Abstract
Background and purpose
Total hip replacement (THR) with a reverse hybrid (RH), a combination of a cemented polyethylene cup and a cementless femoral stem, has been increasingly used in Scandinavia. In a randomized trial, we compared an RH THR with a proximal hydroxyapatite- (HA-) coated stem to a conventional cemented THR. Both groups received the same polyethylene cup.
Patients and methods
51 patients (52 hips) were included. Radiostereometry (RSA) and dual-energy X-ray absorptiometry (DEXA) were performed postoperatively and after 6, 12, and 24 months. 42 patients (43 hips) were followed for 2 years.
Results
Mean cup rotation around the x-axis was 0.13° for the cemented group and –0.24° for the RH group (p = 0.03). Cup migration in the other axes, and stem migration and wear were similar between the 2 study groups. Bone remodeling around the cup was also similar between the groups. Bone loss in Gruen zone 1 was 18% for the cementless stems, as compared to an increase of 1.4% for the cemented ones (p < 0.001). Bone loss was similar in the other Gruen zones. Harris hip score and Oxford hip score were similar pre- and postoperatively in the 2 groups.
Interpretation
In the present study, RH THR with a cementless hydroxyapatite-coated stem and conventional cemented THR did not show any major differences regarding stem migration and bone loss after 2 years of follow-up.
A reverse hybrid (RH) in total hip replacement (THR) is a cemented polyethylene cup with a cementless femoral stem. In the past decade, the use of this method has increased in Norway and Sweden (Swedish Hip Arthroplasty Register 2007, Norwegian Arthroplasty Register 2010). In the Norwegian Arthroplasty Register (NAR), some cementless stems have better survival than cemented ones in patients who are 60 years old or younger, and it has been suggested that the RH method could be an option in young patients due to the good results with cemented cups and with some cementless stems (Havelin et al. 2000). In the Swedish Hip Arthroplasty Register, cemented cups have performed better than cementless cups, and cementless femoral stems have had better survival than cemented stems with aseptic loosening as endpoint (Hailer et al. 2010). A medium-term report from the NAR has shown promising results for certain RH combinations (Lindalen et al. 2011). Some authors have pointed out that hydroxyapatite (HA) particles released from HA-coated implants may increase polyethylene wear (Bloebaum et al. 1994, Røkkum et al. 2002). We have not found any record of randomized trials that have shown that the RH method is superior to conventional cemented THR. In the present study, we compared a RH THR with a proximally HA-coated stem to a cemented THR in a randomized trial. Our null hypothesis was that there would be no differences in clinical results, wear, remodeling of bone, or migration of the components between the study groups.
Patients and methods
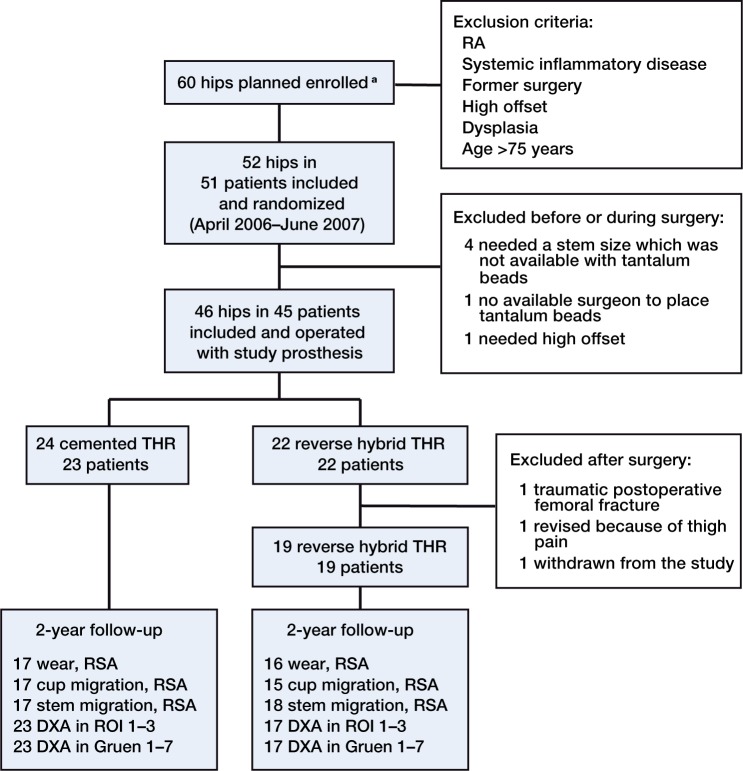
51 patients (52 hips) with osteoarthritis were randomized by a nurse before surgery to either a conventional cemented THR or an RH THR. We used sealed envelopes. The patients were operated on from April 2006 through June 2007. The study was terminated in June 2007 due to delay in delivery of study prostheses. 9 patients (9 hips) were excluded for various reasons (Figure 1).
Figure 1.
Flow chart illustrating the randomized controlled trial. a Due to delay in delivery of study prosthesis inclusion of participants was stopped in some periods and numbers of eligibility are not present
The operations were performed using the posterolateral approach. All patients received a Reflection cemented all-polyethylene cup (Smith and Nephew, Memphis, TN). The cup was made of UHMWPE, ram-extruded from GUR 1050 and sterilized with ethylene oxide. The cup was inserted by third-generation cementing technique with Palacos R+G (Heraeus, Hanau, Germany). No systematic templating of the hips was performed preoperatively.
All patients received thromboprophylaxis with Fragmin (dalteparin). Pre- and postoperative prophylaxis with cefalotin was administered intravenously. 2 patients received clindamycin due to penicllin allergy. 8 surgeons participated in the study.
Cemented stem
The femur was reamed to adequate size and a Spectron EF stem (Smith and Nephew) of the same size was cemented with Palacos R+G (Heraeus). 3 patients received a stem one size larger than the reamed size. The stem had tantalum balls attached to the tip and to the calcar region. To the neck, a metal piece with a cone and a tantalum marker was attached during surgery. A distal cement restrictor of polyethylene was used and pressurization of the cement was performed before insertion of the prostheses. A 28-mm head of cobalt chrome (Smith and Nephew) was used in all cases. In this group, we had all sizes (1–5) of the femoral stem in standard offset.
Cementless stem
In the RH group, a Taperloc (Biomet, Warsaw, IN) cementless femoral stem made of Ti6A14V was used. The stem had a proximal plasma-sprayed HA coating on top of the porous metal coating. The HA coating was 55 ± 15 µm thick and had a crystallinity of 50–70%. The Taperloc had tantalum balls attached to the tip, at the calcar region and at the neck. We used a femoral stem of the same size as the last reamer. In this group, we had sizes 7.5, 10, 12.5, 15, and 17.5 in standard offset. A 28-mm head of cobalt chrome (Biomet) was used in all cases.
During the operation the cup, the periacetabular bone, the greater trochanter, and the lesser trochanter were marked with 1-mm tantalum balls. The implants used in the study were selected since we used the Reflection cemented all-polyethylene cup in combination with either the cemented Spectron EF stem or the cementless Taperloc stem without HA in our department. The patients were mobilized on the first postoperative day, with weight bearing as tolerated. All patients were scored preoperatively and after 2 years using the Harris hip score and Oxford hip score.
The study was conducted in accordance with the Helsinki Declaration and approved by the regional ethics committee (REK) Sør-Øst in Norway. (Clinical Trials.gov Identifier: NCT00526539). All patients were recruited, operated on, and followed according to study protocol at Lovisenberg Diaconal Hospital. RSA and DEXA scans and analysis were performed at Oslo University Hospital. All patients gave informed consent to participate in the study.
RSA
RSA was performed postoperatively and at 6 months, and at 1 and 2 years. We used a uniplanar calibration cage number 43 (RSA Biomedical, Umeå, Sweden). Radiographs were taken using 2 fixed X-ray tubes with the patient in the supine position. Analysis was done with UmRSA Digital Measure 6.0 (RSA Biomedical, Umeå, Sweden). At least 3 tantalum markers had to be identified in order to calculate wear or migration. In addition to the markers attached to the femoral component, the center of the head was used as a reference point. The cut-off for mean error (ME) was set at 0.30 and condition numbers lower than 150 were accepted (Valstar et al. 2005). The precision of the measurements was calculated by double examinations and expressed as an absolute mean plus 2 times the standard deviation (SD) to cover the 95% confidence interval (CI) (Röhrl et al. 2004) (Table 1). 3-dimensional (3D) wear was measured as the vectorial resultant of all 3 (x-, y-, z-) axes.
Table 1.
Precision for point motion (wear) and movement (translation and rotation for cup and stem) in x-, y-, and z-axes. n = 108 double examinations for wear, n = 113 double examinations for cup migration, and n = 140 double examinations for stem migration. RSA was performed at a mean of 7 (4-31) days postoperatively
| Cup |
Stem |
||||
|---|---|---|---|---|---|
| Wear (mm) |
translation (mm) |
rotation (°) a | translation (mm) |
rotation (°) a | |
| x-axis | 0.11 | 0.11 | 0.58 | 0.15 | 0.39 |
| y-axis | 0.10 | 0.11 | 0.43 | 0.09 | 0.59 |
| z-axis | 0.19 | 0.29 | 0.26 | 0.31 | 0.20 |
a degrees around axis
Bone mineral density (BMD)
BMD was measured by dual-energy X-ray absorptiometry (DEXA) on a Prodigy scanner (Lunar), with baseline defined by the scan taken postoperatively. Bone remodeling was measured as change in BMD at 6 months, and at 1 and 2 years. During scanning, the patient was placed in a supine position and a foot brace was used to standardize the position. An area from the lower border of the distal sacroiliac joint to an area distal to the tip of the femoral stem was included in the scan. The paint facility was used to exclude non-bony structures.
BMD around the cup was measured according to 3 regions of interest (ROIs) as modified DeLee and Charnley zones, described by Field et al. (2006). When all ROIs had been positioned in 1 patient, they were copied and placed in the same manner in all other scans of the same individual. In the femur, we used Gruen zones 1–7 (Figure 2). We regarded the cement as a constant factor and did not try to exclude it. Double examinations were performed and the patients were asked to stand up; they were then repositioned between the scans to estimate the coefficient of variation (CV) (Table 2). To calculate the CV, we used the formula: CV % = 100 [(δ/√2)/µ], where δ is the SD of the difference in BMD between the double examinations in each individual. µ is the mean of all BMD measurements for each ROI (Wilkinson et al. 2001, Digas et al. 2006).
Figure 2.
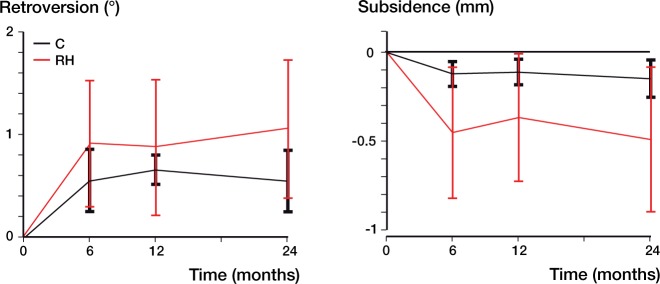
Graphic representation of stem micromovement, retroversion (left panel) and subsidence (right panel), from baseline (postoperatively) to the 2-year follow-up. Mean with 95% CI. n = 18/20, 17/18, and 17/18 for cemented and uncemented stems, respectively, at 6, 12, and 24 months of follow-up. C: cemented; RH: reverse hybrid.
Table 2.
Coefficient of variation (CV) for different ROIs. n = 126 double examinations in ROIs 1–3. CV for Gruen zones 1–7. n = 136 double examinations in zones 1–3 and zones 5–7. n = 130 double examinations in zone 4. DEXA scans were performed at a mean of 7 (4–31) days postoperatively
| ROI |
Gruen zone |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| CV (%) | 2.2 | 2.7 | 4.1 | 1.6 | 1.4 | 1.6 | 1.2 | 1.6 | 2.1 | 2.1 |
Radiography
Anteroposterior pelvic radiographs and lateral views of the femur were taken postoperatively and at 2 years. These radiographs were studied to see if any of the components were loose and to evaluate lucency. Radiolucency was defined as a lucent line between the cement and bone interface. A radiographically loose cup was defined as complete lucency in the cement/bone interface. M-desk version 3.0 (UmRSA Biomedical) was used by one observer (EL) to calculate the implant position and radiolucency.
Statistics
All data were analyzed using SPSS software version 18. Binary outcomes were analyzed using chi-square test and Fisher’s exact test. The Mann-Whitney U test was used to test for differences between the 2 groups. We used the non-parametric Wilcoxon signed-rank test for related samples to test for differences in migration in one group at different times. Based on previous RSA studies (Kärrholm et al. 1997), we chose the sample size as 60 hips, covering for possible dropouts. Bøe et al. (2011) have calculated that to reveal a difference between 2 groups regarding stem translation of 0.5 mm (SD 0.5), rotation of 0.7° (SD 0.7), and a difference in BMD of 10% (SD 10) with a power of 80% and a significance level of 0.05, a sample size of 17 cases in each group would be appropriate. 95% CIs were calculated. Values of p < 0.05 were regarded as statistically significant.
Results
Clinical outcome
Mean pre- and postoperative Harris hip and Oxford hip scores were similar in the 2 groups (Table 3). No infections, dislocations, or nerve injuries were registered. 1 patient with a cementless stem was revised 1 year after the index procedure, due to thigh pain. We had no peroperative femoral fracture in either group. Operating time was 8 min shorter for the RH group (p = 0.05) (Table 3).
| n | Cemented | n | Reverse hybrid |
p-value | |
|---|---|---|---|---|---|
| Age, years | 24 | 68 (65–70) | 22 | 66 (64–68) | |
| Sex, male/female | 24 | 2/22 | 22 | 6/16 | |
| Side operated, right/left | 24 | 11/13 | 22 | 12/10 | |
| Cup size, mm | 24 | 52 (51–53) | 22 | 54 (53–55) | |
| Body mass index | 24 | 26 (25–28) | 22 | 27 (25–29) | |
| Harris hip score preop. | 24 | 56 (52–60) | 19 | 54 (50–59) | |
| Harris hip score 2-year | 24 | 97 (96–99) | 19 | 96 (92–99) | |
| Oxford hip score preop. | 24 | 40 (37–43) | 19 | 39 (35–42) | |
| Oxford hip score 2-year | 24 | 17 (14–19) | 19 | 19 (14–23) | |
| Inclination cup, degrees | 24 | 48 (45–51) | 19 | 46 (43–49) | |
| Offset, mm | 24 | 40 (38–43) | 19 | 38 (36–40) | |
| T value distance, mm | 24 | 4.3(2.7–5.9) | 19 | 6.4(4.6–8.3) | |
| Smoker, yes/no | 24 | 5/19 | 22 | 4/18 | |
| Operation time, min | 24 | 85 (80–90) | 22 | 77 (72–82) | 0.05 a |
| Peroperative bleeding, mL | 24 | 339 (301–376) | 22 | 311 (261–362) | 0.3 a |
| Postoperative bleeding, mL | 24 | 336 (268–403) | 22 | 429 (352–506) | 0.1 a |
| Total bleeding, mL | 24 | 674 (586–763) | 22 | 740 (662–819) | 0.3 a |
| Stem position, varus/neutral/valgus |
24 | 9/14/1 | 19 | 4/15/0 | 0.3b |
| Stem size (numbers) | 1/2/3/4/5 | 7.5/10/12.5/15/17.5 | |||
| (4/9/8/2/1) | (1/5/7/8/1) | ||||
| Surgeons A–H: | |||||
| A | 3 | 6 | |||
| B | 11 | 9 | |||
| C | 1 | 1 | |||
| D | 2 | 0 | |||
| E | 3 | 3 | |||
| F | 1 | 1 | |||
| G | 3 | 1 | |||
| H | 0 | 1 |
a Non-parametric Independent samples Mann Whitney U-test.
b Chi-squared
Wear measurements
Wear for the total material was 0.33 mm (CI: 0.28–0.37) in the proximal direction and 0.39 mm (CI: 0.34–0.44) in the 3D direction. The wear in the proximal direction was 0.32 mm (CI: 0.28–0.36) for the cemented group and 0.33 mm (CI: 0.24–0.42) for the RH group. The 3D wear was 0.37 mm (CI: 0.31–0.43) for the cemented group and 0.40 mm (CI: 0.31–0.50) for the RH group (Table 4).
Table 4.
Wear in mm, including creep. Cup and stem translation in mm and rotation in degrees for the 2 study groups at the 2-year follow-up. The p-values are from the independent samples Mann-Whitney U-test
| Cemented mean (SD) |
Reverse hybrid mean (SD) |
p-value | Mean difference (95% CI) |
|
|---|---|---|---|---|
| Wear, n | 17 | 16 | ||
| x | 0.00 (0.07) | –0.04 (0.17) | 0.9 | 0.04 (–0.06 to 0.13) |
| y | 0.32 (0.08) | 0.33 (0.17) | 0.9 | –0.00 (–0.10 to 0.09) |
| z | –0.03 (0.18) | –0.08 (0.15) | 0.08 | 0.05 (–0.07 to 0.16) |
| 3D | 0.37 (0.11) | 0.40 (0.17) | 0.7 | –0.04 (–0.14 to 0.07) |
| Cup translation, n | 17 | 15 | ||
| x | –0.05 (0.21) | –0.09 (0.32) | 0.9 | 0.04 (–0.15 to 0.24) |
| y | 0.16 (0.39) | 0.11 (0.24) | 0.8 | 0.04 (–0.19 to 0.28) |
| z | –0.03 (0.21) | 0.02 (0.29) | 0.4 | –0.05 (–0.23 to 0.13) |
| 3D | 0.34 (0.38) | 0.37 (0.35) | 0.9 | –0.02 (–0.29 to 0.24) |
| Cup rotation | ||||
| x | 0.13 (0.40) | –0.24 (0.56) | 0.03 | 0.37 (0.02 to 0.72) |
| y | –0.12 (0.27) | –0.03 (0.24) | 0.2 | –0.09 (–0.28 to 0.09) |
| z | –0.38 (0.98) | –0.33 (0.80) | 0.3 | –0.06 (–0.71 to 0.60) |
| Stem translation, n | 17 | 18 | ||
| x | 0.05 (0.11) | 0.09 (0.18) | 0.3 | –0.04 (–0.14 to 0.06) |
| y | –0.15 (0.21) | –0.49 (0.82) | 0.6 | 0.34 (–0.07 to 0.76) |
| z | –0.24 (0.26) | –0.32 (0.29) | 0.5 | 0.08 (–0.11 to 0.27) |
| Stem rotation | ||||
| x | –0.22 (0.33) | –0.12 (0.35) | 0.3 | –0.10 (–0.33 to 0.13) |
| y | 0.55 (0.59) | 1.05 (1.35) | 0.5 | –0.51 (–1.23 to 0.22) |
| z | 0.09 (0.24) | –0.08 (0.32) | 0.1 | 0.17 (–0.03 to 0.36) |
Stability of the cup
Mean rotation of the cup around the x-axis was 0.13° (CI: –0.08 to 0.34) for the cemented group and –0.24° (CI: –0.55 to 0.07) for the RH group (p = 0.03). In the other axes, cup migration was similar in both groups (Table 4).
Stability of the stem
Mean subsidence for the cemented stem was 0.15 mm (CI: 0.04–0.25) and for the cementless stem it was 0.49 mm (CI: 0.08–0.90). Mean retroversion for the cemented stem was 0.55° (CI: 0.25–0.85) and for the cementless stem it was 1.05° (CI: 0.38–1.73) (Table 4, and Figure 2). Comparing the subsidence of the cementless stem from 6 and 12 months up to 2 years we found no statistically significant differences (p = 0.7 and p = 0.2, respectively). In the cemented group, the cone containing a tantalum marker was not stable in 4 cases and it was therefore removed. The resulting high condition numbers were the main reason for excluding these cemented stems.
BMD
Bone remodeling around the cup between baseline (postoperatively) and 2 years was similar in the 2 groups. In Gruen zone 1, we found a bone loss of 18% (CI: 11–24) for the cementless stem as compared to an increase of 1.4% (CI: –3.2 to 5.9) for the cemented stem (p < 0.001). There were no statistically significant differences in the other Gruen zones (Figure 3).
Figure 3.
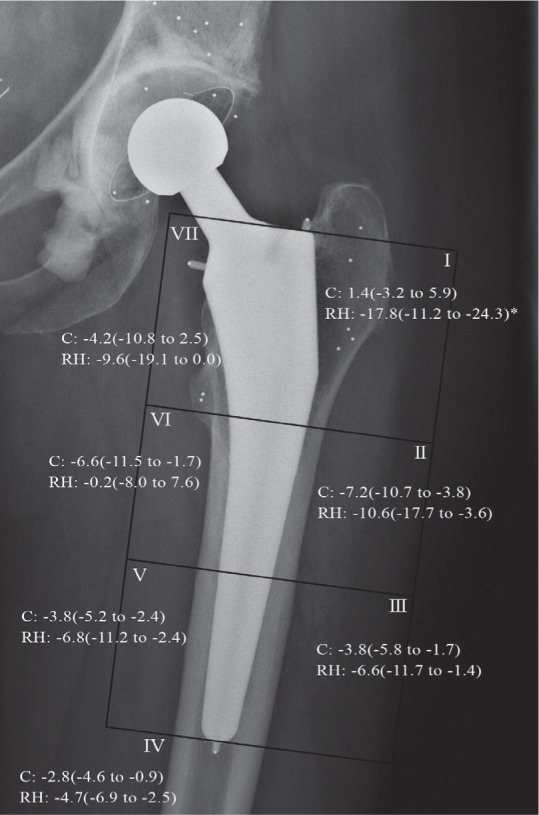
Mean percentage changes in BMD relative to postoperatively in Gruen zones 1–7 at 2 years, with 95% CI of mean. * Statistically significant difference in zone I (p < 0.001, independent-samples Mann-Whitney U test). RH: reverse hybrid; C: cemented.
Radiography
There were no radiographically loose cups. Comparing inclination, stem alignment, and radiolucency around the cup and stem, there were no statistically significant differences between the 2 study groups.
Discussion
We found only minor differences between a reverse hybrid and a cemented THR regarding clinical outcome, wear, prosthesis migration, and change in BMD around the prosthesis. The mean operating time in the RH group was 8 min shorter than for the cemented THR group. In the greater trochanter, the cementless stem gave BMD loss of 18%, as compared to an increase of 1% for the cemented stem.
One strength of this randomized study was the use of high-precision measuring methods. RSA is well established and DEXA has been used in several studies for measuring BMD around hip implants (Wilkinson et al. 2001, Digas et al. 2005, 2006, Field et al. 2006). A weakness of the present study was the missing data for some patients, with a consequent reduction in sample size (Figure 1). Reduction in sample size may have reduced the statistical power, and this could have affected our ability to reveal any differences between the study groups. However, we had enough power to detect a difference in bone remodeling of 10% between the 2 study groups, and in migration of the stems. Other possible confounding factors were the relatively high number of surgeons participating in the study and the fact that there was no systematic templating. The patients were mobilized with weight bearing as tolerated, and RSA was performed after mobilization. There could also be a possible bias in measuring the migration of the uncemented femoral component with the index RSA scan taken after mobilization.
A threshold for clinically important linear wear using UHMWPE has been proposed to be 0.1 mm/year (Dumbleton et al. 2002). We measured the mean difference between study groups regarding wear in the vertical direction (Table 4). A wear difference of 0.1 mm is therefore not within the upper part of the 95% CI, indicating that there was no clinically important differences in wear between the 2 study groups.
All the Reflection cups were made of UHMWPE and sterilized with ethylene oxide. The mean wear, including creep, at the 2-year follow-up was high—both in the proximal direction and in total 3D direction. This finding is in accordance with earlier reports (Digas et al. 2003, Röhrl et al. 2004). Although acceptable according to international standards, the NAR found inferior results for the Reflection cemented all-poly cup in combination with the Spectron EF stem compared to some other prostheses (Espehaug et al. 2009). High wear rates for the Reflection all-poly cemented cup might hypothetically contribute to osteolysis and increased revision rates for the Spectron stem. With longer-term follow-up, one can anticipate the same problem with this cup in RHs as reported for all-cemented THRs.
We found a difference in rotation of the cup around the x-axis (tilt) between the 2 groups. The reason for this finding is uncertain, but the initial position of the stems and cups would probably influence the change around this axis over time.
Excellent long-term survival has been documented for HA-coated femoral stems (Hallan et al. 2007). In an editorial, Morscher (1991) raised concerns about HA-coated implants. HA particles may separate from the prosthesis and lead to third body wear (Bloebaum et al. 1994, Morscher et al. 1998). Røkkum et al. (2002) suggested that thick HA coatings may delaminate and that HA coatings may be a reservoir for HA particles. In our study with 2-year follow-up, the magnitude of wear was similar between a proximal HA-coated stem and a cemented stem using the same cup. This finding is in accordance with the work of Önsten et al (1998), who found no difference in wear in a cementless HA-coated cup and a cemented all-polyethylene cup, but the cups used were from two different companies.
Both stems were initially designed as monoblock types and later modified with different offsets and modularity. The Taperloc stem with and without HA has documented good long-term survival (Parvizi et al. 2004, Hallan et al. 2007, McLaughlin and Lee 2010). The monoblock Spectron stem has shown good long-term survival in a randomized study (Garellick et al. 1999). In the present study, we used the modular version with standard offset for both stems. The Spectron EF stem has a grit-blasted proximal area. Concern has been raised about this prosthesis because of early loosening and severe metallosis (Gonzalez et al. 2006). Thien and Kärrholm (2010) investigated this prosthesis with data from the Swedish Arthroplasty Register and found increased revision rates for the smallest Spectron stem, and also with increasing offset. In our study, using standard offset we found that both stems were well fixed up to 2 years. There were no statistically significant differences in migration between the 2 stems, but the cementless stem rotated slightly more in retroversion and migrated more distally than in a previous study (Bøe et al. 2011). In the present study, we used a posterolateral approach while Bøe et al. (2011) used a direct lateral approach. In an RSA study of a cemented stem, Glyn-Jones et al. (2006) found increased rotation in retroversion with the posterolateral approach, compared to the direct lateral approach.
We did not find any continuous subsidence for the cementless stem after 6 months and up to 2 years. An initial subsidence and migration into retroversion, which stabilized later, has been described using RSA for a clinically well-proven cementless stem (Hallan et al. 2007, Campbell et al. 2011). Continuous migration has been associated with inferior results for cemented stems (Kärrholm et al. 1994). The cemented Spectron stem subsided and migrated into retroversion, with comparable results to that described by Kadar et al. (2011). The subsidence did not exceed the limits described by Kärrholm et al. (1994) for cemented stems.
Using the same cup and demonstrating similar wear between the 2 stem designs, our study is ideal for comparison of changes in BMD around the 2 stems. We found higher bone loss in Gruen zone 1 for the Taperloc stem during the 2-year follow-up. The magnitude of this bone loss in Gruen zone 1 is comparable to that found by Bøe et al. (2011) when they investigated the same prosthesis. Comparison of bone remodeling around a cementless stem and a cemented stem might be biased to some degree by the cement. Exclusion of the stem from the cement may be difficult, and regarding the cement as a constant factor may result in higher initial BMD in the different Gruen zones compared to a stem without cement. This would probably influence the percent change in BMD over time. Considering the good long-term survival for the Taperloc stem reported in the literature, it is unlikely that the reduction in BMD is an important clinical finding.
Bone remodeling around the cup was similar between the 2 study groups. We found that we had good precision in each ROI. Many factors can influence precision, and regarding the cement as a constant factor—and not excluding it by using the paint facility—may improve precision. This is supported by studies that have investigated this for the femoral component (Wilkinson et al. 2001, Digas et al. 2005).
The study groups were similar regarding age, sex, and BMI, and all patients operated had primary arthritis. Activity was not measured and this could have led to bias. High activity levels may lead to higher wear rates (Schmalzried et al. 2000).
In summary, we found that a partially HA-coated stem does not cause more wear than a cemented stem, with up to 2 years of follow-up. The cementless femoral stem had more bone loss in Gruen zone 1 than the cemented stem. Wear of the cemented all-polyethylene Reflection cup was high and comparable to that found in other studies. We did not find major differences between the 2 study groups, but long-term follow-up of the RH concept is necessary.
Acknowledgments
EL: planning, RSA analysis, DEXA analysis, interpretation of data, statistics, and writing of manuscript. J.D: study idea and study design, planning, interpretation of data, and revision of the manuscript with final approval. SMR: planning, supervision/performance of RSA analysis together with EL, interpretation of data, and revision of manuscript with final approval. LN, FS, ØH: planning, interpretation of data, and revision of manuscript with final approval.
We thank the radiographers attached to the Center for Implant and Radiostereometric Research at Oslo University Hospital (CIRRO) for performing the RSA examinations. Professor Ingar Holme of Oslo University Hospital gave statistical advice. Nils Rasmus Vasli helped to illustrate Figure 2 and the South-Eastern Norway Regional Health Authority provided financial support. This study was supported by an independent research grant of 50,000 Norwegian kroner from Smith and Nephew. Smith and Nephew took no part in organizing the study, in analysis, or in writing of the manuscript.
No competing interests declared.
References
- Bloebaum RD, Beeks D, Dorr LD, Savory CG, Dupont JA, Hofmann AA. Complications with hydroxyapatite particulate separation in total hip arthroplasty. Clin Orthop. 1994;(298):19–26. [PubMed] [Google Scholar]
- Bøe BG, Röhrl SM, Heier T, Snorrason F, Nordsletten L. A prospective randomized study comparing electrochemically deposited hydroxyapatite and plasma-sprayed hydroxyapatite on titanium stems. Acta Orthop. 2011;82(1):13–9. doi: 10.3109/17453674.2010.548027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D, Mercer G, Nilsson KG, Wells V, Field JR, Callary SA. Early migation characteristics of a hydroxyapatite-coated femoral stem:an RSA study. Int Orthop. 2011;35(4):483–8. doi: 10.1007/s00264-009-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digas G, Thanner J, Nivbrant B, Röhrl S, Ström H, Kärrholm J. Increase in early polyethylene wear after sterilization with ethylene oxide: radiostereometric analyses of 201 total hips. Acta Orthop Scand. 2003;74(5):531–41. doi: 10.1080/00016470310017910. [DOI] [PubMed] [Google Scholar]
- Digas G, Thanner J, Anderberg C, Kärrholm J. Fluoride-containing acrylic bone cement in total hip arthroplasty. Randomized evaluation of 97 stems using radiostereometry and dual-energy x-ray absorptiometry. J Arthroplasty. 2005;20(6):784–92. doi: 10.1016/j.arth.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Digas G, Kärrholm J, Thanner J. Different loss of BMD using uncemented press-fit and whole polyethylene cups fixed with cement: repeated DXA studies in 96 hips randomized to 3 types of fixation. Acta Orthop. 2006;77(2):218–26. doi: 10.1080/17453670610045948. [DOI] [PubMed] [Google Scholar]
- Dumbleton KA, Chalmers RL, McNally J, Bayer S, Fonn D. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17(5):649–61. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- Espehaug B, Furnes O, Engesaeter LB, Havelin LI. 18 years of results with cemented primary hip prostheses in the Norwegian Arthroplasty Register. Acta Orthop. 2009;80(4):402–12. doi: 10.3109/17453670903161124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field RE, Cronin MD, Singh PJ, Burtenshaw C, Rushton N. Bone remodeling around the Cambridge cup: a DEXA study of 50 hips over 2 years. Acta Orthop. 2006;77(5):726–32. doi: 10.1080/17453670610012908. [DOI] [PubMed] [Google Scholar]
- Garellick G, Malchau H, Regner H, Herberts P. The Charnley versus the Spectron hip prosthesis: radiographic evaluation of a randomized, prospective study of 2 different hip implants. J Arthroplasty. 1999;14(4):414–25. doi: 10.1016/s0883-5403(99)90096-7. [DOI] [PubMed] [Google Scholar]
- Glyn-Jones S, Alfaro-Adrian J, Murray DW, Gill HS. The influence of surgical approach on cemented stem stability: an RSA study. Clin Orthop. 2006;(448):87–91. doi: 10.1097/01.blo.0000224006.25636.cc. [DOI] [PubMed] [Google Scholar]
- Gonzalez D. V, Rana A, Nestor B, Bostrom M, Westrich G, Salvati EA. Metallic shedding, surface finish changes, and extensive femoral osteolysis in the loose Spectron EF stem. Clin Orthop. 2006;(442):165–70. doi: 10.1097/01.blo.0000181145.01306.f9. [DOI] [PubMed] [Google Scholar]
- Hailer NP, Garellick G, Kärrholm J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop. 2010;81(1):34–41. doi: 10.3109/17453671003685400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallan G, Lie SA, Furnes O, Engesaeter LB, Vollset SE, Havelin LI. Medium- and long-term performance of 11,516 uncemented primary femoral stems from the Norwegian arthroplasty register. J Bone Joint Surg (Br) 2007;89(12):1574–80. doi: 10.1302/0301-620X.89B12.18969. [DOI] [PubMed] [Google Scholar]
- Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand. 2000;71(4):337–53. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- Kadar T, Hallan G, Aamodt A, Indrekvam K, Badawy M, Havelin LI, Stokke T, Haugan K, Espehaug B, Furnes O. A randomized study on migration of the Spectron EF and the Charnley flanged 40 cemented femoral components using radiostereometric analysis at 2 years. Acta Orthop. 2011;82(5):538–44. doi: 10.3109/17453674.2011.618914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärrholm J, Borssén B, Lowenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter? 4-7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg (Br) 1994;76(6):912–7. [PubMed] [Google Scholar]
- Kärrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J. Radiostereometry of hip prostheses. Review of methodology and clinical results. Clin Orthop. 1997;(344):94–110. [PubMed] [Google Scholar]
- Lindalen E, Havelin LI, Nordsletten L, Dybvik E, Fenstad AM, Hallan G, Furnes O, Hovik O, Röhrl SM. Is reverse hybrid hip replacement the solution? Acta Orthop. 2011;82(6):639–45. doi: 10.3109/17453674.2011.623569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JR, Lee KR. Uncemented total hip arthroplasty with a tapered femoral component: a 22- to 26-year follow-up study. Orthopedics. 2010;33(9):639. doi: 10.3928/01477447-20100722-40. [DOI] [PubMed] [Google Scholar]
- Morscher EW. Hydroxyapatite coating of prostheses. J Bone Joint Surg (Br) 1991;73(5):705–6. doi: 10.1302/0301-620X.73B5.1894652. [DOI] [PubMed] [Google Scholar]
- Morscher EW, Hefti A, Aebi U. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg (Br) 1998;80(2):267–72. doi: 10.1302/0301-620x.80b2.8316. [DOI] [PubMed] [Google Scholar]
- Norwegian Arthroplasty Register Annual report. 2010. p. 22.
- Onsten I, Carlsson AS, Besjakov J. Wear in uncemented porous and cemented polyethylene sockets: a randomised, radiostereometric study. J Bone Joint Surg (Br) 1998;80(2):345–50. doi: 10.1302/0301-620x.80b2.8032. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Sharkey PF, Hozack WJ, Orzoco F, Bissett GA, Rothman RH. Prospective matched-pair analysis of hydroxyapatite-coated and uncoated femoral stems in total hip arthroplasty. A concise follow-up of a previous report. J Bone Joint Surg (Am) 2004;86(4):783–6. doi: 10.2106/00004623-200404000-00017. [DOI] [PubMed] [Google Scholar]
- Röhrl SM, Nivbrant B, Ström H, Nilsson KG. Effect of augmented cup fixation on stability, wear, and osteolysis: a 5-year follow-up of total hip arthroplasty with RSA. J Arthroplasty. 2004;19(8):962–71. doi: 10.1016/j.arth.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Røkkum M, Reigstad A, Johansson CB. HA particles can be released from well-fixed HA-coated stems: histopathology of biopsies from 20 hips 2-8 years after implantation. Acta Orthop Scand. 2002;73(3):298–306. doi: 10.1080/000164702320155293. [DOI] [PubMed] [Google Scholar]
- Schmalzried TP, Shepherd EF, Dorey FJ, Jackson WO, dela RM, Fa’vae F, McKellop HA, McClung CD, Martell J, Moreland JR, Amstutz HC. The John Charnley Award. Wear is a function of use, not time. Clin Orthop. 2000;(381):36–46. doi: 10.1097/00003086-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Swedish Hip Arthroplasty Register Annual Report. 2007. pp. 11–5.
- Thien TM, Kärrholm J. Design-related risk factors for revision of primary cemented stems. Acta Orthop. 2010;81(4):407–12. doi: 10.3109/17453674.2010.501739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valstar ER, Gill R, Ryd L, Flivik G, Borlin N, Karrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop. 2005;76(4):563–72. doi: 10.1080/17453670510041574. [DOI] [PubMed] [Google Scholar]
- Wilkinson JM, Peel NF, Elson RA, Stockley I, Eastell R. Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg (Br) 2001;83(2):283–8. doi: 10.1302/0301-620x.83b2.10562. [DOI] [PubMed] [Google Scholar]