, et al. Altered zinc homeostasis and caspase-3 activity in murine allergic airway inflammation. Am J Respir Cell Mol Biol. 2002;27:286–96. doi: 10.1165/rcmb.2001-0014OC. [DOI] [PubMed] [Google Scholar]
- 103.Devergnas S, Chimienti F, Naud N, Pennequin A, Coquerel Y, Chantegrel J, et al. Differential regulation of zinc efflux transporters ZnT-1, ZnT-5 and ZnT-7 gene expression by zinc levels: A real-time RT-PCR study. Biochem Pharmacol. 2004;68:699–709. doi: 10.1016/j.bcp.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 104.Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol. 2008;83:368–80. doi: 10.1189/jlb.0307148. [DOI] [PubMed] [Google Scholar]
- 105.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–7. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 106.Truong-Tran AQ, Ruffin RE, Zalewski PD. Visualization of labile zinc and its role in apoptosis of primary airway epithelial cells and cell lines. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1172–83. doi: 10.1152/ajplung.2000.279.6.L1172. [DOI] [PubMed] [Google Scholar]
- 107.Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am J Respir Cell Mol Biol. 2009;41:207–16. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taylor CG, Bray TM. Effect of hyperoxia on oxygen free radical defense enzymes in the lung of zinc-deficient rats. J Nutr. 1991;121:460–6. doi: 10.1093/jn/121.4.460. [DOI] [PubMed] [Google Scholar]
- 109.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med. 2009;37:1380–8. doi: 10.1097/CCM.0b013e31819cefe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang ZL, Wasserloos KJ, Liu X, Stitt MS, Reynolds IJ, Pitt BR, et al. Nitric oxide decreases the sensitivity of pulmonary endothelial cells to LPS-induced apoptosis in a zinc-dependent fashion. Mol Cell Biochem. 2002;234-235:211–7. [PubMed] [Google Scholar]
- 111.Thambiayya K, Wasserloos KJ, Huang Z, Kagan VE, St Croix CM, Pitt BR. LPS-induced decrease in intracellular labile zinc, [Zn]i, contributes to apoptosis in cultured sheep pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L624–32. doi: 10.1152/ajplung.00376.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L165–77. doi: 10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- 113.Kroncke KD, Fehsel K, Schmidt T, Zenke FT, Dasting I, Wesener JR, et al. Nitric oxide destroys zinc-sulfur clusters inducing zinc release from metallothionein and inhibition of the zinc finger-type yeast transcription activator LAC9. Biochem Biophys Res Commun. 1994;200:1105–10. doi: 10.1006/bbrc.1994.1564. [DOI] [PubMed] [Google Scholar]
- 114.Berendji D, Kolb-Bachofen V, Meyer KL, Grapenthin O, Weber H, Wahn V, et al. Nitric oxide mediates intracytoplasmic and intranuclear zinc release. FEBS Lett. 1997;405:37–41. doi: 10.1016/s0014-5793(97)00150-6. [DOI] [PubMed] [Google Scholar]
- 115.St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am J Physiol Lung Cell Mol Physiol. 2002;282:L185–92. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- 116.St Croix CM, Stitt MS, Leelavanichkul K, Wasserloos KJ, Pitt BR, Watkins SC. Nitric oxide-induced modification of protein thiolate clusters as determined by spectral fluorescence resonance energy transfer in live endothelial cells. Free Radic Biol Med. 2004;37:785–92. doi: 10.1016/j.freeradbiomed.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Stitt MS, Wasserloos KJ, Tang X, Liu X, Pitt BR, St Croix CM. Nitric oxide-induced nuclear translocation of the metal responsive transcription factor, MTF-1 is mediated by zinc release from metallothionein. Vascul Pharmacol. 2006;44:149–55. doi: 10.1016/j.vph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 118.Bernal PJ, Bauer EM, Cao R, Maniar S, Mosher M, Chen J, et al. A role for zinc in regulating hypoxia-induced contractile events in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2011;300:L874–86. doi: 10.1152/ajplung.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duffy JY, Miller CM, Rutschilling GL, Ridder GM, Clegg MS, Keen CL, et al. A decrease in intracellular zinc level precedes the detection of early indicators of apoptosis in HL-60 cells. Apoptosis. 2001;6:161–72. doi: 10.1023/a:1011380508902. [DOI] [PubMed] [Google Scholar]
- 120.Tzeng E, Kim YM, Pitt BR, Lizonova A, Kovesdi I, Billiar TR. Adenoviral transfer of the inducible nitric oxide synthase gene blocks endothelial cell apoptosis. Surgery. 1997;122:255–63. doi: 10.1016/s0039-6060(97)90016-7. [DOI] [PubMed] [Google Scholar]
- 121.Ceneviva GD, Tzeng E, Hoyt DG, Yee E, Gallagher A, Engelhardt JF, et al. Nitric oxide inhibits lipopolysaccharide-induced apoptosis in pulmonary artery endothelial cells. Am J Physiol. 1998;275:L717–28. doi: 10.1152/ajplung.1998.275.4.L717. [DOI] [PubMed] [Google Scholar]
- 122.St Croix CM, Leelavaninchkul K, Watkins SC, Kagan VE, Pitt BR. Nitric oxide and zinc homeostasis in acute lung injury. Proc Am Thorac Soc. 2005;2:236–42. doi: 10.1513/pats.200501-007AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spahl DU, Berendji-Grun D, Suschek CV, Kolb-Bachofen V, Kroncke KD. Regulation of zinc homeostasis by inducible NO synthase-derived NO: Nuclear metallothionein translocation and intranuclear Zn2+ release. Proc Natl Acad Sci U S A. 2003;100:13952–7. doi: 10.1073/pnas.2335190100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cortese-Krott MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Nitric oxide-mediated protection of endothelial cells from hydrogen peroxide is mediated by intracellular zinc and glutathione. Am J Physiol Cell Physiol. 2009;296:C811–20. doi: 10.1152/ajpcell.00643.2008. [DOI] [PubMed] [Google Scholar]
- 125.Rishi P, Kaur P, Virdi JS, Shukla G, Koul A. Amelioratory effects of zinc supplementation on Salmonella-induced hepatic damage in the murine model. Dig Dis Sci. 2008;53:1063–70. doi: 10.1007/s10620-007-9958-2. [DOI] [PubMed] [Google Scholar]
- 126.Szuster-Ciesielska A, Lokaj I, Kandefer-Szerszen M. The influence of cadmium and zinc ions on the interferon and tumor necrosis factor production in bovine aorta endothelial cells. Toxicology. 2000;145:135–45. doi: 10.1016/s0300-483x(00)00147-5. [DOI] [PubMed] [Google Scholar]
- 127.Lizard G, Deckert V, Dubrez L, Moisant M, Gambert P, Lagrost L. Induction of apoptosis in endothelial cells treated with cholesterol oxides. Am J Pathol. 1996;148:1625–38. [PMC free article] [PubMed] [Google Scholar]
- 128.Bortner CD, Oldenburg NB, Cidlowski JA. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995;5:21–6. doi: 10.1016/s0962-8924(00)88932-1. [DOI] [PubMed] [Google Scholar]
- 129.Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, et al. Zinc is a potent inhibitor of the apoptotic protease, caspase-3.A novel target for zinc in the inhibition of apoptosis. J Biol Chem. 1997;272:18530–3. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 130.Chai F, Truong-Tran AQ, Ho LH, Zalewski PD. Regulation of caspase activation and apoptosis by cellular zinc fluxes and zinc deprivation: A review. Immunol Cell Biol. 1999;77:272–8. doi: 10.1046/j.1440-1711.1999.00825.x. [DOI] [PubMed] [Google Scholar]
- 131.Morana SJ, Wolf CM, Li J, Reynolds JE, Brown MK, Eastman A. The involvement of protein phosphatases in the activation of ICE/CED-3 protease, intracellular acidification, DNA digestion, and apoptosis. J Biol Chem. 1996;271:18263–71. doi: 10.1074/jbc.271.30.18263. [DOI] [PubMed] [Google Scholar]
- 132.Wei Q, Wang J, Wang MH, Yu F, Dong Z. Inhibition of apoptosis by Zn2+ in renal tubular cells following ATP depletion. Am J Physiol Renal Physiol. 2004;287:F492–500. doi: 10.1152/ajprenal.00083.2004. [DOI] [PubMed] [Google Scholar]
- 133.Zalewski PD, Forbes IJ, Giannakis C. Physiological role for zinc in prevention of apoptosis (gene-directed death) Biochem Int. 1991;24:1093–101. [PubMed] [Google Scholar]
- 134.Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240:419–24. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 135.Taylor CG, McCutchon TL, Boermans HJ, DiSilvestro RA, Bray TM. Comparison of Zn and vitamin E for protection against hyperoxia-induced lung damage. Free Radic Biol Med. 1997;22:543–50. doi: 10.1016/s0891-5849(96)00390-5. [DOI] [PubMed] [Google Scholar]
- 136.Taylor CG, Towner RA, Janzen EG, Bray TM. MRI detection of hyperoxia-induced lung edema in Zn-deficient rats. Free Radic Biol Med. 1990;9:229–33. doi: 10.1016/0891-5849(90)90033-f. [DOI] [PubMed] [Google Scholar]
- 137.Levy MA, Tsai YH, Reaume A, Bray TM. Cellular response of antioxidant metalloproteins in Cu/Zn SOD transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L172–82. doi: 10.1152/ajplung.2001.281.1.L172. [DOI] [PubMed] [Google Scholar]
- 138.Philcox JC, Coyle P, Michalska A, Choo KH, Rofe AM. Endotoxin-induced inflammation does not cause hepatic zinc accumulation in mice lacking metallothionein gene expression. Biochem J. 1995;308(Pt 2):543–6. doi: 10.1042/bj3080543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843–8. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lichten LA, Liuzzi JP, Cousins RJ. Interleukin-1beta contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2009;296:G860–7. doi: 10.1152/ajpgi.90676.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maret W. Redox biochemistry of mammalian metallothioneins. J Biol Inorg Chem. 2011;16:1079–86. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 142.Fukada T, Kambe T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics. 2011;3:662–74. doi: 10.1039/c1mt00011j. [DOI] [PubMed] [Google Scholar]
- 143.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515–22. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


