Abstract
This study aims to evaluate the safety and feasibility of obtaining wedged pulmonary artery (PA) samples and investigate the differential vascular beds’ distribution of select inflammatory and cellular adhesion molecules that are implicated in pulmonary arterial hypertension (PAH) pathogenesis. This is a cross-sectional study of adult patients. Serum samples were simultaneously drawn from three different vascular sites during right heart catheterization as part of PAH evaluation: The superior vena cava, distal pulmonary artery prior to wedging, and distal pulmonary artery after (and distal to) wedging. The study group was comprised of patients with either PAH or chronic thromboembolic pulmonary hypertension (i.e., WHO/Dana Point Group 1 or 4). The internal control group included patients whose hemodynamics were not consistent with pulmonary hypertension. The external control group consisted of healthy volunteers who had a peripheral venous sample drawn. The mean age of the 25 study patients was 55 ± 14 years and mean BMI was 31 ± 10, and those of the 25 internal control patients were 49 ± 14 years and 26 ± 5, respectively. There were no complications resulting from obtaining wedged PA samples. Obtaining adequate wedged samples was successful in 80% of patients. More severe pulmonary hypertension was associated with lower success rates. There were no significant differences in the concentrations of the different biomarkers studied amongst the different vascular sites (n = 25 study patients). There was a nonsignificant trend of decreasing biomarkers concentrations from peripheral to wedged to un-wedged PA samples. Compared to the healthy external controls, sVCAM-1 levels were higher in the study group. Obtaining wedged PA blood samples is safe and feasible in adult patients with pulmonary hypertension. There were no differences in the distribution of markers between the vascular beds within patients.
Keywords: biomarkers, pulmonary hypertension, right heart catheterization, wedged sample
Pulmonary arterial hypertension (PAH) continues to be a progressive and incurable disease.[1] Though significant strides have been made in the last two decades with regard to availability and efficacy of therapy,[2] the morbidity and mortality rates associated with PAH continue to be unacceptably high[3,4] and our understanding of PAH remains incomplete. PAH affects tens of thousands of patients in the United States alone. The pathophysiology of PAH is diverse but it commonly involves intimal hyperplasia and proliferation, smooth muscle hypertrophy, and in situ microthrombosis.[5–7] Although multiple molecules have been identified and implicated in the pathogenesis of PAH, only three molecular pathways were successfully targeted therapeutically-namely nitric oxide, endothelin-1, and prostaglandin I-2.[2] The lack of curative therapies leaves a huge gap in this field, requiring further research into innovative techniques for disease evaluation and therapy.
The primary site of the pathophysiology of PAH is believed to be the pulmonary arterioles and capillaries.[8] Although, some molecules have been shown to play a pivotal role in the pathogenesis of PAH such as tumor necrosis factor (TNF-α), their concentrations are not consistently shown to be elevated in peripheral blood samples.[9] The concentrations of many biomarkers in the peripheral venous circulation may not reflect the severity or pathobiology of the disease because of theoretical suspicion that these molecules may get “diluted” once in the systemic circulation and away from their “factory of origin” (the pulmonary circulation).
In theory, blood samples from the distal pulmonary arterioles, pulmonary capillaries, and even postcapillary venules would be the ideal source for investigating the multiple inflammatory and cellular adhesion molecules that have been implicated in the pathogenesis of PAH, short of a biopsy in those patients. So-called “wedged” pulmonary artery (PA) blood samples would be the ideal blood samples for such studies. Wedged PA blood samples have been studied in other diseases and they have been shown to accurately reflect pulmonary capillary vasculature in humans.[10,11] However, this technique has not been studied in PAH patients, and so the safety and the feasibility of such a technique is not known in this population.
Using this technique, we planned on studying multiple inflammatory and cellular adhesion molecules, but focused only on three of these molecules: sP-Selectin, vascular endothelial growth factor (VEGF), and soluble vascular cell adhesion molecule (sVCAM). These three molecules have been studied in animal PAH models and in humans (tissue biopsies, explanted lungs, and blood samples) and are believed to play key roles in the pathogenesis of PAH.[6,9,12,13]
The primary goals of this cross-sectional study are to explore the safety and feasibility of collecting wedged blood samples from the distal pulmonary vasculature in patients with pulmonary hypertension (PH) and to better understand the utility of such a technique in evaluating the distribution of select inflammatory and cellular adhesion biomarkers in PH. Secondarily, we hypothesized that the concentrations of the above mentioned molecules would be higher in patients with PH (compared to healthy controls) and in the wedged PA samples (compared to the peripheral venous samples).
MATERIALS AND METHODS
Study design and population
This is a single center, cross-sectional controlled study. The lab technician running the multi-plex ELISA analyses was masked to the source (i.e., study group) of the serum samples. The subject population was derived from patients evaluated in the Pulmonary Hypertension Program at the University of North Carolina at Chapel Hill (UNC) and were referred for right heart catheterization (RHC) for evaluation of PH between February 2009 and April 2011. Those patients whose hemodynamics and clinical settings were consistent with PH and were either World Health Organization (WHO) Group 1 (i.e., PAH) or 4 (i.e., chronic thromboembolic PH) comprised our study group (Fig. 1). This study was approved by the Institutional Review Board (IRB study #: 09-0003) and each participant signed an informed consent form prior to samples collection.
Figure 1.
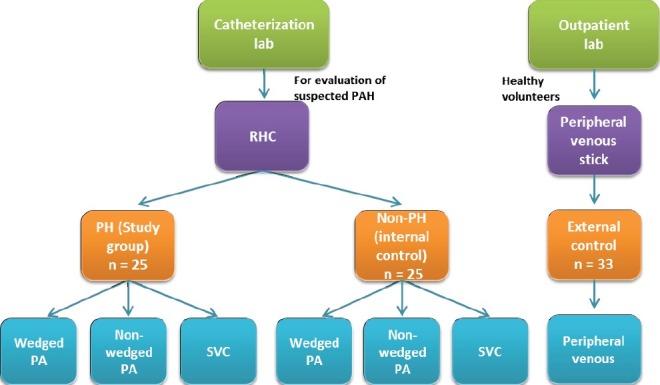
Study design. The PH study group and the internal control group comprised of patients that had a right heart catheterization (RHC) as part of evaluation for PH. The external control group comprised of healthy volunteers from whom a peripheral venous sample was obtained. The external control group did not have a RHC.
The un-wedged PA samples were drawn from the distal PA catheter port with the PA catheter balloon deflated. The wedged samples were also drawn from the distal PA catheter port but while the PA catheter balloon was inflated. Wedging status was confirmed by a clear change in the waveform showing a typical wedge pressure waveform, with or without fluoroscopy confirmation (to confirm lack of movement of the inflated PA catheter balloon within the distal PA). For quality control and avoidance of practice variation, all the RHCs and blood samples collection were done exclusively by two attending physicians. Guide-wires to stiffen and facilitate advancing the catheter into the pulmonary artery were not used.
Only peripheral venous serum samples were drawn from external healthy controls (Fig. 1). Serum samples from all three groups were stored at -80°C and they were all analyzed at the same time by the same lab personnel.
Statistical analysis
The first two goals of this study are exploratory in nature: (1) Safety and feasibility of obtaining wedged PA blood samples in this patient population; and (2) Exploring potential differential distribution of wedged and peripheral samples’ biomarkers concentrations. A total of at least 20-25 patients are typically deemed adequate for such exploratory analyses.
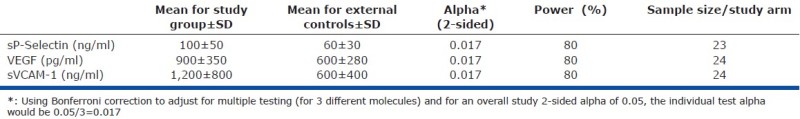
For our subsequent analysis and taking into consideration that we are primarily focusing on testing three different molecules (sP-Selectin, VEGF, and sVCAM-1), the estimated sample size for each study group is 24 patients for the PH study group and the external control group (Appendix A). This would give a power of 80%, assuming an overall study 2-sided alpha of 0.05 adjusting for multiple testing (of three hypotheses).
Sample descriptive data were expressed as means (± standard deviation [sd]) for continuous and normally distributed variables (including the concentrations of the different biomarkers) and as counts and percentages for categorical variables (including race and gender for example). Fisher's exact test was used to compare the fraction of successful “wedging” in the study and internal control groups. We used the Wilcoxon signed-rank test or the student paired t-test as appropriate to compare the biomarkers concentrations within patients. A 2-sample student t-test was used to compare the mean PA pressure and the biomarkers concentrations between different study groups.
An extension of the Wilcoxon rank-sum test (a nonparametric test with incorporated correction for ties)[14] was used to test for trend across the three venous sites within patients. Because of the size of our study population and to ensure stability of the linear regression model, we had room to adjust for only one covariable. This was a priori decided to be body mass index (BMI). All statistical analyses were performed using STATA software version 11 IC for Windows (STATACorp, College Station, Tex.; 2010).
RESULTS
Patients’ characteristics
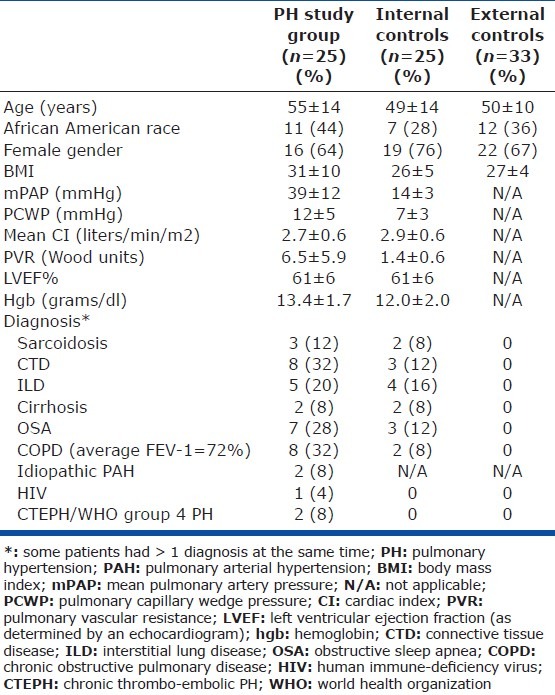
The study and the internal control groups comprised those whom we were able to get a wedged PA sample from. The three study groups were overall comparable. The PH study group was slightly older and had a higher BMI than the internal control and the healthy external control groups (Table 1). Twenty-two out of 25 patients in the study PH group were treatment-naïve, and those on treatment were on oral PAH-specific therapies. We were successful in getting 10 milliliters (ml) of blood from the PA during wedging (wedged PA sample) from 80% of the patients that had an RHC and consented to the study (72% of patients with PH vs. 88% of patients without PH; P = 0.11).
Table 1.
Patients demographics and characteristics
Hemodynamic data
The mean PA pressure and the pulmonary vascular resistance in the group whom we were able to obtain a wedged sample from were lower than those whom we could not obtain a wedged sample from (26 vs. 39 mmHg, P = 0.0009; and 3.3 vs. 6.8 Wood units, P = 0.0018, respectively). All patients that had a wedged PA sample drawn or attempted had no complications during or after the procedure (followed until the time of discharge to home from the outpatient procedure center).
Biomarkers data
There were no statistically significant differences between the concentrations of the biomarkers between the wedged PA samples and their peripheral counterparts in the PH study patients (Table 2) or in the internal control group. Except for sVCAM-1 (higher in the PH study group relative to external controls), there were no significant differences either between the concentrations of these biomarkers in the peripheral venous samples of the PH study patients versus external controls (Table 3) or versus internal controls (data not shown). Analysis of covariance (ANCOVA) was used to compare these biomarkers concentrations adjusted for BMI, and this did not change the above findings.
Table 2.
Concentrations in the peripheral venous system compared to wedged PA in the PH study group (n=24*)
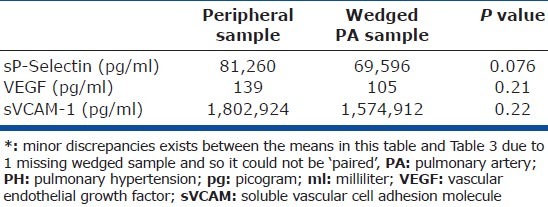
Table 3.
Concentrations of peripheral venous samples from the study PH group and healthy external control group expressed as mean (SD)
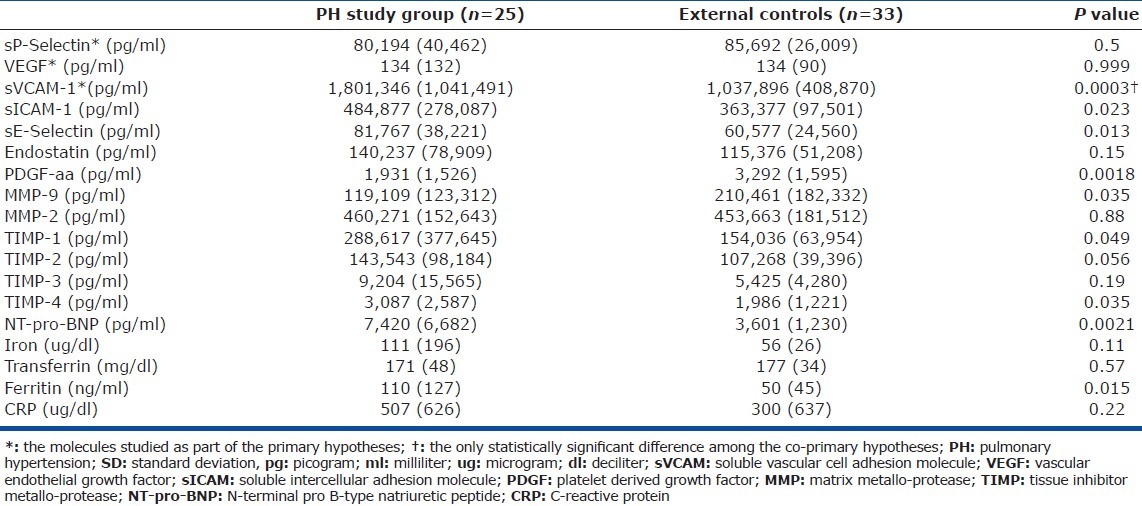
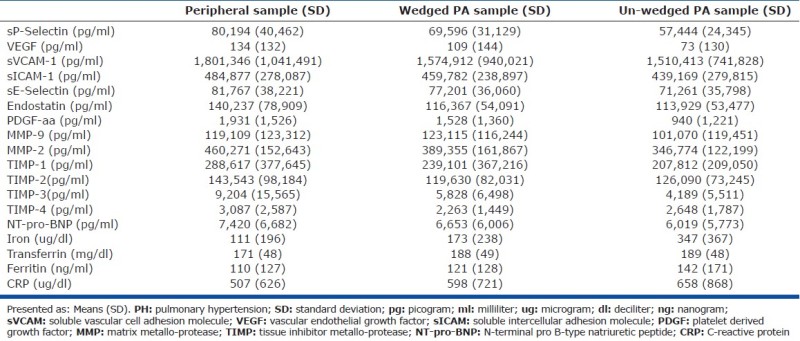
There was a nonsignificant trend of decreasing biomarkers concentrations from the peripheral to wedged PA to un-wedged PA samples (Table 2; Appendix B). The raw means and standard deviations of the concentrations of all 18 different biomarkers studied are presented to facilitate sample size calculations for future studies by other investigators (Appendix B).
DISCUSSION
Obtaining wedged blood samples from the pulmonary arteries of adult patients with PH is safe and feasible. None of the patients who had a wedged PA sample attempted had a complication, and the success rate for obtaining such a sample was 80%. The group of patients whom we were not able to get a wedged blood sample from had a significantly higher mean PA pressure than the group whom we were successful in obtaining the wedged blood sample. This finding might argue that the severity of pulmonary vasculopathy could play a key role in the feasibility of obtaining such samples.
Our exploratory data do not support the hypothesis that local distal PA serum levels of sP-Selectin, VEGF, or sVCAM-1 are different from the peripheral venous circulation when drawn from the distal pulmonary arterioles/capillaries/venules using a wedged PA blood sample. The levels of sP-Selectin and VEGF were also comparable between the PH study and control groups. There were large variations among endpoints as manifested by unexpectedly wide standard deviations for each of the molecules studied (Appendix B) which could undermine the power of this study.
One possibility for the lack of difference in the VEGF level could be that the signal was lost in serum separation (VEGF being released from platelets upon centrifugation). Plasma (instead of serum) collection might have been a better choice.
The meaningfulness, if any, of the trend of decreasing concentrations from the peripheral to wedged PA to un-wedged PA is unknown. It is interesting that in more than half of the biomarkers studied, the concentrations in the peripheral samples were higher than those in the wedged samples and those were in turn higher than in the un-wedged samples (admittedly without statistical significance). The trend of the wedged samples concentrations being higher than those of the un-wedged samples raises the theoretical possibility that the balloon inflation itself pressing on the vessel wall is potentially causing the release of some of these inflammatory biomarkers and cellular adhesion molecules.
In our investigation, there were no significant differences in indices of iron homeostasis between patients with PH and external controls. This may be somewhat discordant with previous studies. Iron availability was shown to affect PA pressures with an infusion of the chelator deferoxamine elevating values.[15] Hypoxia-induced PH was attenuated by increasing iron availability and exacerbated by decreasing it.[16,17] Disruptions in iron homeostasis were described among cohorts diagnosed with PAH. Iron deficiency without overt anemia was observed among patients with idiopathic PAH using serum iron, transferrin saturation, transferrin receptor, ferritin, hepcidin, erythropoietin, and zinc protoporphyrin.[18–20] However, there was no evidence of iron deficiency among our patients with PH using some of these same endpoints. It is not possible to suggest that inflammation skewed a potential relationship between iron and PH in our investigation since other blood endpoints exclude significant systemic inflammation (e.g., C-reactive protein). It may be that an association between iron homeostasis and PH exists in specific cohorts (e.g., hypoxia-induced PH) but not all.
One limitation of our study is that we do not know whether or not the 20% of patients that we could not get a wedged PA sample from are systematically different from our study patients. Such a potential difference could have confounded the biomarkers relationship, and potentially moved the study results closer to the null hypothesis, i.e., favoring lack of association between the biomarkers concentrations and the vascular bed. Although, their clinical and demographic characteristics were comparable to our study groups, they did have a higher mean PA pressure and presumably worse pulmonary vasculopathy.
Another limitation is that a relatively large proportion of our PH patients had pulmonary comorbidities including mild COPD. This may have confounded the relationship between the PH status and the inflammatory biomarkers. Similarly, one third of our PH population had a connective tissue disease (mostly scleroderma) associated with their PH. Since, the source of pathology or inflammation in connective tissue diseases is not primarily or necessarily the distal pulmonary arterioles, this would alter the differential venous distribution of the inflammatory and cell adhesion biomarkers. Another potential limitation is that fluoroscopy was not consistently used to confirm the wedging status of the PA catheter. A clear and typical wedge tracing and/ or a change in the color of the aspirated blood from dark (precapillary) to bright red (capillary or postcapillary) obviated the need for fluoroscopy confirmation in most of the patients.
In conclusion, obtaining wedged blood samples from the pulmonary arteries of adult patients with PH is safe and feasible. This could potentially open a new frontier of evaluation of patients, since in theory it could better reflect the local milieu in the precapillary arterioles, capillaries, and postcapillary venules. There were no significant differences in the concentrations of select inflammatory and cellular adhesion molecules between peripheral venous samples and wedged PA samples in patients with PH.
Appendix A: Basis of sample size estimation
Appendix B: Mean values (with standard deviations) of all the biomarkers studied for each vascular bed in the PH study group
Footnotes
Source of Support: This study was funded by Gilead Sciences Inc., NIH grant # 5T32HL007106-34 (Dr. Fares), and by the Division of Pulmonary and Critical Care Medicine at the University of North Carolina, at Chapel Hill
Conflict of Interest: None declared.
REFERENCES
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S78–84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: A reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–87. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Yaici A, Montani D, O’Callaghan DS, Jaïs X, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–55. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 5.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–9. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest. 2005;115:2691–4. doi: 10.1172/JCI26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23–42. doi: 10.1016/j.ccm.2006.11.010. 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaseanu C, Tudor S, Tamsulea I, Marta D, Manea G, Moldoveanu E. Vascular endothelial growth factor, lipoporotein-associated phospholipase A2, sP-selectin and antiphospholipid antibodies, biological markers with prognostic value in pulmonary hypertension associated with chronic obstructive pulmonary disease and systemic lupus erithematosus. Eur J Med Res. 2007;12:145–51. [PubMed] [Google Scholar]
- 10.Safian RD, Come SE, Kadin M, Lorell BH. Use of pulmonary capillary wedge aspirates for the antemortem diagnosis of pulmonary microvascular tumor. Cathet Cardiovasc Diagn. 1989;17:112–5. doi: 10.1002/ccd.1810170212. [DOI] [PubMed] [Google Scholar]
- 11.Brewster H, McIlroy MB. Blood gas tensions and pH of pulmonary “wedge” samples in patients with heart disease. J Appl Physiol. 1973;34:413–6. doi: 10.1152/jappl.1973.34.4.413. [DOI] [PubMed] [Google Scholar]
- 12.Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, et al. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica. 2008;93:20–6. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 13.Cella G, Vianello F, Cozzi F, Marotta H, Tona F, Saggiorato G, et al. Effect of bosentan on plasma markers of endothelial cell activity in patients with secondary pulmonary hypertension related to connective tissue diseases. J Rheumatol. 2009;36:760–7. doi: 10.3899/jrheum.080542. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 15.Balanos GM, Dorrington KL, Robbins PA. Desferrioxamine elevates pulmonary vascular resistance in humans: Potential for involvement of HIF-1. J Appl Physiol. 2002;92:2501–7. doi: 10.1152/japplphysiol.00965.2001. [DOI] [PubMed] [Google Scholar]
- 16.Smith TG, Talbot NP, Dorrington KL, Robbins PA. Intravenous iron and pulmonary hypertension in intensive care. Intensive Care Med. 2011;37:1720. doi: 10.1007/s00134-011-2325-y. [DOI] [PubMed] [Google Scholar]
- 17.Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, et al. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: Two randomized controlled trials. JAMA. 2009;302:1444–50. doi: 10.1001/jama.2009.1404. [DOI] [PubMed] [Google Scholar]
- 18.Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respira J. 2011;37:1386–91. doi: 10.1183/09031936.00100510. [DOI] [PubMed] [Google Scholar]
- 19.Decker I, Ghosh S, Comhair SA, Farha S, Tang WH, Park M, et al. High levels of zinc-protoporphyrin identify iron metabolic abnormalities in pulmonary arterial hypertension. Clin Transl Sci. 2011;4:253–8. doi: 10.1111/j.1752-8062.2011.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili E, Gibbs JS. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: Clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58:300–9. doi: 10.1016/j.jacc.2011.02.057. [DOI] [PubMed] [Google Scholar]


