Abstract
Prostacyclin analogs therapy has been associated with development of thrombocytopenia. Little is known whether this treatment increases the risk of intracranial hemorrhage in pulmonary artery hypertension (PAH) patients. We queried the Cleveland Clinic billing database to identify cases of nontraumatic sudural hematoma (SDH) in patients with PAH. We identified those individuals who were receiving prostacyclin analogs therapy at the time of the neurological event and assessed whether these patients were also taking antiplatelet or anticoagulation therapies. We identified three cases of nontraumatic SDH in 856-patient-year of prostacylin analog treatment. All patients were women, had low normal platelet counts or thrombocytopenia, and were concomitantly receiving anticoagulation therapy in the appropriate therapeutic anticoagulation range. All three patients were managed conservatively and had no neurologic sequelae. Nontraumatic acute subdural hematoma is a rare event in patients with PAH treated with prostacyclin analogs. All affected patients were concomitantly receiving anticoagulation therapy.
Keywords: anticoagulation, prostacyclin analogs, pulmonary hypertension, subdural hematoma
Pulmonary arterial hypertension (PAH) is a disease process that can lead to right heart failure and death.[1] The latest 4th World Symposium on Pulmonary Hypertension supports the use of prostacyclin analogs (including epoprostenol, treprostinil, and iloprost) for the treatment of PAH patients in New York Heart Association functional class (NYHA) III/IV.[2] This recommendation is based on several studies that showed improvements in symptoms, 6-Minute Walk Distance, and pulmonary hemodynamics in patients with PAH who were treated with different prostacyclin analogs.[3–6] In addition, intravenous epoprostenol was associated with a 12-week survival benefit when compared with conventional therapy in PAH patients.[7]
Patients with PAH have abnormalities in platelet aggregation, and prostacyclin analogs are associated with the development of thrombocytopenia even after adjustment for severity of hemodynamic abnormalities.[8,9] In fact, thrombocytopenia, defined as a platelet count <150,000/mL has been observed in 34-65% of the patients treated with epoprostenol.[9,10] Furthermore, prostacyclin analogs inhibit platelet aggregation and cause platelets to be less responsive to their natural agonists.[11–14]
A variable proportion of patients with PAH also receive anticoagulation therapy concomitantly with protacyclin analogs. This practice is based on retrospective studies, before the use of PAH-specific therapies, which showed thrombotic lesions in the pulmonary circulation and an increase in survival in PAH patients who received anticoagulation.[15,16] Patients receiving anticoagulation therapy are at an increased risk of bleeding; however, it is unknown whether the combination of prostacyclin analogs with this treatment further increases the risk of bleeding. We hereby report the occurrence of subdural hematomas in three patients with PAH who were concomitantly treated with prostacyclin analogs and therapeutic anticoagulation. This study was approved by the Cleveland Clinic Institutional Review Board (protocol approval number 11-021). Informed consent was waived. We identified the patients in this report by searching the Cleveland Clinic billing database using ICD 9 diagnostic codes that corresponded to PAH (416.0 and 416.8) and subdural hematoma (432.1) between January 2003 and January 2012. We then reviewed the medical charts of all these patients (n = 99) and identified three patients who developed non-traumatic subdural hematoma while receiving treatment with prostacyclin analogs. We excluded one patient treated with specific-PAH therapies who had a traumatic subdural hematoma. All patients had confirmed PAH by right heart catheterization. We recorded the use of anticoagulation and/or anti-platelet therapies around the time of intracranial bleed. We also collected laboratory values such as platelet counts and coagulation profiles just before the bleeding event.
CASE REPORTS
Patient 1
The first patient is a 64-year-old Caucasian female with a diagnosis of idiopathic PAH made in July 2005. Her other past medical history includes essential hypertension, hyperlipidemia, type II diabetes mellitus, and coronary artery disease status post percutaneous coronary intervention with stent placement. The patient was in NYHA Functional Class III. Right heart catheterization showed a mean pulmonary artery pressure of 41 mmHg, cardiac index of 2.2 l/min/m2, and mixed venous O2 saturation of 72%. Echocardiogram showed moderate-to-severe right ventricular dilation with moderate right ventricular dysfunction. The patient started treatment for PAH with the intravenous prostacyclin analog treprostinil in August of 2005. In addition to the prostacyclin analog, the patient received aspirin 81 mg daily, clopidogrel 75 mg daily, and warfarin 3 mg daily. After seven months of this treatment and without any trauma, the patient presented to her local hospital with new onset confusion. Her dose of treprostinil at this time was 58 ng/kg/min. A computed tomography (CT) scan showed an acute subdural hematoma (Fig. 1, panel A). Her blood work prior to her intracranial bleed showed a low normal platelet count of 162,000/uL and an international normalized ratio (INR) of 2.7. The patient's clopidogrel and warfarin were discontinued. Her neurologic status remained stable. She was continued on treprostinil for treatment of her pulmonary hypertension. She had no residual neurological deficits as result of her bleed.
Figure 1.
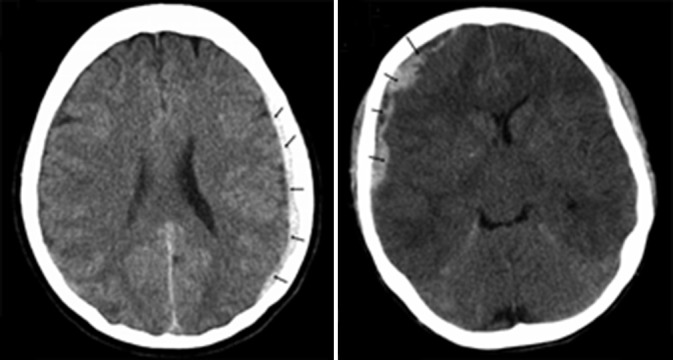
Subdural hematoma. Computed tomographies revealing subdural hematomas (arrows) in the first (panel A) and second cases (panel B).
Patient 2
The second patient is a 46-year-old female with a diagnosis of mixed pulmonary arterial and pulmonary venous hypertension made in February of 2008. She began treatment with the inhaled prostacyclin analog iloprost in April of 2008. Her other past medical history included paroxysmal atrial fibrillation and history of rheumatic heart disease status post mitral and aortic valve replacement with mechanical prosthetic valve. The patient was in NYHA Functional Class III. Right heart catheterization showed a right atrial pressure of 17 mmHg and a right ventricular pressure of 132/2 mmHg. Pulmonary artery pressure could not be directly measured due to the inability to advance the catheter into the pulmonary artery. Her echocardiogram showed moderately dilated right ventricle with moderately decreased right ventricular systolic function. The patient was admitted to our hospital in December of 2009 for evaluation of dyspnea when she developed new onset headaches. The dose of iloprost at that time was 6 inhalations per day of 5 mcg. A CT scan of the brain showed multiple acute and subacute subdural hematomas overlying the cerebral hemispheres and left posterior fossae (Fig. 1, panel B). At the time of her bleed, the patient was also taking aspirin 81 mg daily. She was receiving therapeutic anticoagulation with heparin for her mechanical valve with an activated partial thromboplastin time (aPTT) value of 71.2 seconds. She was noted to have mild thrombocytopenia with a count of 145,000/uL. The patient's anticoagulation therapy was held and she did not have any neurologic sequelae from her bleed. Her anticoagulation therapy was later resumed with a lowered target INR of 1.5-2. She had no further known episodes of bleeding afterwards.
Patient 3
The third patient is a 58-year-old female with idiopathic PAH who had been receiving intravenous epoprostenol since November 2010. She was in NYHA Functional Class I/II. Right heart catheterization showed mean right atrial pressure of 4 mmHg, pulmonary artery pressure of 65/32, mean pulmonary artery pressure of 43 mmHg, a cardiac index of 2.2 l/min/m2, and mixed venous O2 saturation of 60%. Her echocardiogram showed moderate right ventricle dilation with moderate right ventricle systolic dysfunction. Her other relevant past medical history includes essential hypertension, type II diabetes mellitus on insulin therapy, and depression. Five months after the initiation of epoprostenol, the patient presented to local hospital with headache, nausea, disorientation, and gait difficulties. The dose of epoprostenol was 30 ng/kg/min. She underwent CT scan of the head which showed subacute subdural hematomas involving the left frontoparietal and right frontal areas. The patient was receiving warfarin at the time of her bleed. Blood work revealed thrombocytopenia with a platelet count of 94,000/uL and INR of 2.4. Anticoagulation therapy was discontinued. She was followed by our neurosurgical team who observed interval resolution of her hematomas. Patient recovered without significant neurologic deficits. She was subsequently continued on epoprostenol.
DISCUSSION
Of the patients with PAH treated with prostacyclin analogs who are regularly followed at Cleveland Clinic since 2003 (856-patient-year), we identified only three individuals who developed non-traumatic acute subdural hematoma. This gives us an incidence of non-traumatic SDH of 3.5 cases of non-traumatic SDH per 1,000 patient-years of prostacyclin analog treatment. All patients were women who had low normal platelet count or thrombocytopenia and were receiving anticoagulation therapy with an appropriate INR or aPTT range. Two patients were also under treatment with aspirin or clopidogrel at the time of the neurologic event. In all cases, the subdural hematoma was managed conservatively without the need for surgical intervention. None of the patients had any neurologic sequelae and all continued treatment with prostacyclin analog therapy.
Multiple studies have examined the safety of intravenous and inhaled formulations of prostacyclin analogs.[5,17,18] As of yet, there is limited data describing the relative risks of hemorrhage in PAH patients treated with these medications. Bleeding complications such as GI bleeding have been described during continuous infusion of treprostinil.[4] Ogawa et al. reported the occurrence of alveolar hemorrhage in nine out of 31 consecutive patients with idiopathic PAH treated with epoprotenol and anticoagulation during a mean follow-up time of up to two years.[19] To our knowledge, no trial has described the development of subdural hematomas as a result of prostacyclin analog treatment.
Since prostacyclin analogs are associated with the development of thrombocytopenia[9,10] and these medications inhibit platelet aggregation,[14,20] it is reasonable to think that treated patients may be at a higher risk of developing intracranial hemorrhage especially if they are receiving concomitant anticoagulation therapy. In the present study all patients who developed non-traumatic subdural hematomas were women receiving concomitant anticoagulation therapy and had either low or low-normal platelet counts. The prostacyclin analog doses were not unusually high. The INR or aPTT levels were within the optimal range in all patients, suggesting that the bleeding was not related to anticoagulation overdose.
Intracranial hemorrhage (primarily intracerebral) is overall an uncommon complication of antithrombotic therapy with rates that range from 0.3 to 0.6% per year of treatment.[21] However, the precise incidence of this complication is unknown as most of the trials studying this medication excluded patients at risk for intracerebral hemorrhage. Usually this complication is associated with advance age, high INR (≥ 3), and concomitant use of antiplatelet therapy.[21,22] Anticoagulation therapy is also a known risk factor for the development of subdural hematoma predominantly in patients with INR ≥ 4.[23,24] Interestingly in our study, none of the PAH patients who received prostacylin analogs without anticoagulation therapy developed non-traumatic acute subdural hematomas. Thus, it appears that the risk for this neurological complication is low and likely related to the concomitant use of anticoagulation.
A limitation of the present study is that cases were retrospectively collected thus we cannot be entirely sure that we included all patients with this complication. In addition, it is possible that some patients had subclinical subdural hematomas that were not detected.
In conclusion, nontraumatic acute subdural hematoma is a rare event in patients with PAH treated with prostacyclin analogs. Its development appears to be associated with thrombocytopenia and simultaneous use of anticoagulation treatment.
Footnotes
Source of Support: Dr. Adriano Tonelli is supported by CTSA KL2 Grant # RR024990 (Adriano R Tonelli) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research
Conflict of Interest: None declared.
REFERENCES
- 1.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, loyd JE, Manes A, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S78–84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132:425–34. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: A double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 5.Tapson VF, Gomberg-Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman AD, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: A prospective, multicenter, open-label, 12-week trial. Chest. 2006;129:683–8. doi: 10.1378/chest.129.3.683. [DOI] [PubMed] [Google Scholar]
- 6.Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesh DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The primary pulmonary hypertension study group. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 8.Aytekin M, Aulak KS, Haserodt S, Chakravarti R, Cody J, Minai OA, et al. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: Role of nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2012;302:L512–20. doi: 10.1152/ajplung.00289.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin KM, Channick RN, de Lemos JA, Kim NH, Torres F, Rubin LJ. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135:130–6. doi: 10.1378/chest.08-1323. [DOI] [PubMed] [Google Scholar]
- 10.Hargett CW. Thrombocytopenia associated with chronic intravenous epoprostenol therapy. Chest. 2004;126:760S. [Google Scholar]
- 11.Sinzinger H, Horsch AK, Silberbauer K. The behaviour of various platelet function tests during long-term prostacyclin infusion in patients with peripheral vascular disease. Thromb Haemost. 1983;50:885–7. [PubMed] [Google Scholar]
- 12.Beghetti M, Reber G, de MP, Vadas L, Chiappe A, Spahr-Schopfer I, et al. Aerosolized iloprost induces a mild but sustained inhibition of platelet aggregation. Eur Respir J. 2002;19:518–24. doi: 10.1183/09031936.02.00094302. [DOI] [PubMed] [Google Scholar]
- 13.Yardumian DA, Machin SJ. Altered platelet function in patients on continuous infusions of epoprostenol. Lancet. 1984;1:1357. doi: 10.1016/s0140-6736(84)91855-5. [DOI] [PubMed] [Google Scholar]
- 14.Whittle BJ, Moncada S, Vane JR. Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and D2 on platelet aggregation in different species. Prostaglandins. 1978;16:373–88. doi: 10.1016/0090-6980(78)90216-2. [DOI] [PubMed] [Google Scholar]
- 15.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: Natural history and the importance of thrombosis. Circulation. 1984;70:580–7. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 16.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 17.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: Prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–8. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 18.Hoeper MM, Schwarze M, Ehlerding S, Adler-Schuermeyer A, Spiekerkoetter E, Niedermeyer J, et al. Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. N Engl J Med. 2000;342:1866–70. doi: 10.1056/NEJM200006223422503. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa A, Matsubara H, Fujio H, Miyaji K, Nakamura K, Morita H, et al. Risk of alveolar hemorrhage in patients with primary pulmonary hypertension-anticoagulation and epoprostenol therapy. Circ J. 2005;69:216–20. doi: 10.1253/circj.69.216. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RJ, MacDermot J, Wilkins AJ. Prostacyclin analogues reduce ADP-ribosylation of the alpha-subunit of the regulatory Gs-protein and diminish adenosine (A2) responsiveness of platelets. Br J Pharmacol. 1987;90:501–10. doi: 10.1111/j.1476-5381.1987.tb11199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy: Recent data and ideas. Stroke. 2005;36:1588–93. doi: 10.1161/01.STR.0000170642.39876.f2. [DOI] [PubMed] [Google Scholar]
- 22.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–4. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 23.Levine MN, Hirsh J, Landefeld S, Raskob G. Hemorrhagic complications of anticoagulant treatment. Chest. 1992;102:352S–63S. doi: 10.1378/chest.102.4_supplement.352s. [DOI] [PubMed] [Google Scholar]
- 24.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]


