Abstract
Opiate dependence (OD) and Borderline Personality Disorder (BPD), separately and together, are significant public health problems with poor treatment outcomes. BPD is associated with difficulties in emotion regulation, and brain imaging studies in BPD individuals indicate differential activation in prefrontal-cingulate cortices and their interactions with limbic regions. Likewise, a similar network is implicated in drug cue responsivity in substance abusers. The present, preliminary study uses functional magnetic resonance imaging (fMRI) to examine activation of this network in comorbid OD/BPD participants when engaged in an “oddball” task that requires attention to a target in the context of emotionally negative distractors. Twelve male OD/BPD participants and 12 male healthy controls participated. All OD/BPD participants were taking the opiate replacement medication Suboxone, and a subset of participants were positive for substances of abuse on scan day. Relative to controls, OD/BPD participants demonstrated reduced activation to negative stimuli in the amygdala and anterior cingulate. Unlike previous studies that demonstrated hyperresponsivity in neural regions associated with affective processing in individuals with BPD versus healthy controls, comorbid OD/BPD participants were hyporesponsive to emotional cues. Future studies that also include BPD-only and OD-only groups are necessary to help clarify the individual and potentially synergistic effects of these two conditions.
Keywords: Borderline Personality Disorder, Opiate Dependence, fMRI, Amygdala, Emotion
Borderline personality disorder (BPD) is characterized by functional impairment across interpersonal, behavioral, cognitive, and especially emotional domains (Rosenthal, et al., 2008; Skodol, et al., 2002). In addition to difficulties associated with the disorder itself, BPD frequently co-occurs with Axis I disorders including substance dependence (Grant, et al., 2008; Skodol, et al., 2002). Comorbidity between opiate dependence (OD) and BPD is particularly problematic. Within treatment-seeking opiate dependent populations, comorbidity with BPD ranges from 5.2% (Brooner, King, Kidorf, Schmidt, & Bigelow, 1997) to 44% (Sansone, Whitecar, & Wiederman, 2008), and among individuals identified with BPD, approximately 38% meet criteria for drug abuse or dependence (Trull, Sher, Minks-Brown, Durbin, & Burr, 2000). Among those in treatment for OD, the presence of BPD is associated with poorer outcomes including higher rates of mood disorder, heroin overdose, suicide, and injection-related health problems at follow-up (Darke, et al., 2007; Kosten, Kosten, & Rounsaville, 1989). One potential contributor to the high rate of comorbidity between BPD and OD is a desire among BPD individuals to “self-medicate” as a way of coping with overwhelming negative affect (Trull, et al., 2000). A greater understanding of affective processes that underlie comorbid OD/BPD has the potential to inform the understanding and treatment of this difficult condition.
Disruptions in affective processing have been observed in both BPD and OD. In BPD, studies using self-report and experience sampling techniques have demonstrated greater affective instability (Ebner-Priemer, et al., 2007; Koenigsberg, et al., 2002; Stein, 1996; Trull, et al., 2008). Psychophysiological studies of emotion in BPD are mixed, with some studies suggesting heightened physiological reactivity to emotion (Ebner-Priemer, et al., 2005; Ebner-Priemer, et al., 2007) and others suggesting hypoarousal (Herpertz, Kunert, Schwenger, & Sass, 1999; Herpertz, et al., 2000) In addition, individuals with BPD show heightened sensitivity to identification of emotional cues in laboratory studies (Domes, et al., 2008; Lynch, et al., 2006; Wagner & Linehan, 1999). In contrast to heightened sensitivity to emotion in BPD, OD individuals show impaired ability to identify facial expressions of emotion (Kornreich, et al., 2003; polysubstance users including a high percentage of opiate users, Verdejo-Garcia, Rivas-Perez, Vilar-Lopez, & Perez-Garcia, 2007). Though studies are inconsistent in reports of heightened or reduced subjective ratings of emotional cues relative to non-substance-using controls, OD individuals show an attenuated heart rate, blood pressure, and endocrine response to negative stimuli relative to controls (Gerra, et al., 2003), anomalous zygomatic muscle reactivity to negative images (Lubman, et al., 2009), and exaggerated attentional blink (i.e., a deficit in perceiving the second of two stimuli presented in close succession) to negative and neutral (but not addiction-related) stimuli following an initial stimulus (Liu, Li, Sun, & Ma, 2008). They also fail to demonstrate valence or arousal effects on attention as measured by P300 ERP responses to novel cues in positive, negative, and neutral emotional contexts (Marques-Teixeira & Santos Barbosa, 2005). These findings have led to the suggestion that substance-dependent individuals, including OD, demonstrate a reduced sensitivity to emotional stimuli (Verdejo-Garcia, Perez-Garcia, & Bechara, 2006). Given findings of emotion hypersensitivity in BPD and hyposensitivity in OD, it is unclear how emotion sensitivity may be affected in a comorbid population.
Consistent with behavioral and self-report studies, BPD is characterized by functional changes in brain regions serving emotional processes. BPD individuals show reduced ventromedial prefrontal activation, including orbitofrontal cortex, and increased limbic/striatal activation when engaging in a go/no go task under emotional load, suggesting a neural link between emotion dysregulation and impulsive behavior (Silbersweig, et al., 2007). Likewise, heightened activity in amygdala (Donegan, et al., 2003; Driessen, et al., 2004; Herpertz, et al., 2001; Koenigsberg, et al., 2009; Minzenberg, Fan, New, Tang, & Siever, 2007), medial prefrontal cortex (Herpertz, et al., 2001; Schnell & Herpertz, 2007), and orbitofrontal cortex (Driessen, et al., 2004) have been observed in BPD participants (relative to both healthy controls and non-BPD individuals with a history of trauma or abuse) when engaging with emotional stimuli. Disturbed connectivity between prefrontal regions (associated with emotion regulation and impulse control) and limbic regions including amygdala (associated with emotion reactivity) has been implicated in BPD (New, et al., 2007). Overall, neuroimaging studies largely provide support for the presence of an underlying biologically-based emotional dysfunction in BPD, including enhanced frontolimbic activation to emotionally aversive cues.
Less is known about neural responding to emotional cues in OD individuals. In OD, heightened activity in response to drug-related cues has been observed in the same fronto-limbic regions associated with emotional processing, including amygdala, medial prefrontal, and orbitofrontal cortex (e.g., Yang, et al., 2009). In a recent study of reactivity to affective cues in heroin dependent and non-dependent control groups, the heroin dependent participants showed reduced activation in right amygdala to negative versus neutral images (Z. X. Wang, et al., 2010). It has been suggested that in drug dependence, neural circuits typically activated by attention, motivation, and emotion are “hijacked,” increasing the salience of drug cues at the expense of other cues (Daglish, et al., 2003).
The goal of the current study was to compare responses to affective stimuli between a comorbid OD/BPD sample and healthy controls using functional magnetic resonance imaging (fMRI). The experimental task asked participants to attend to a neutral target image in the context of affective and other distractor images. We hypothesized that group differences will be observed in fronto-limbic affective processing regions when viewing emotional versus neutral distractors. However, given that OD is associated with dampened emotional responding while BPD is associated with heightened emotional responding, the direction of that difference (i.e., heightened versus reduced) is unknown.
Methods
Participants
Twelve male subjects (2 left handed, mean age=30.8) who met full diagnostic criteria for both opiate dependence (OD) and borderline personality disorder (BPD) and 12 healthy control subjects (2 left-handed, mean age=32.8) participated in the study. Participants in the OD/BPD group were recruited from an outpatient treatment study comparing Dialectical Behavior Therapy and standard Individual/Group Drug Counseling as treatments for OD in individuals with BPD. Participants from both treatment conditions were included. All participants were recruited and scanned at the University of Washington. Control participants were recruited via local and university web site and flyer advertisements. The inclusion criteria for the OD/BPD treatment study were: (a) diagnosis of primary OD, as assessed by the Structured Clinical Interview for DSM-IV Disorders – Axis I (SCID I; First et al., 1995); (b) diagnosis of BPD, as assessed by the Structured Clinical Interview for DSM-IV Disorders – Axis II (SCID II; First et al., 1996); (c) no diagnosis of bipolar disorder or psychotic disorders, as assessed by the SCID I; (d) estimated verbal IQ of 75 or greater, as assessed by the Peabody Picture Vocabulary Test (Dunn & Dunn, 1997); and (e) no current use of prescribed psychiatric medications (e.g., antidepressants). Axis I conditions not specifically ruled out as stated above were permitted (e.g., major depressive disorder, eating disorders, anxiety disorders were allowed). OD/BPD participants had been in treatment for an average of 14.8 weeks, and all OD/BPD participants were taking the opiate replacement medication buprenorphine/naloxone (Suboxone). No other psychoactive medications (e.g., antidepressants) were prescribed or permitted in the protocol, and all previously prescribed psychoactive medications were discontinued/tapered before participants entered the treatment study. Control subjects were lifetime-free of substance abuse/dependence and opiate use, as assessed by the SCID I, and did not meet criteria for BPD, as assessed by the SCID II. Further inclusion criteria for all participants included no conditions that would interfere with the safety or quality of MRI scanning (e.g., implanted metal, history of neurological injury, claustrophobia) and male gender. The study was restricted to a single gender in order to reduce gender-related variance in emotion reactivity (Cahill, 2003), and male gender was selected because males are under-represented in studies of BPD.
Detailed demographic and clinical assessments for the two groups are listed in Table 1. Groups did not differ in age or race/ethnicity, but the control group had a higher average education level than the OD/BPD group. The study was approved for ethical treatment of human subjects by the Institutional Review Boards at the University of Washington and Duke University Medical Center (where pilot testing and data analyses were conducted), and all subjects provided written informed consent after the procedures had been fully explained.
Table 1.
Demographic and Questionnaire Information for Control and OD/BPD Participants
| Control (n = 12) | OD/BPD (n = 12) | |
|---|---|---|
|
|
||
| Age Mean (SD) | 32.8 (13.9) a | 30.8 (9.5) a |
| Education Group: Mean (SD) | 7.5 (1.8)a | 4.5 (1.5) b |
| Education Group: Mode | Some college (6/12) | Some college (5/12) |
| Racial/Ethnic Background | ||
| White/Caucasian | 50% a | 66.7% a |
| White/Latino | 8.3% | 8.3% |
| Asian | 41.56% | 0% |
| Native American | 0% | 8.3% |
| Left-handed | n = 2 a | n = 2 a |
| Dissociation score (SD) | 0.25 (.58) a | 1.91 (1.73) b |
Note. Education Groups range from 2 = some high school to 10 = doctoral degree. Differing superscripts across rows indicate a significant difference at p < .05; identical subscripts indicate no statistical difference.
Scan day
Urinalysis (UA) was performed at the beginning of the scan session for both OD/BPD and control groups. UA measured detectable levels of opiates, amphetamines, methamphetamines, barbiturates, benzodiazepines, cocaine, PCP, marijuana, methadone, and tricyclics (Triage TOX system, Biosite Inc., San Diego CA). OD/BPD participants received their prescribed dose of buprenorphine/naloxone immediately before the scan. Dissociative symptoms were measured by the four-item Dissociative Symptoms Scale (Stiglmayr, Shapiro, Stieglitz, Limberger, & Bohus, 2001) before and after the scan. Anatomical and functional images were collected in the same scan session.
Experimental design
An “emotional oddball” task (L. Wang, et al., 2008; L. Wang, McCarthy, Song, & LaBar, 2005) was administered in which participants were asked to watch for a target image in the context of standard scrambled images as well as negative, neutral, and drug image distractors. All of the distractors were trial-unique. The presentation frequency for targets and negative, drug, and neutral distractors was 2.85% each, with standards comprising the remaining 88.6% of stimuli presented. Participants pressed a response button using their right index finger upon detection of a target oddball stimulus (circle) and their right middle finger to all other stimuli. The imaging session consisted of 10 runs, each containing 175 stimuli (stimulus duration=1500 ms, inter-stimulus interval= 2000 ms). The interval between successive rare stimuli (targets and/or distractors) was randomized between 16–20 s to allow hemodynamic responses to return to baseline.
The present study focuses on negative image distractor trials; activation tables for target and drug cue trials are reported in supplemental materials available online.
Stimuli
The stimuli and design of the emotional oddball task were similar to that described previously (Wang et al., 2005). Briefly, distractor images were chosen from the International Affective Picture Set (IAPS; Lang, Bradley & Cuthbert, 2005) and images used in previous studies (Wang et al., 2005). Negative images included pictures of injury, conflict and facial displays of negative emotions, while neutral images included everyday scenes of work and shopping as well as neutral facial displays. Drug pictures included scenes of drug use, drug paraphernalia and supplies, social contexts, and money. Distractor images were categorized as negative, neutral, and drug-related based on pilot testing by 11 control and 7 OD/BPD participants (who did not participate in the main study). On a nine-point visual analogue scale, negative images averaged valence scores of 2.3 and arousal scores of 6.1; neutral images averaged valence scores of 4.7 and arousal scores of 3.1. The attentional targets were circles of varying sizes and luminance, and the standard stimuli were phase-scrambled and luminance-matched versions of the distractors. All images were converted to grayscale.
Image acquisition and analysis
MR images were acquired on a 3.0 Tesla Phillips Achieva scanner and were analyzed as described previously (Wang et al., 2005). High-resolution T1 images were collected using a 3D MPRAGE pulse sequence (a Turbo Field Echo (TFE) sequence with an inversion pulse) and an R/L SENSE factor of 1.5. Parameters for anatomical scanning were modified over the course of the study; however, all data were normalized to standard space following coregistration, so the impact of these changes should be minimal.1 Functional 2D radiant echo EPI images were acquired transaxially with the following parameters: TR = 2000 ms, TE = 30 ms, FOV = 24 × 24 cm, flip angle = 76°, matrix = 64×64, 32 contiguous images, slice thickness = 3.8 mm, resulting in 3.75 × 3.75 × 3.8 mm voxels.
Image pre-processing was conducted using temporal realignment for interleaved slice acquisition and spatial realignment to adjust for motion using affine transformation routines implemented in SPM (Wellcome Department of Cognitive Neurology, London, UK). The realigned images were co-registered to the anatomic images obtained for each participant and normalized to SPM’s template image, which conforms to the Montreal Neurologic Institute’s standardized brain space. The voxel size was 3.5×3.5×3.5 mm3 after normalization. The functional data were spatially smoothed with an 8-mm isotropic Gaussian kernel prior to statistical analysis.
The voxel-wise and region-of-interest (ROI) analyses used custom MATLAB scripts (Pelphrey et al., 2003, Dolcos and McCarthy, 2006, Wang et al., 2006). Event epochs were extracted for a voxel-based event-related analysis and a functional ROI analysis. The hemodynamic response was time-locked to the onset of each image of interest (negative and neutral distractors). The whole epoch of each event was extracted from 4 s before the onset of the stimulus to 14 s post-onset. Voxel-based signal percentage change at each post-onset time point (from 0 s to +14 s) was calculated for each subject by subtracting the mean pre-stimulus baseline activity (activity to scrambled pictures presented from 4 s to 0 s prior to each event) and then averaging across all trials with the same event type. We validated the hemodynamic time course at each voxel by testing the correlation of the hemodynamic response across time with the canonical gamma hemodynamic response for each event in each subject.
Statistical contrasts at each time point were set up using a random-effect analysis to calculate signal differences between the conditions of interest across each group of participants. Statistical t maps at each time point were derived for the events of interest, resulting in a t statistic for every voxel. This sequential approach accounts for inter-subject variability and permits generalization to the population at large. Only the results at peak time point 6 s post-stimulus are reported here. Only those voxels whose hemodynamic responses were significantly correlated with the canonical hemodynamic response (false discovery rate-corrected p < 0.01 with a spatial extent of five contiguous voxels) were entered into further within-and between-group analyses. Two-sample t-tests were conducted to compare voxel-wise signal changes at the peak time point (6 s post-stimulus) between OD/BPD and controls at for each negative image, threshholded at p < 0.001 uncorrected with a spatial extent of five contiguous voxels. Regions were identified using Harvard-Oxford and Talairach atlases.
To visualize the hemodynamic response profile for each functional region, an ROI analysis was performed. The middle frontal gyrus (MFG), anterior cingulate gyrus (ACG), amygdala, and hippocampus were chosen as ROIs based on previous study results (L. Wang, LaBar, & McCarthy, 2006; L. Wang, et al., 2008; L. Wang, et al., 2005). The mean signal change within each ROI was computed for each time point for each event. Only regions demonstrating voxel-based cluster group differences in t values at p < .01 in the present study were further analyzed. To confirm the voxel-based findings, a statistical analysis using ANOVA was conducted on the ROIs, focused on the mean percent signal change by hemisphere at the peak time point (6 s post-stimulus). A 2 (Type: negative, neutral) × 2 (Group: OD/BPD, control) ANOVA was performed.
Results
Sample characteristics
Urinalysis results were negative for opiates for 8 of 12 OD/BPD participants. The most common positive non-opiate result was for THC (9/12 positive), followed by cocaine (2/12), benzodiazepines (1/12), and amphetamine (1/12). Among controls, one participant was positive for THC, and the remaining 11 were negative for all substances.
Reaction time and self-report
T tests were conducted to compare square-root transformed in-scanner reaction times (RT) between groups. The OD/BPD and control groups did not differ in their reaction times to negative, t(22) = 0.31, p = .75, or neutral stimuli t(22) = 0.76, p = .45. In rating the valence and arousal levels elicited by each image after the scan, data for two control participants and three OD/BPD participants were discarded due to systematically repeated ratings (used the same number for all ratings) and notably short (< 300 ms) reaction times. Subjective valence and arousal ratings for negative and neutral distractors were analyzed using 2 (Distractor Valence: negative, neutral) × 2 (Group: OD/BPD, control) MANOVAs for valence and arousal ratings. For valence ratings, there was a significant main effect of Distractor Valence, F(1,17) = 38.83, p < .00001, such that negative images were rated more negative than neutral images. The main effect of Group was not significant, F(1,17) = 0.91, p = .34, nor was the Distractor Valence x Group interaction, F(1,17) = 0.79, p = 44. Similarly for arousal ratings, there was a significant main effect of Distractor Valence, F(1,17) = 29.72, p = .00004, such that negative images were rated more arousing than neutral images. The main effect of Group was not significant, F(1,17) = 0.04, p = .84, nor was the Distractor Valence x Group interaction, F(1,17) = 0.03, p = 86. Average image ratings and reaction times are included in Supplemental Table 3. Finally, pre- and post-scan dissociative symptom scores were averaged, and t tests were used to compare scores between groups. Consistent with dissociation as a symptom of BPD, dissociation scores were higher in the OD/BPD group than in controls, t(22) = 3.16, p = .005. Scores are reported in Table 1.
fMRI results
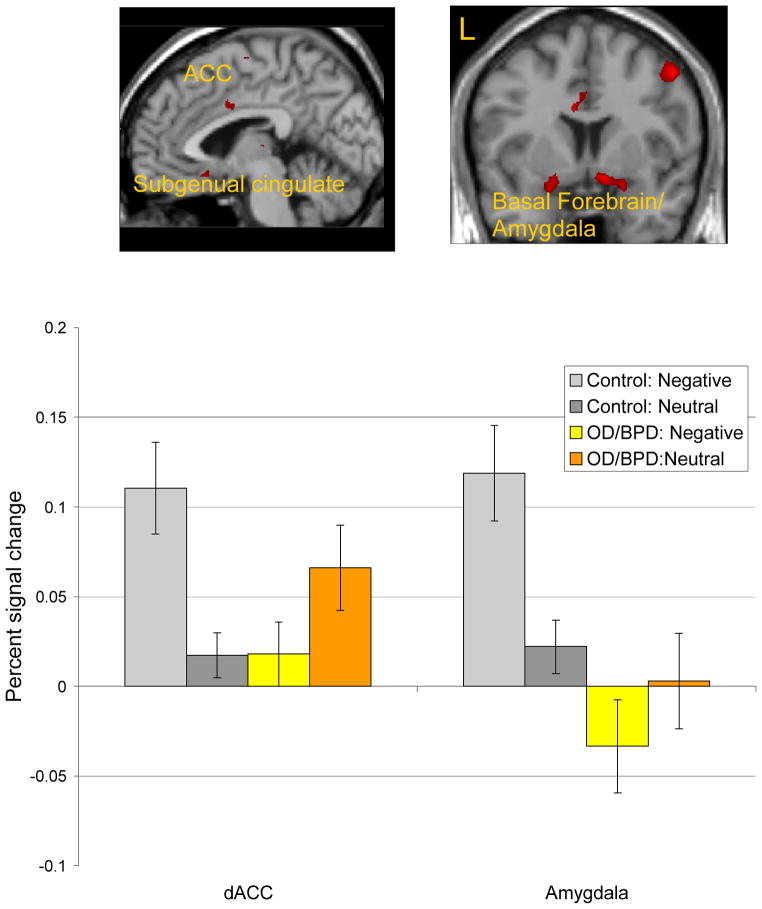
A direct statistical comparison between the OD/BPD and control groups revealed activity reductions in the OD/BPD group to negative images (Negative-Neutral distractor contrast) in emotion processing regions including bilateral amygdala, extending to hippocampus and ACG, as well as left subgenual cingulate. The OD/BPD group showed greater activation than controls to negative images in left inferior frontal gyrus (Table 2, Figure 1). Follow-up 2 (Group: OD/BPD, control) × 2 (Distractor: negative, neutral) ANOVAs were performed for the fronto-cingulate-limbic ROIs. P values were Bonferroni-corrected to a significance value of p < .007. Group x Distractor interactions were observed in dorsal ACG, left and right amygdala (basal frontal and extended amygdala), right MFG, and left hippocampus, whereby controls, but not OD/BPD participants, showed greater activation to negative than neutral stimuli.2
Table 2.
Brain Regions Associated with Activation to Negative vs. Neutral Targets (Threshold p < 0.001)
| Region | BA | Hemisphere | Maximum t | MNI coordinates | Cluster size | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Control> OD/BPD | ||||||||
| Subgenual cingulate | 25 | L | 3.93 | −4 | 14 | 11 | 6 | |
| Superior frontal gyrus | 6 | R | 4.05 | 16 | 12 | 62 | 6 | |
| Anterior cingulate gyrus | 24 | Midline | 5.02 | 0 | 4 | 35 | 8 | |
| Amygdala | - | R | 5.01 | 25 | 4 | −18 | 19 | |
| - | L | 4.71 | −25 | 4 | −18 | 14 | ||
| Precentral gyrus | 6 | R | 5.27 | 44 | −2 | 50 | 23 | |
| Precentral gyrus | 6 | R | 4.51 | 42 | −7 | 49 | 7 | |
| Thalamus | - | R | 4.03 | 7 | −18 | 7 | 6 | |
| Cerebellum | - | R | 4.10 | 14 | −46 | −11 | 9 | |
| Superior parietal sulcus | 7 | L | 4.86 | −35 | −60 | 60 | 19 | |
| OD/BPD >Control | ||||||||
| Inferior frontal gyrus | 45 | L | 4.87 | −53 | 39 | 7 | 12 | |
Figure 1.
Voxel-based random effect analysis for negative targets at the peak time point. Significantly decreased activation in the OD/BPD group compared with the control group from a two-sample t test on activation to the negative minus neutral contrast at the peak time point (p < .001, uncorrected, spatial extent of five contiguous voxels). The mean percentage signal change in dorsal ACC and amygdala (averaged over left and right hemispheres) at the peak time point is shown in the graphs below each image. Error bars reflect standard error of the mean. ACC = anterior cingulate cortex; L = left hemisphere; R = right hemisphere.
Effects of recent substance use and dissociation
In order to address the potential effects of recent substance use on activation in fronto-cingulate-limbic ROIs, the number of substances with positive UAs at the MRI scan was coded for all participants (range: 0–4). Follow-up 2 (Group: OD/BPD, control) × 2 (Distractor: negative, neutral) ANOVAs for ROIs were repeated, adding the positive UA variable as a covariate. There were no significant effects of, or interactions with, the positive UA variable. The overall pattern of results was consistent when positive UAs were used as a covariate, using a conventional p < .05 criteria for significance. Similarly, the presence or absence of a positive screen for opiates was added as a covariate in a separate set of analyses. Again, there were no significant effects of, or interactions with, the positive opiate variable.
Dissociative Symptoms Scale (DSS) scores were used as a covariate in an additional series of Group x Distractor ANOVAs predicting ROI activation levels. Again, the overall pattern of results was unchanged when including DSS scores as a covariate, using a conventional p < .05 criteria for significance. For dorsal ACG, left and right amygdala, and right MFG, there was no significant main effect of DSS score or interaction between DSS scores and image valence. For left hippocampus, a significant main effect of dissociation was observed, F(1,21) = 14.23, p = .001, as was an interaction between image valence and DSS scores, F(1.21) = 5.22, p = .03. There was a significant negative correlation between DSS scores and hippocampal activation to both negative and neutral distractors, r = .54, p = .007 and r = .58, p = .003, respectively.
Discussion
The goal of the present study was to compare affective processing between OD/BPD and control groups. The OD/BPD group showed reduced neural reactivity to negative distractors in regions associated with affective processing (amygdala, ACG) when compared with controls. These results are inconsistent with previous studies of emotion reactivity in BPD, which have generally found relatively heightened amygdala activation (Donegan, et al., 2003; Driessen, et al., 2004; Herpertz, et al., 2001; Koenigsberg, et al., 2009; Minzenberg, et al., 2007). However these results are consistent with findings of reduced recognition of, and physiological reactivity to, negative cues in OD (Gerra, et al., 2003; Kornreich, et al., 2003; Z. X. Wang, et al., 2010). In the presence of comorbid OD/BPD, it appears that neural activity to emotional cues follows patterns more consistent with substance use than with personality disorder processes.
One potential explanation for reduced ACC and amygdala activation in the OD/BPD group is that opiate use in BPD is driven, in part, by an attempt to reduce hyper-responsivity to negative affective cues (c.f., Trull, et al., 2000), which may in fact be successful. Also, buprenorphine administration, a component of the buprenorphine/naloxone maintenance medication used by study participants, is thought to reduce negative affect via its effect on opiate receptors (Gerra, et al., 2006), though buprenorphine/naloxone-maintained opiate dependent subjects still rated negative affect higher than controls following a chemical stressor (Kakko, et al., 2008). It should be noted that in the present study, participant groups did not differ in their reported emotional responses to negative images. Effects of buprenorphine on neural activity are mixed, with a PET study showing broad reductions in cortical glucose metabolism following buprenorphine injection (Walsh, et al., 1994) and an fMRI study showing increases in dorsal ACC activation in methadone or buprenorphine/naloxone-maintained opiate depended subjects during a cognitive task (Yucel, et al., 2007). Acute buprenorphine administration has been observed to potentiate striatal activation and attenuate somatosensory cortex and thalamic activation in the context of a pain stressor, but effects on ACC and amygdala are not reported (Upadhyay, et al., 2009). Thus, it is unclear what specific effect buprenorphine/naloxone had on study results. The degree of dissociative symptoms, though higher in the OD/BPD group, did not account for reduced ACC and amygdala activations. Whether as a result of short-term administration of maintenance medication, long-term opiate use, or other factors, individuals with comorbid OD/BPD do not show the expected amygdalar, cingulate, or medial prefrontal hyperactivation to negative cues characteristic of BPD.
This is the first fMRI study of which we are aware that examines comorbid OD/BPD – a severe condition with poor treatment outcomes. The use of male subjects provides a unique contribution to the BPD literature, which often focuses exclusively on female participants (e.g., Driessen, et al., 2004; Driessen, et al., 2000; Herpertz, et al., 2001; Schnell & Herpertz, 2007; only 1 male BPD participant in Silbersweig, et al., 2007). However, it is uncertain if gender differences contributed to the finding of reduced emotion network activation in the OD/BPD sample, and further replication in a mixed-gender sample is in order. There were additional limitations to the study, which restrict its interpretability and require further replication and expansion. Recent substance use by the OD/BPD group may have caused differences in stimulus perception or regional blood flow that are more related to state effects than trait or diagnosis-specific effects. Continued substance use is a problem in OD research using populations not in restricted treatment environments; it is frequently observed in OD individuals engaged in outpatient treatment (Gruber, Delucchi, Kielstein, & Batki, 2008; Linehan, et al., 2002) and has been observed in previous neuroimaging studies of OD individuals in outpatient treatment (e.g., Ersche, et al., 2006). However, as reported above, entering the number of UA-positive substances or presence of recent opiate use as a covariate in ROI analyses did not reveal any significant main effects or interactions, suggesting that the impact of acute substance use was not pronounced. Future studies comparing individuals using opiate replacement medications to those not using medications are required to determine their impact. As in much of the clinical fMRI literature, the sample size is modest. Future larger-scale studies also would do well to include BPD-only and OD-only comparison groups to help tease apart the relative contribution of the two disorders, versus any synergistic effects of comorbidity within individuals.
In conclusion, this study, despite its preliminary nature, is an important first step in characterizing the neurological basis of emotional processing in comorbid personality and substance dependence disorders, and in this clinically challenging population in particular. Reduced activation was seen to emotional stimuli, hewing more closely to effects seen in OD than in BPD, a finding which may help to inform interventions focused on emotion regulation in this population. It is critical to expand the study of the neurological underpinnings of disorders beyond single-diagnosis samples if we are to fully understand the nature of disordered real-world populations.
Supplementary Material
Acknowledgments
This project was supported by NIDA R01 DA014997-03S1 (PI: Linehan) and R01 DA017372-03S1 (PI: Lynch). MJS was partially supported by NIMH T32 MH070448. Dr. Linehan receives royalties from Guilford Press for books she has written on dialectical behavior therapy (DBT) and also receives DBT training fees and royalties for DBT training materials from Behavioral Tech, LLC. The authors would like to thank Prudence Cuper, Jimmy Dias, Syam Gadde, Neva Oskin, Todd Richards, and Jim Voyvodic for their assistance in establishing scanner parameters, data collection, and analysis. We also thank our participants for the generous use of their time.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/per
For the first four subjects, images were collected in the sagittal plane with the following parameters: repetition time (TR) = 7.0 ms, echo time (TE) = 3.25 ms, 160 contiguous images, slice thickness = 1 mm. The last 20 subjects were scanned with the following T1 parameters: repetition time (TR) = 7.6 ms, TE = 3.7 ms, 64 contiguous images, slice thickness = 1.9 mm. Ten subjects were collected in the axial plane, and the remaining subjects were collected in oblique axial orientation, parallel to the AC-PC line. For all subjects, field of view (FOV) = 24 × 24 cm, flip angle = 8°, reconstruction matrix = 256×256.
When education level was used as a covariate in ROI analyses, there were no significant effects of, or interactions with, the education variable. The overall pattern of results was consistent when education level was used as a covariate.
References
- Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Archives of General Psychiatry. 1997;54(1):71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Annals of the New York Academy of Sciences. 2003;985:163–173. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Lingford-Hughes A, et al. Functional connectivity analysis of the neural circuits of opiate craving: “more” rather than “different”? Neuroimage. 2003;20(4):1964–1970. doi: 10.1016/j.neuroimage.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Williamson A, Mills KL, Havard A, Teesson M. Borderline personality disorder and persistently elevated levels of risk in 36-month outcomes for the treatment of heroin dependence. Addiction. 2007;102(7):1140–1146. doi: 10.1111/j.1360-0443.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- Domes G, Czieschnek D, Weidler F, Berger C, Fast K, Herpertz SC. Recognition of facial affect in Borderline Personality Disorder. J Pers Disord. 2008;22(2):135–147. doi: 10.1521/pedi.2008.22.2.135. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54(11):1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Driessen M, Beblo T, Mertens M, Piefke M, Rullkoetter N, Silva-Saavedra A, et al. Posttraumatic stress disorder and fMRI activation patterns of traumatic memory in patients with borderline personality disorder. Biological Psychiatry. 2004;55(6):603–611. doi: 10.1016/j.biopsych.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Badeck S, Beckmann C, Wagner A, Feige B, Weiss I, et al. Affective dysregulation and dissociative experience in female patients with borderline personality disorder: a startle response study. J Psychiatr Res. 2005;39(1):85–92. doi: 10.1016/j.jpsychires.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Welch SS, Grossman P, Reisch T, Linehan MM, Bohus M. Psychophysiological ambulatory assessment of affective dysregulation in borderline personality disorder. Psychiatry Res. 2007;150(3):265–275. doi: 10.1016/j.psychres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Roiser JP, Fryer TD, London M, Robbins TW, et al. Differences in orbitofrontal activation during decision-making between methadone-maintained opiate users, heroin users and healthy volunteers. Psychopharmacology (Berl) 2006;188(3):364–373. doi: 10.1007/s00213-006-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Baldaro B, Zaimovic A, Moi G, Bussandri M, Raggi MA, et al. Neuroendocrine responses to experimentally-induced emotions among abstinent opioid-dependent subjects. Drug & Alcohol Dependence. 2003;71(1):25–35. doi: 10.1016/s0376-8716(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Gerra G, Leonardi C, D’Amore A, Strepparola G, Fagetti R, Assi C, et al. Buprenorphine treatment outcome in dually diagnosed heroin dependent patients: A retrospective study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30(2):265–272. doi: 10.1016/j.pnpbp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2008;69(4):533–545. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug & Alcohol Dependence. 2008;94(1–3):199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 2001;50(4):292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Kunert HJ, Schwenger UB, Sass H. Affective responsiveness in borderline personality disorder: a psychophysiological approach. Am J Psychiatry. 1999;156(10):1550–1556. doi: 10.1176/ajp.156.10.1550. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Schwenger UB, Kunert HJ, Lukas G, Gretzer U, Nutzmann J, et al. Emotional responses in patients with borderline as compared with avoidant personality disorder. J Pers Disord. 2000;14(4):339–351. doi: 10.1521/pedi.2000.14.4.339. [DOI] [PubMed] [Google Scholar]
- Kakko J, von Wachenfeldt J, Svanborg KD, Lidstrom J, Barr CS, Heilig M. Mood and neuroendocrine response to a chemical stressor, metyrapone, in buprenorphine-maintained heroin dependence. Biological Psychiatry. 2008;63(2):172–177. doi: 10.1016/j.biopsych.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou V, Schmeidler J, New AS, Goodman M, et al. Characterizing affective instability in borderline personality disorder. American Journal of Psychiatry. 2002;159(5):784–788. doi: 10.1176/appi.ajp.159.5.784. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Siever LJ, Lee H, Pizzarello S, New AS, Goodman M, et al. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res. 2009;172(3):192–199. doi: 10.1016/j.pscychresns.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich C, Foisy ML, Philippot P, Dan B, Tecco J, Noel X, et al. Impaired emotional facial expression recognition in alcoholics, opiate dependence subjects, methadone maintained subjects and mixed alcohol-opiate antecedents subjects compared with normal controls. Psychiatry Research. 2003;119(3):251–260. doi: 10.1016/s0165-1781(03)00130-6. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Kosten TR, Rounsaville BJ. Personality disorders in opiate addicts show prognostic specificity. Journal of Substance Abuse Treatment. 1989;6(3):163–168. doi: 10.1016/0740-5472(89)90003-2. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Dimeff LA, Reynolds SK, Comtois KA, Welch SS, Heagerty P, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug & Alcohol Dependence. 2002;67(1):13–26. doi: 10.1016/s0376-8716(02)00011-x. [DOI] [PubMed] [Google Scholar]
- Liu N, Li B, Sun N, Ma Y. Effects of addiction-associated and affective stimuli on the attentional blink in a sample of abstinent opiate dependent patients. Journal of Psychopharmacology. 2008;22(1):64–70. doi: 10.1177/0269881107077804. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, et al. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Archives of General Psychiatry. 2009;66(2):205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Lynch TR, Rosenthal MZ, Kosson DS, Cheavens JS, Lejuez CW, Blair RJ. Heightened sensitivity to facial expressions of emotion in borderline personality disorder. Emotion. 2006;6(4):647–655. doi: 10.1037/1528-3542.6.4.647. [DOI] [PubMed] [Google Scholar]
- Marques-Teixeira JE, Santos Barbosa MF. Emotional states and informational brain processing in drug addicts free of drugs: an ERPs study. International Journal of Psychiatry in Clinical Practice. 2005;9(3):213–220. doi: 10.1080/13651500510029101. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;155(3):231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, et al. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32(7):1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- Rosenthal MZ, Gratz KL, Kosson DS, Cheavens JS, Lejuez CW, Lynch TR. Borderline personality disorder and emotional responding: a review of the research literature. Clinical Psychology Review. 2008;28(1):75–91. doi: 10.1016/j.cpr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Whitecar P, Wiederman MW. The prevalence of borderline personality among buprenorphine patients. International Journal of Psychiatry in Medicine. 2008;38(2):217–226. doi: 10.2190/PM.38.2.h. [DOI] [PubMed] [Google Scholar]
- Schnell K, Herpertz SC. Effects of dialectic-behavioral-therapy on the neural correlates of affective hyperarousal in borderline personality disorder. Journal of Psychiatric Research. 2007;41(10):837–847. doi: 10.1016/j.jpsychires.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. American Journal of Psychiatry. 2007;164(12):1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: psychopathology, comorbidity, and personality structure. Biological Psychiatry. 2002;51(12):936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Stein KF. Affect instability in adults with a borderline personality disorder. Archives of Psychiatric Nursing. 1996;10(1):32–40. doi: 10.1016/s0883-9417(96)80084-7. [DOI] [PubMed] [Google Scholar]
- Stiglmayr CE, Shapiro DA, Stieglitz RD, Limberger MF, Bohus M. Experience of aversive tension and dissociation in female patients with borderline personality disorder -- a controlled study. Journal of Psychiatric Research. 2001;35(2):111–118. doi: 10.1016/s0022-3956(01)00012-7. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: a review and integration. Clinical Psychology Review. 2000;20(2):235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Solhan MB, Tragesser SL, Jahng S, Wood PK, Piasecki TM, et al. Affective instability: measuring a core feature of borderline personality disorder with ecological momentary assessment. J Abnorm Psychol. 2008;117(3):647–661. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Anderson J, Schwarz AJ, Baumgartner R, Coimbra A, George E, et al. fMRI Measures of Drug Modulation of CNS Activation by Noxious Stimuli: Differential effects of a Failed Analgesic (fosaprepitant) vs. an Opioid Analgesic (Buprenorphine) Neuroimage. 2009;47(Supp 1):S62. [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M, Bechara A. Emotion, decision-making and substance dependence: a somatic-marker model of addiction. Current Neuropharmacology. 2006;4(1):17–31. doi: 10.2174/157015906775203057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Rivas-Perez C, Vilar-Lopez R, Perez-Garcia M. Strategic self-regulation, decision-making and emotion processing in poly-substance abusers in their first year of abstinence. Drug Alcohol Depend. 2007;86(2–3):139–146. doi: 10.1016/j.drugalcdep.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Wagner AW, Linehan MM. Facial expression recognition ability among women with borderline personality disorder: implications for emotion regulation? Journal of Personality Disorders. 1999;13(4):329–344. doi: 10.1521/pedi.1999.13.4.329. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Gilson SF, Jasinski DR, Stapleton JM, Phillips RL, Dannals RF, et al. Buprenorphine reduces cerebral glucose metabolism in polydrug abusers. Neuropsychopharmacology. 1994;10(3):157–170. doi: 10.1038/npp.1994.18. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, McCarthy G. Mood alters amygdala activation to sad distractors during an attentional task. Biological Psychiatry. 2006;60(10):1139–1146. doi: 10.1016/j.biopsych.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski MJ, Rosenthal MZ, Dolcos F, Lynch TR, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research. 2008;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, McCarthy G, Song AW, LaBar KS. Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion. 2005;5(1):12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Zhang JX, Wu QL, Liu N, Hu XP, Chan RC, et al. Alterations in the processing of non-drug-related affective stimuli in abstinent heroin addicts. Neuroimage. 2010;49(1):971–976. doi: 10.1016/j.neuroimage.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xie J, Shao YC, Xie CM, Fu LP, Li DJ, et al. Dynamic neural responses to cue-reactivity paradigms in heroin-dependent users: an fMRI study. Human Brain Mapping. 2009;30(3):766–775. doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, et al. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12(7):611, 691–702. doi: 10.1038/sj.mp.4001955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.