EXECUTIVE SUMMARY
1.0 PREAMBLE
2.0 METHODOLOGY
-
3.0 DEFINITIONS
3.1 General definitions
3.2 Operational definition of CA-MRSA
3.3 Definition limitations
-
4.0 EPIDEMIOLOGY
4.1 The rise of CA-MRSA
4.2 CA-MRSA in Canada
4.3 Origin of CA-MRSA and its ability to disseminate
4.4 Populations at risk
4.5 Transmission
-
5.0 MICROBIOLOGY
5.1 S aureus and MRSA
5.2 Virulence factors
5.3 Nomenclature of strains
-
5.4 Resistance to non-beta-lactam antibiotics
5.4.1 Clindamycin
5.4.2 Erythromycin
5.4.3 Quinolones
5.5 Differences between CA-MRSA and HA-MRSA
-
6.0 MANAGEMENT
-
6.1 Diagnostic evaluation
6.1.1 When to suspect CA-MRSA
6.1.2 When to obtain cultures
-
6.2 Treatment
6.2.1 Minor SSTIs (folliculitis, furuncles and small abscesses without cellulitis)
6.2.2 Empirical therapy of non-lifethreatening infections other than minor skin infections, potentially due to CA-MRSA
6.2.3 Empirical therapy of lifethreatening infections potentially due to CA-MRSA
6.2.4 Confirmed non-life-threatening CA-MRSA infections other than minor skin infections
6.2.5 Confirmed CA-MRSA lifethreatening infections
6.2.6 Adjunctive therapy
-
-
7.0 SCREENING AND DECOLONIZATION
7.1 Screening for CA-MRSA
7.2 Decolonization
7.3 Guidelines for the use of decolonization regimens
-
8.0 POPULATION SURVEILLANCE
8.1 Population surveillance program for CA-MRSA
8.2 Laboratory support
-
9.0 PREVENTION
-
9.1 Prevention of transmission of CA-MRSA
9.1.1 Role of the individual
9.1.2 Role of health care practitioners
9.1.3 Role of health authorities
-
9.2 Prevention in specific settings
9.2.1 Households with CA-MRSA infection
9.2.2 Daycare centres and schools
9.2.3 Sports settings
9.2.4 Pets and other animals
9.2.5 Correctional facilities or shelters
9.2.6 Newborn care facilities
-
-
10.0 DIRECTIONS FOR FUTURE RESEARCH
10.1 Epidemiology
10.2 Biology of CA-MRSA: Organism versus host
10.3 Clinical outcomes and management
10.4 Infection prevention and control
10.5 Knowledge translation
11.0 CONCLUSION
APPENDIX
REFERENCES
EXECUTIVE SUMMARY
Methicillin resistance among community isolates of Staphylococcus aureus has reached a staggering high of 75% in some communities in the United States (US) (1). These organisms, which are resistant to the entire class of beta-lactam antibiotics, have evaded an important component of the physician’s armamentarium and may require clinicians to change their management of presumed staphylococcal infections. The recent emergence of community-associated (CA) methicillin-resistant S aureus (MRSA) strains as dominant clones signals their adaptation to survive and spread outside the hospital setting. Descriptions of severe disease and characterization of their virulence factors warn of their potential to inflict significant morbidity and mortality. Thus, we are faced with an emerging and formidable foe – a pathogen combining virulence, resistance and an ability to disseminate at large (2). At present, the prevalence of CA-MRSA in Canada is unknown but thought to be low based on the collective clinical experience of infectious disease experts across the country. If Canada is to delay or prevent the emergence of CA-MRSA in its communities, vigilance and determined control efforts are needed.
The purpose of the present document is to convey basic information regarding the epidemiology and microbiology of CA-MRSA, as well as to suggest recommendations related to the clinical management, prevention and control of CA-MRSA infections. It complements existing publications on hospital-associated (HA) MRSA and CA-MRSA, including a recent statement from the US Centers for Disease Control and Prevention (CDC) (3).
Sources of information and recommendations were derived from a comprehensive literature review, a Working Group meeting of Canadian and US experts, and extensive discussions within an expert panel writing group. When available, published and unpublished Canadian data are presented. The highlights of the present document include clinician-oriented treatment guidelines addressing the various presentations of presumed and confirmed CA-MRSA infection and their management. Guidelines for infection prevention and control in a variety of settings, such as homes, daycare centres and schools, sports settings, pet-owning households, prisons and homeless shelters, and neonatal care facilities, are included. The document does not address health care settings other than nurseries; existing guidelines for infection control in hospitals and clinics should be followed in these settings. Directions for future research are also suggested. The content of the present document will be modified and updated as microbiologists and public health specialists find evolving regional prevalence of CA-MRSA, and as new studies are published.
Front-line physicians need to be aware of the increasing prevalence and potential severity of CA-MRSA infections. They are advised to obtain specimens for culture from all serious skin and soft tissue infections (SSTIs), including abscesses and other infected sites. The management of presumed S aureus infection should include the use of surgical drainage when appropriate, and empirical antibiotic therapy should be adjusted when regional rates of clinical infections due to CA-MRSA increase. Judicious use of antibiotics is emphasized as a prevention strategy. Families, school and day-care centre personnel, and sports teams should be actively encouraged to practice meticulous handwashing, the most important measure to control or attenuate community transmission of CA-MRSA.
1.0. PREAMBLE
A dramatic increase in the rate of methicillin resistance among community isolates of S aureus has recently been observed in the US (4). CA-MRSA has emerged as the dominant pathogen in some communities in the US, with a prevalence as high as 75% of all S aureus isolates (1). These CA strains are genetically and clinically distinct from strains of HA-MRSA, which has been a familiar problem in health care institutions for several decades. Currently, the prevalence of CA-MRSA in Canada is unknown but thought to be low based on the collective clinical experience of infectious disease experts. However, as the prevalence of CA-MRSA increases, clinicians may need to change their approach to the management of presumed S aureus infections. Furthermore, if Canada is to limit the emergence of CA-MRSA in its communities, vigilance and determined control efforts are needed.
To date, there are no Canadian consensus guidelines for the management and prevention of CA-MRSA infections in children and adults. Recent reports (unpublished data, 5,6) of serious invasive disease and mortality due to CA-MRSA in Canada emphasize the need for such guidelines. Focus has centred for years on the challenge of controlling the spread of MRSA in hospitals and chronic care institutions. The present document addresses MRSA in the community and complements previously published guidelines (Appendix). The reader is specifically referred to other excellent existing documents on CA-MRSA, including a statement from the CDC (3), the BC Centre for Disease Control (7) and the Canadian Paediatric Society (8). The present CA-MRSA document is unique because it has a national focus; underwent a rigorous methodology, which included a Working Group meeting of national experts and an expert panel review process; consists of practical, clinician-oriented treatment guidelines addressing multiple possible presentations of CA-MRSA; and has a multidisciplinary focus on the prevention of transmission.
The goal of the present document is to provide information about the epidemiology and microbiology of CA-MRSA in Canada as well as recommendations on its treatment, prevention and control. The document is directed toward health care workers, including public health practitioners, laboratory personnel, clinicians, nurses, infection control practitioners, veterinary medicine personnel and other health care practitioners involved in outbreak management and direct patient care. While the guidelines provide suggestions for specific patient management, they are not meant to replace clinical judgment in the care of the individual patient. The scope of the present document includes the following:
definitions and a general description of the epidemiology highlighting the Canadian experience, where available;
microbiology of MRSA emphasizing differences between traditional HA strains and emerging CA strains;
clinical management guidelines;
recommendations for screening and decolonization;
recommendations for the prevention and management of outbreaks and infections occurring in the community; and
directions for future research, based on ideas generated at the Working Group meeting.
2.0. METHODOLOGY
The information and recommendations presented in the current document result from a comprehensive literature review, a Working Group meeting of Canadian and US experts, and discussions of an expert panel writing committee.
A review of the English-language medical literature from 1980 to March 2006 was conducted. Data sources included MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials. Published abstracts of papers presented at local and international infectious disease or microbiology conferences were cited when they were the only available information from ongoing trials or emerging reports.
An interdisciplinary Working Group meeting held on October 27 and 28, 2005, in Toronto, Ontario, assembled 70 Canadian experts in pediatric and adult infectious disease, infection control, microbiology and public health, as well as US experts in CA-MRSA from Texas and from the CDC. The meeting was supported by the Public Health Agency of Canada, the Canadian Committee on Antibiotic Resistance, and the Ontario Ministry of Health and Long-Term Care. A rich dialogue emerged around issues in CA-MRSA treatment, prevention and control, including important questions for future research.
Recommendations were developed based on the literature review, the Working Group meeting and the opinions of the expert panel. Recommendations were graded on the basis of strength and quality of the supporting evidence (Table 1) (9). The consensus statements were proposed, debated, revised and agreed on by members of the expert panel through conference calls and face-to-face meetings. The document was rigorously reviewed and debated by the expert panel committee using electronic mail in an iterative process with multiple revision steps. Suggestions were then evaluated by the panel and incorporated into the final document.
TABLE 1.
Strength of recommendations and quality of evidence
| Strength | Definition |
| A | Strong recommendation |
| Should always be offered | |
| Experts agree | |
| B | Moderate recommendation |
| Should usually be offered | |
| Most experts agree | |
| C | Optional recommendation |
| May be offered | |
| Expert opinion varies | |
| Grade | Definition |
|
| |
| I | Evidence from at least one proper randomized, controlled trial |
| II | Evidence from at least one well-designed clinical trial without randomization from cohort or case-controlled analytical studies, or from dramatic results of uncontrolled experiments |
| III | Evidence from opinions of respected authorities based on clinical experience, descriptive studies or reports of expert committees |
Data from reference 9
The present document was approved for publication by the Guidelines Committee of the Association of Medical Microbiology and Infectious Disease Canada. These guidelines will be reviewed annually by the CA-MRSA expert panel and will be considered current unless they are revised or withdrawn from distribution.
3.0. DEFINITIONS
3.1. General definitions
MRSA: MRSA demonstrates resistance to the semisynthetic penicillins (methicillin, oxacillin and cloxacillin). It is also resistant to cephalosporins, monobactams and carbapenems. Resistance to other antibiotic classes may occur, but it is strain dependent.
HA-MRSA: MRSA strains that circulate and are transmitted to individuals within health care facilities.
CA-MRSA: MRSA isolates obtained from individuals in the community who have not had recent exposure to the health care system, or from patients in health care facilities in whom the infection was present or incubating at the time of admission.
3.2. Operational definition of CA-MRSA
The case definition for CA-MRSA endorsed by the expert panel, consistent with that used by the US CDC, is MRSA infection in a person who has none of the following risk factors for HA-MRSA: isolation of MRSA more than 48 h after hospital admission; history of hospitalization, surgery, dialysis or residence in a long-term care facility within one year of the MRSA culture date; the presence of an indwelling catheter or a percutaneous device at the time of culture; or previous isolation of MRSA (10).
3.3. Definition limitations
Using a standard definition is important for consistently estimating the burden of CA-MRSA infection (11); however, operational definitions of CA-MRSA have varied among studies. MRSA detected within 24 h, 48 h or 72 h of admission has been variably considered to be of community origin (12). Isolates from patients with health care contact, such as recent hospitalization, hemodialysis or indwelling catheters, have been excluded in some studies but not in others (12).
It is not always possible to identify the source of MRSA with certainty, making the classification of ‘CA’ and ‘HA’ strains based on epidemiological criteria somewhat imprecise. Because genetic and molecular distinctions between CA and HA strains have been described, molecular markers can now be used to define isolates as CA-MRSA or HA-MRSA. The onset of MRSA disease in the community may be attributable to bacterial strains acquired by discharged inpatients or health care personnel and subsequently transmitted to close contacts in the community (13,14). In a meta-analysis, approximately one-half of community-based patients colonized with MRSA had a health care-associated risk factor, suggesting a hospital origin of the isolates (12). Conversely, the ability to track strains using molecular epidemiological markers has enabled investigators to describe the spread of CA-MRSA strains within the hospital (15–17). Because hospital strains have moved into the community and community strains have spread within hospitals, it has become increasingly difficult to distinguish CA-MRSA and HA-MRSA by epidemiological criteria (18). Figure 1 illustrates a classification scheme for MRSA based on molecular and epidemiological characteristics, and the challenge of accurately discriminating between ‘community’ and ‘hospital’ isolates.
Figure 1.
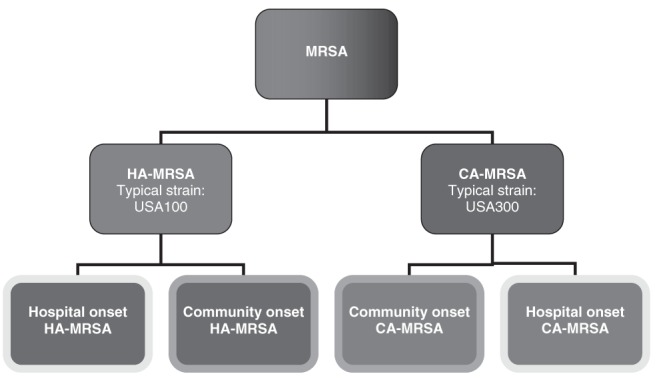
Classification scheme for methicillin-resistant Staphylococcus aureus (MRSA). Molecular techniques allow MRSA strains to be identified as community-associated (CA) MRSA (eg, the epidemic USA300 strain) or hospital-associated (HA) MRSA (eg, the USA100 strain [strain nomenclature described in section 5.3]). Epidemiological information can be used to further determine whether the infection arose in the community or in the hospital. Although, typically, HA-MRSA strains are isolated within health care facilities (ie, hospital-onset HA-MRSA), ‘spillover’ of these strains into the community (ie, community-onset HA-MRSA) has been observed (12). Conversely, CA-MRSA strains were first described in the population at large (ie, community-onset CA-MRSA) but have since been observed in health care settings (ie, hospital-onset CA-MRSA) (15–17)
4.0. EPIDEMIOLOGY
4.1. The rise of CA-MRSA
MRSA was first described in 1961 (19), shortly after the introduction of semisynthetic penicillins (methicillin, oxacillin and cloxacillin). MRSA has long been recognized as a nosocomial pathogen. In the US, up to 40% of hospital S aureus strains are methicillin-resistant, while in Canada, nosocomial MRSA rates have increased from 0.95% in 1995 to 10.4% in 2003 (20). A new phenomenon has been observed over the past 10 years: MRSA strains have emerged, increased in prevalence and become the dominant strains in some US communities. These clones are genetically distinct from HA-MRSA, are well adapted to spread within the community and have the potential to cause severe disease. The rise of MRSA, first in hospitals and now in the community, has been likened to the historically similar trend of resistance of S aureus to penicillin, which emerged first in hospitals in the 1940s and later in the community throughout the 1960s (21).
Cases of severe MRSA infection in patients without contact with health care institutions were reported in a remote Aboriginal population in Australia in 1993 (22). The pathogen gained North American attention when the CDC reported four pediatric deaths from CA-MRSA in 1999 (23). Since then, methicillin resistance among isolates of S aureus outside of health care institutions has reached epidemic proportions in some US communities (24–27). Over a 10-year period, the Driscoll Children’s Hospital in Corpus Christi, Texas, documented an increase in the rate of methicillin resistance among community isolates from 2.9% in 1990 to 40.3% in 2001 (4). The Texas Children’s Hospital in Houston now reports that over 75% of community isolates are methicillin resistant, so MRSA, rather than methicillin-sensitive S aureus (MSSA), is now the dominant community pathogen (1). Increasingly, CA-MRSA is being detected around the globe (28), with multiple reports from Europe (29–31), Southeast Asia (32–35) and Australia (36,37).
To date, a spectrum of clinical manifestations of CA-MRSA infection have been described. The most common manifestation of CA-MRSA infection is SSTI (38). However, there are accumulating reports of severe disease, including sepsis (39), necrotizing fasciitis (40), purpura fulminans (41), toxic shock syndrome (42,43), necrotizing pneumonia (44,45) and empyema (46,47), caused by CA-MRSA. These severe presentations may occur in otherwise healthy children and young adults.
4.2. CA-MRSA in Canada
The first reported cases of CA-MRSA in Canada occurred in an Aboriginal community in Alberta between 1986 and 1989 (48). A cluster of 15 cases of CA-MRSA infections, predominantly soft tissue in origin, with organisms that remained relatively susceptible to non-beta-lactam antibiotics was reported from a small, rural town in southern Manitoba from 1997 to 1998 (49). This strain is rapidly emerging in several neighbouring communities in northern Manitoba and Saskatchewan (50). In 1998, the first case of CA-MRSA disease and transmission in a child care centre was reported in Toronto, Ontario (51). In Ontario in 2004, 11% of 10,301 MRSA isolates were thought to be CA (52). However, it is possible that the overall prevalence of this emerging pathogen has been underestimated.
This pathogen’s potential to cause severe disease has been demonstrated in several Canadian reports. Severe soft tissue infections due to CA-MRSA have been reported in western Canada, including an outbreak in the Calgary Health Region in Alberta in 2004 involving illicit drug users and homeless people (53). The first case of fatal necrotizing pneumonia in a young, otherwise healthy adult was reported recently from the Calgary Health Region (5), and the first fatal pediatric case was described in Ontario (unpublished data). Several additional cases of severe necrotizing pneumonia without clinical or laboratory evidence of antecedent viral respiratory tract infection have been reported in southern Alberta (6).
4.3. Origin of CA-MRSA and its ability to disseminate
Because CA-MRSA has emerged very recently, several questions regarding its origin and ability to spread in the community have been the focus of intensive investigation. Where did CA-MRSA come from? Among other hypotheses, horizontal gene transfer of the resistance determinants from coagulase-negative staphylococci to CA strains of MSSA has been postulated (54). Did antibiotic pressure contribute to the emergence of this pathogen? S aureus and coagulase-negative staphylococci may coexist on the skin of patients treated with beta-lactam antibiotics, providing an environment conducive to the selection of CA-MRSA strains after horizontal transfer of resistance genes (55). What properties of CA-MRSA have allowed it to propagate in the community and indeed arise as the dominant clone in some settings? CA-MRSA strains are genetically distinct and do not simply represent the ‘spillover’ of hospital strains into the community (55,56). CA-MRSA grows more rapidly in vitro than does HA-MRSA (57), likely because HA-MRSA strains carry many antibiotic resistance genes and have a high ‘fitness cost’ of resistance (58). The genome of the epidemic CA-MRSA USA300 strain (strain nomenclature described in section 5.3) has recently been sequenced in its entirety and reveals a novel mobile genetic element – the arginine catabolic mobile element, also present in the ubiquitous skin commensal Staphylococcus epidermidis – that may enhance fitness and pathogenicity (59). Thus, CA-MRSA may be better suited to competition with other bacteria in the environment, whereas HA-MRSA dominates in hospital settings under intense antibiotic pressure. Interested readers are referred to several comprehensive reviews of these topics for more detailed explanations (54,55,60).
4.4. Populations at risk
CA transmission of MRSA has been documented in several identifiable populations (23,38,61–97). These groups, listed in Table 2, are considered to be at high risk for CA-MRSA.
TABLE 2.
Risk factors for community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections
| Risk categories and factors | References |
|---|---|
| High-risk populations | |
| Young people | Age distribution for CA-MRSA younger than for hospital-associated MRSA (38) High rate of CA-MRSA in children younger than two years (61) CA-MRSA more common in Canadian children than adults (62) |
| Minority populations | |
| Native or Aboriginal, and African-American people | Higher risk in Aboriginal communities in midwestern United States (23,63,64) Higher risk in Alaskan natives (65) More common in African-Americans (61,66,67) Aboriginal communities in Canada (48,49,62,68,69) |
| Athletes – mainly those involved in contact sports | Outbreaks in football teams (70–74) Outbreak in wrestling team (75) Outbreaks in other competitive sports (71,76) |
| Intravenous drug users | San Francisco intravenous drug users (77) Western Canadian report of USA300 strain outbreak documents higher risk in intravenous drug users (78) |
| Men who have sex with men | CA-MRSA described in HIV-positive population of men who have sex with men (79,80) |
| Military personnel | 3% of United States army soldiers colonized (81) |
| Inmates of correctional facilities | Reports of outbreaks in United States prisons (82–86) Two outbreaks (total of 10 inmates) in Hamilton, Ontario (87) |
| Previous positive MRSA cultures | |
| MRSA carriage | Colonized soldiers more likely to get CA-MRSA disease (81) |
| Past MRSA infection | Prior abscess a risk factor for CA-MRSA (88) |
| Medical history | |
| Chronic skin disorder | Dermatological condition most common underlying medical disorder for CA-MRSA infection (38) Classroom contact of an index CA-MRSA case had chronic dermatitis (51) |
| Recurrent or recent antibiotic use | Antibiotic use associated with CA-MRSA infection in rural Alaska (65) |
| Environmental risks | |
| Low socioeconomic status | Medically underserved populations at higher risk of CA-MRSA (88,89) |
| Overcrowding | Close contact implicated in jail outbreak (84), neonatal intensive care unit transmission (90) |
| Contact with colonized pet | Family dog a source of recurrent infection (91) |
| Veterinary work | Cases documented in veterinarians working with horses (92–94), small animal veterinarians (95,96) and pig farmers (97) |
4.5. Transmission
The spread of CA-MRSA, like S aureus in general, occurs through direct contact between an infected person and an uninfected person, or by indirect contact through touching contaminated objects or surfaces that are part of the infected person’s environment. Zoonotic transmission (from animals to humans) has also been documented (91,93,95,96,98–103).
5.0. MICROBIOLOGY
5.1. S aureus and MRSA
S aureus is a Gram-positive coccus that tends to form clusters. Resistance to methicillin and the entire class of beta-lactam antibiotics in S aureus is determined by altered penicillin binding protein 2a. This enzyme is encoded by the gene mecA, which is located within a larger mobile genetic element called the staphylococcal chromosomal cassette mec (SCCmec). Currently, there are five types of SCCmec distinguished by their genetic sequence, labelled SCCmec I to SCCmec V. CA-MRSA strains usually contain SCCmec IV or V, whereas HA-MRSA strains usually contain SCCmec I, II or III (104).
5.2. Virulence factors
Factors produced by S aureus that may play a role in virulence are shown in Table 3. The role of each of these factors in clinical disease is not clear, although considerable attention has been focused on the Panton-Valentine leukocidin (PVL) (104). The PVL is an extracellular product of S aureus that is encoded by the genes lukS-PV and lukF-PV (104). This factor, by its cytolytic pore-forming activity, damages the cell membranes of neutrophils, monocytes and macrophages (Figure 2). Infection caused by PVL-positive strains tends to occur in children and young adults, and is associated with higher mortality (105,106). In patients with staphylococcal osteomyelitis, the presence of PVL-positive isolates is associated with a higher likelihood of complications (107,108).
TABLE 3.
Virulence factors of Staphylococcus aureus
| Category | Toxin |
|---|---|
| Cytolytic toxins | Panton-Valentine leukocidin S and F |
| Fibronectin-binding proteins A and B | |
| Leukocidin R | |
| Superantigenic toxins | Enterotoxins (A to J) |
| Epidermolytic toxins | |
| Toxic shock syndrome toxin-1 | |
| Enhanced growth and survival | Arginine catabolic mobile element |
Figure 2.
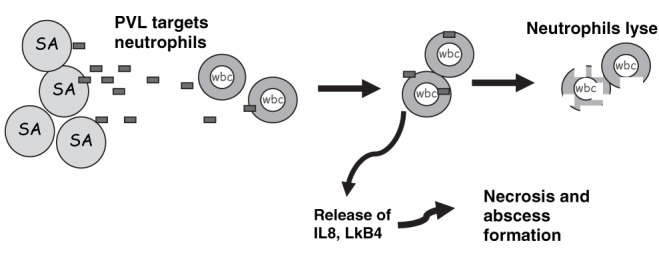
Mechanism of action of the Panton-Valentine leukocidin (PVL). PVL is a putative virulence factor associated with severe clinical disease. The toxin is released from Staphylococcus aureus (SA) and targets the cell membrane of neutrophils (wbc). This causes the release of cytokines, such as interleukin-8 (IL8) and leukotriene B4 (LkB4), which produce necrosis and abscess formation. The toxin also causes lysis of neutrophils such that infection with PVL-positive strains may be associated with neutropenia
5.3. Nomenclature of strains
Independent studies of the molecular epidemiology of MRSA have resulted in a confusing nomenclature of circulating strains (60). In Canada, based on Sma1 macrorestriction patterns from pulsed-field gel electrophoresis, 10 major clusters have been labelled CMRSA-1 to CMRSA-10 (109). In the US, also using pulsed-field gel electrophoresis profiles, 11 major epidemic strains of MRSA, labelled USA100 to USA1100, have been described to date (110). The USA300 (equivalent to CMRSA-10) strain is the dominant circulating clone of CA-MRSA in North America (17,39,40,73,111,112). Another molecular method, multilocus sequence typing, has been used internationally to unambiguously categorize MRSA strains by using the sequence of internal fragments of seven chromosomal housekeeping genes (60). Table 4 shows the common circulating strains and the relationship among the different systems of nomenclature. Also provided are the associated SCCmec types and the presence or absence of PVL genes in the various strains.
TABLE 4.
Nomenclature of Staphylococcus aureus strains
| US PFGE | CMRSA strain | MLST | SCCmec type | PVL presence |
|---|---|---|---|---|
| USA100 | 2 | 5 | II | Negative |
| USA200 | 3,6 | 36 | II | Negative |
| USA300 | 10 | 8 | IVa | Positive |
| USA400 | 7 | 1 | IVa | Positive |
| USA500 | 5,9 | 8 | IV | Negative |
| USA600 | 1 | 45 | II | Negative |
| USA700 | – | 72 | IVa | Negative |
| USA800 | 2 | 5 | IV | Negative |
| USA1000 | – | 59 | IV | Positive |
| USA1100 | – | 30 | IV | Positive |
CMRSA Canadian methicillin-resistant Staphylococcus aureus; MLST Multilocus sequence typing; PFGE Pulsed-field gel electrophoresis; PVL Panton-Valentine leukocidin; SCCmec Staphylococcal chromosomal cassette mec; US United States
5.4. Resistance to non-beta-lactam antibiotics
5.4.1. Clindamycin
While clindamycin resistance is common in HA-MRSA (observed in 79% of isolates) (38), CA-MRSA has a low baseline resistance to clindamycin in some communities. Clindamycin resistance rates for CA-MRSA vary across the US, from 2% (Texas in 2001) to 17% (Minnesota in 2000) (61,113). In Canada, 49% of pediatric and 85% of adult MRSA isolates were resistant to clindamycin between 1995 and 2002 (62), but this reflects a preponderance of hospital strains within the Canadian Nosocomial Infection Surveillance Program.
Laboratory testing for clindamycin resistance should include the double disk diffusion test (D test) for inducible clindamycin resistance (114). A clindamycin disk is placed at a fixed distance from an erythromycin disk, and a D-shaped zone of inhibition around the clindamycin disk indicates that resistance has been induced by the diffusion of erythromycin (ie, MLSB phenotype) (115). Clinical failures have been documented in CA-MRSA infection when clindamycin was used to treat strains with inducible clindamycin resistance (positive D test) (115,116). Therefore, laboratories should routinely test for inducible clindamycin resistance, and clindamycin should be avoided when inducible resistance is detected.
5.4.2. Erythromycin
Both HA-MRSA and CA-MRSA are frequently resistant to erythromycin. For example, resistance was detected in vitro in 91% and 56% of HA-MRSA and CA-MRSA isolates, respectively, in Minnesota in 2000 (61). A large, laboratory-based survey indicated that 93% of Canadian MRSA isolates demonstrated resistance to erythromycin (117).
5.4.3. Quinolones
Emergence of resistance during therapy leading to treatment failure may occur when quinolones are used for the treatment of S aureus infections, including CA-MRSA (118).
5.5. Differences between CA-MRSA and HA-MRSA
In summary, CA-MRSA strains are genetically and phenotypically distinct from HA-MRSA strains, as highlighted in Table 5.
TABLE 5.
Contrasting community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) and hospital-associated (HA) MRSA
| CA-MRSA | HA-MRSA | |
|---|---|---|
| Typical demographic characteristics | Younger Minority population |
Older Nursing home resident |
| Common SCCmec type | IV | II |
| PVL virulence factor | Common | Unusual |
| Predominant strains | USA300 (CMRSA-10) USA400 (CMRSA-7) |
USA100 (CMRSA-1) USA200 (CMRSA-3,6) |
| Multidrug resistance (other than beta-lactam) | Rare | Common |
CMRSA Canadian MRSA; PVL Panton-Valentine leukocidin; SCCmec Staphylococcal chromosomal cassette mec; USA United States
6.0. MANAGEMENT
6.1. Diagnostic evaluation
At the initial clinical presentation, CA-MRSA may not be easily distinguished from HA-MRSA, MSSA or streptococci as the agent of infection. Epidemiological risk factors (Table 2) should heighten suspicion of CA-MRSA. Furthermore, microbiological diagnosis can be helpful in guiding management and may prove helpful in monitoring local rates of CA-MRSA as this pathogen emerges in the community. The following principles of management are intended to assist the clinician faced with a possible or proven case of CA-MRSA infection and address clinical presentations that are potentially consistent with S aureus infection.
6.1.1. When to suspect CA-MRSA
Recommendations
In areas where approximately 10% to 15% of community isolates of S aureus are methicillin resistant, CA-MRSA should be suspected in any patient who presents with an SSTI. (BIII)
Suspect CA-MRSA in severe infections compatible with S aureus (eg, sepsis [39], necrotizing fasciitis [40], necrotizing pneumonia [44,45] and empyema [46,47]). (AIII)
Suspect CA-MRSA when risk factors for CA-MRSA are present (Table 2). (AIII)
Suspect CA-MRSA when there is a poor response to beta-lactam therapy in individuals with presumed staphylococcal infection. (AIII)
6.1.2. When to obtain cultures
Recommendations
Cultures should be obtained from SSTIs, as well as other sites where S aureus infection is suspected, that have not responded to initial therapy. (AIII)
Culture recurrent furuncles or abscesses (two or more in six months). (AIII)
Obtain cultures in any severe presentation of the disease (should include blood cultures). (AIII)
Obtain cultures when an outbreak is suspected in consultation with public health. (AIII)
Consider an attempt to obtain material for culture from areas of cellulitis by aspiration of the area, with or without preceding saline injection, particularly for patients who are going to be admitted for inpatient therapy or whose cellulitis progresses on treatment. (BIII)
Specimens for culture of SSTIs should not be routinely obtained for all individuals presenting with minor skin infections and without previous CA-MRSA infection. (AIII)
6.2. Treatment
Studies have demonstrated that antibiotics may not be necessary in patients with minor SSTIs due to CA-MRSA (119). When systemic antimicrobial therapy is indicated for an infection consistent with S aureus, clinicians should bear in mind the possibility of methicillin resistance. The prevalence of CA-MRSA in the community is an important factor in guiding empirical antibiotic choice, but is unknown in most areas in Canada. The rate of methicillin resistance among community strains of S aureus is assumed to be low at the present time because of the relatively few cases of antibiotic failures reported to date, but may rise in the near future, as has occurred in many US cities. The threshold of resistance that should prompt a change in empirical therapy is thought to be approximately 10% to 15% (113). However, even in communities in which the rate is lower than 10% to 15%, clinicians may wish to include antimicrobial coverage for CA-MRSA in cases in which the infection is severe or life-threatening. In the absence of data to suggest a high prevalence of methicillin resistance in Canada, no change in empirical therapy of presumed S aureus infections is advocated at the present time. As resistance rates become better defined, recommendations for empirical therapy may change.
Table 6 summarizes the treatment principles and antimicrobial options for various presentations of CA-MRSA infection. Further details of the antimicrobial agents useful in the treatment of CA-MRSA are presented in Table 7. The following recommendations are categorized according to empirical therapy for suspected CA-MRSA infection versus therapy for confirmed infection, and according to severity and location of infection.
TABLE 6.
Guidelines for the management of infections due to community-associated methicillin-resistant Staphylococcus aureus (MRSA)
| Clinical disease | Key features | Management principles | Antimicrobial choices* |
|---|---|---|---|
| Skin and soft tissue infection (SSTI) | |||
|
| |||
| Mild | Localized disease Infected scratches Insect bites Furuncles Small abscesses Absence of systemic illness |
Culture selectively† No antibiotic therapy recommended except for young or immunocompromised host Cover draining lesions Emphasize personal hygiene Close follow-up Return if worsening |
Generally not indicated Topical antiseptic or antibacterial (eg, bacitracin) therapy may be considered. Systemic antimicrobial therapy may be considered in the young infant or immunocompromised host. |
| Moderate | Cellulitis Moderate abscesses Minimal or no associated systemic features |
Culture (blood if febrile, site if purulent) Drainage of abscess or needle aspiration Oral therapy in older child or adult Consider parenteral therapy for young or immunocompromised host Appropriate infection control measures Imaging for extent and complications (case by case) Close follow-up Return if worsening |
ET includes clindamycin 150 mg to 450 mg every 6 h po and ped dose of 30 mg/kg/day ÷ every 6 h to 8 h po, or TMP-SMX one double-strength tablet or two regular-strength tablets every 12 h po and ped dose of 8 mg/kg/day to 12 mg/kg/day (based on TMP component) ÷ every 12 h po/IV plus coverage for group A streptococcus, or doxycycline‡ 100 mg every 12 h po. If parenteral therapy is necessary, see choices for severe SSTI. Treat proven MRSA as above, based on sensitivity testing. If parenteral therapy is necessary, see choices for severe SSTI. |
| Severe | Extensive cellulitis Large or multiple abscesses Associated systemic features |
Culture (blood if febrile, site if purulent) Drainage of abscess Hospitalize Parenteral therapy Appropriate infection control measures Infectious disease consultation Imaging for extent and complications |
ET includes vancomycin 1 g every 12 h IV and ped dose of 40 mg/kg/day to 60 mg/kg/day ÷ every 6 h IV. Some experts recommend adding cloxacillin or a first-generation cephalosporin while awaiting culture and sensitivity results (superior for MSSA). Clindamycin may be added in cases of toxin-mediated syndrome. Treatment for proven MRSA includes vancomycin 1 g every 12 h IV and ped dose of 40 mg/kg/day to 60 mg/kg/day ÷ every 6 h IV. Alternatives include clindamycin 600 mg to 900 mg every 8 h IV/IM (if sensitive) and ped dose of 30 mg/kg/day to 40 mg/kg/day ÷ every 6 h to 8 h IV or TMP-SMX* 8 mg/kg/day to 10 mg/kg/day (based on TMP component) ÷ every 12 h IV (if sensitive) and ped dose of 8 mg/kg/day to 12 mg/kg/day (based on TMP component) ÷ every 6 h IV. Clindamycin is bacteriostatic and should not be used alone if a bactericidal drug is required. |
| Musculoskeletal infection (MSI) | |||
|
| |||
| Osteomyelitis | Preceding trauma Tendency for multifocal lesions Disease in adjacent muscle not uncommon Progression to chronic osteomyelitis possible May be complicated by DVT |
Cultures (blood, bone and tissue) Involve surgical team (early debridement and drainage) Infectious disease consultation Parenteral therapy Consider combination therapy for severe cases or if slow to respond Infection control measures Look for other infected sites (imaging) |
ET includes vancomycin 1 g every 12 h IV and ped dose of 40 mg/kg/day to 60 mg/kg/day ÷ every 6 h IV, or clindamycin 600 mg to 900 mg every 8 h IV/IM/po and ped dose of 30 mg/kg/day to 40 mg/kg/day ÷ every 6 h to 8 h IV or po, or TMP-SMX 8 mg/kg/day to 10 mg/kg/day (based on TMP component) ÷ every 12 h IV and ped dose of 8 mg/kg/day to 12 mg/kg/day (based on TMP component) ÷ every 6 h IV. Treat proven MRSA as above, based on sensitivity testing. Addition of rifampin may be considered for osteomyelitis. |
| Pyomyositis | May be extensive Tendency for multifocal involvement |
Cultures (blood, tissue) Surgical drainage Infectious disease consultation Parenteral therapy Infection control measures Imaging |
Treat similiar to osteomyelitis. |
| Necrotizing fasciitis | Clinically indistinguishable from GAS disease Toxic High complication rate |
Cultures (blood and tissue) Surgical debridement Infectious disease consultation Parenteral therapy Infection control measures Imaging |
ET includes vancomycin 1 g every 12 h IV and ped dose of 40 mg/kg/day to 60 mg/kg/day ÷ every 6 h IV. Some experts recommend adding cloxacillin or a first-generation cephalosporin while awaiting culture and sensitivity results (superior for MSSA). Clindamycin may be added in case of toxin-mediated syndrome. Adjuncts such as IVIG should be considered on a case-by-case basis in conjunction with ID specialist. Treat proven MRSA as above, based on sensitivity testing. |
| Respiratory tract infection (RTI) | |||
|
| |||
| Necrotizing pneumonia | Influenza-like prodrome, hemoptysis, fever, shock, leukopenia, pneumatoceles, abscesses, consolidation Respiratory failure High mortality |
Cultures (blood, pleural fluid and sputum) ID consultation Intensive care unit care Infection control measures Combination parenteral therapy Chest drainage if empyema |
ET includes vancomycin 1 g every 12 h IV and ped dose of 40 mg/kg/day to 60 mg/kg/day ÷ every 6 h IV. Treat proven MRSA as above, based on sensitivity testing. Consider linezolid (superior for hospital-associated MRSA pneumonia [123,124]), as guided by ID opinion. |
| Other | |||
|
| |||
| Sepsis syndrome | Shock Multiorgan failure May have purpura fulminans Associated SSTI, MSI, RTI May be complicated by Waterhouse-Friedrichsen syndrome High mortality |
Blood cultures Culture any pus or fluid collection Look for primary or secondary focus ID consultation Intensive care unit care Imaging: look for occult abscesses, bone infection or endocarditis Involve surgery and other specialists as needed Infection control measures Parenteral, multidrug therapy Prolonged therapy for endovascular infections |
ET includes vancomycin 1 g every 12 h IV and ped dose of 40 mg/kg/day to 60 mg/kg/day ÷ every 6 h IV. Some experts recommend adding cloxacillin or a first-generation cephalosporin while awaiting culture and sensitivity results (superior for MSSA). Clindamycin may be added in case of toxin-mediated syndrome. Adjuncts such as IVIG should be considered on a case-by-case basis in conjunction with ID specialist Proven MRSA treatment includes vancomycin 1 g every 12 h IV and ped dose of 40 mg/kg/day to 60 mg/kg/day ÷ every 6 h IV. Alternatives include clindamycin 600 mg to 900 mg every 8 h IV/IM (if sensitive) and a ped dose of 30 mg/kg/day to 40 mg/kg/day ÷ every 6 h to 8 h IV or TMP-SMX 8 mg/kg/day to 10 mg/kg/day (based on TMP component) ÷ every 12 h IV (if sensitive) and ped dose of 8 mg/kg/day to 12 mg/kg/day (based on TMP component) ÷ every 6h IV. Clindamycin is bacteriostatic and should not be used alone if a bactericidal drug is required. |
| Septic thrombophlebitis, DVT | Complicates musculoskeletal infection | Management as for sepsis syndrome, plus Doppler ultrasound, often found on magnetic resonance imaging Anticoagulation, under direction of hematology (159) |
Treat as above For endovascular infections, combination antimicrobial therapy is recommended (ie, vancomycin plus either gentamicin or rifampin) Prolonged therapy is required for endovascular infection, and expert advice from an ID specialist should be considered. |
| Endocarditis | Suspect if persistent bacteremia Pre-existing valvular heart disease may not be present |
Management as for sepsis syndrome, plus Echocardiogram Cardiology consultation ID consultation Parenteral therapy Monitor for complications (embolic phenomena and hemodynamic instability) |
|
Choice of antimicrobial therapy depends on local susceptibility patterns;
Patients with risk factors, as a part of an outbreak investigation, and patients with slowly responding or recurrent lesions;
Not recommended for pediatric patients younger than eight years or during pregnancy. (AIII) Further information on antibiotic dosages and adverse effects can be found in Table 7. DVT Deep vein thrombosis; ET Empirical therapy; GAS Group A streptococcal; ID Infectious disease; IM Intramuscularly; IV Intravenously; IVIG Intravenous immunoglobulin; MSSA Methicillin-sensitive Staphylococcus aureus; ped Pediatric; po Orally; TMP-SMX Trimethoprim-sulfamethoxazole
TABLE 7.
Recommended antibiotics and doses
| Drug | Dose (adult) | Dose (pediatric)* | Adverse reaction | Notes |
|---|---|---|---|---|
| Oral options for mild to moderate SSTI with no systemic features | ||||
|
| ||||
| Clindamycin | 150 mg to 450 mg every 6 h po | 30 mg/kg/day ÷ every 6 h to 8 h po | Pseudomembranous colitis | Resistance may occur Check susceptibility with D test |
| TMP-SMX | 1 DS or 2 RS tabs (160 mg TMP/800 mg SMX) every 12 h po | 8 mg/kg/day to 12 mg/kg/day (based on TMP component) ÷ every 12 h po | Allergy (skin rash) Bone marrow suppression |
Not recommended for group A streptococcus |
| Doxycycline | 100 mg every 12 h po | 2 mg/kg/day to 4 mg/kg/day ÷ every 12 h to maximum 100 mg every 12 h po Not for children younger than eight years |
Photosensitivity Erosive esophagitis Teeth staining |
Not for children younger than eight years Not for use in pregnancy |
| Linezolid | 400 mg every 12 h po | 20 mg/kg/day ÷ every 12 h po (160) | Dose-dependent bone marrow suppression Peripheral neuropathy Optic neuritis (rare) |
Selected cases only Expensive ID consultation recommended |
| Parenteral therapy for systemic and severe infections | ||||
|
| ||||
| Vancomycin | 1 g every 12 h IV | 40 mg/kg/day to 60 mg/kg/day ÷ every 6 h to a maximum 4 g/day IV | Renal toxicity | Lower efficacy in pneumonia Monitor levels |
| Clindamycin | 600 mg to 900 mg every 8 h IV/IM | 30 mg/kg/day to 40 mg/kg/day ÷ every 6 h to 8 h IV | Pseudomembranous colitis | Resistance may occur Check susceptibility with D test Bacteriostatic |
| TMP-SMX | 8 mg/kg/day to 10 mg/kg/day ÷ every 12 h IV | 8 mg/kg/day to 12 mg/kg/day (based on TMP component) ÷ every 12 h IV | Allergy (skin rash) Bone marrow suppression |
Not recommended for group A streptococcus |
| Linezolid | 600 mg every 12 h IV/po | Children younger than 12 years: 30 mg/kg/day ÷ every 8 h IV (160) Children older than 12 years: 20 mg/kg/day ÷ every 12 h IV |
Dose-dependent bone marrow suppression Peripheral neuropathy Optic neuritis (rare) |
Selected cases only Expensive ID consultation recommended Bacteriostatic |
| Adjunctive therapy | ||||
|
| ||||
| Rifampin | 600 mg po qd | 10 mg/kg/day to 20 mg/kg/day ÷ every 12 h to 24 h po/IV | Hepatotoxicity Rash Discoloured urine Stains soft contact lenses |
Consider in high bacterial burden Never use alone Potential for drug interactions |
| Fusidic acid | 500 mg tid po/IV | No data for children | Skin rash Jaundice |
No cerebrospinal fluid penetration Not excreted in urine Never use alone (161) Bacteriostatic |
Doses in the neonate may be different. DS Double strength; D test Double disk diffusion test; ID Infectious disease; IM Intramuscularly; IV Intraveneously; po Orally; qd Once daily; RS Regular strength; SSTI Skin and soft tissue infection; tid Three times daily; TMP-SMX Trimethoprim-sulfamethoxazole
6.2.1. Minor SSTIs (folliculitis, furuncles and small abscesses without cellulitis)
Recommendations
-
One or more of the following measures may be used:
local therapy using hot soaks and elevation; (AIII)
incision and drainage without antimicrobial therapy (119); (AII)
topical mupirocin or bacitracin; (AIII) and/or
topical antiseptics. (BIII)
For young infants and for the immunocompromised host, antimicrobial therapy is recommended in addition to local measures, incision and drainage. (BIII)
In follow-up, routine screening for colonization of the nares or other body sites is not recommended. (AIII)
6.2.2. Empirical therapy of non-life-threatening infections other than minor skin infections, potentially due to CA-MRSA
Recommendations
Antibiotic choice should be based on the severity of illness at presentation, clinical judgment and regional susceptibilities of strains. Antibiotic chosen should include an agent effective against group A streptococcus. (AIII)
At present, cloxacillin or cefazolin remain appropriate empirical antibiotic choices for moderate infections (serious enough to require systemic antibiotics but not considered life threatening), consistent with S aureus. (AIII)
6.2.3. Empirical therapy of life-threatening infections potentially due to CA-MRSA
Recommendations
6.2.4. Confirmed non-life-threatening CA-MRSA infections other than minor skin infections
Recommendations
For patients older than eight years, first-line oral agents include clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX) or doxycycline. Fusidic acid in combination with another agent may also be considered. (AIII)
Treatment should be guided by the susceptibility pattern. Clindamycin should only be considered an appropriate choice if susceptibility is confirmed using the D test. (AIII)
For children younger than eight years, first-line oral agents include clindamycin and TMP-SMX. Doxycycline is contraindicated in children younger than eight years, as are all tetracyclines, and there are limited data regarding the use of fusidic acid in children (122). (AIII)
Do not use doxycycline in pregnancy. (AIII)
Patients should be followed, and the antimicrobial therapy should be reconsidered if there is evidence of treatment failure. (CIII)
In follow-up, routine screening for colonization of the nares or other body sites is not recommended. (AIII)
6.2.5. Confirmed CA-MRSA life-threatening infections
Recommendations
Treatment options include parenteral vancomycin, clindamycin (provided susceptibility confirmed with D test) and TMP-SMX. (AIII)
Some experts recommend against the use of bacteriostatic agents such as clindamycin alone for the treatment of life-threatening infections. (CIII) Fusidic acid and rifampin should not be used alone because of rapid emergence of resistance. (AIII)
Newer drug therapies, such as linezolid, tigecycline or quinupristin-dalfopristin, should be guided by an infectious disease specialist. In particular, drugs such as quinupristin-dalfopristin and daptomycin should be used with caution in pediatric populations in which there are limited data for their use. (CIII)
Linezolid is preferred over vancomycin for the treatment of MRSA pneumonia because of its superiority in clinical trials for adults with HA-MRSA pneumonia (123), possibly explained by better penetration into the lung parenchyma (124). (BII)
In follow-up, routine screening for colonization of the nares or other body sites is not recommended. (AIII)
6.2.6. Adjunctive therapy
Recommendations
Combination of first-line drugs with rifampin or gentamicin to enhance killing in serious invasive disease should be decided on a case-by-case basis in discussion with an infectious disease specialist (125). (BIII)
Other adjunctive therapy, such as intravenous immunoglobulin, may play a role in neutralizing toxin-mediated effects and may be considered for selected patients with severe disease as guided by an infectious disease or critical care specialist (126). (CIII)
7.0. SCREENING AND DECOLONIZATION
7.1. Screening for CA-MRSA
S aureus may asymptomatically colonize body surfaces, particularly the nares. Rates of colonization in a recent US population-based survey (127) were 31.6% for S aureus and 0.84% for MRSA. These asymptomatic carriers may act as a reservoir for infection; therefore, identifying S aureus carriers and eradicating the carriage state may theoretically prevent recurrent S aureus infections or person-to-person spread. However, at present, there is insufficient evidence to support the use of eradication regimens; thus, there is no clear role for screening (128).
Recommendations
In the nonoutbreak setting, routinely screening individuals infected with CA-MRSA or their contacts for colonization of nares or other sites is not recommended. (AIII)
-
In selected circumstances, following consultation with public health or an infectious disease consultant, nasal and/or additional site screening may be considered. These selected circumstances include the following:
Individuals with recurrent S aureus skin infections (two or more in six months), in whom eradication therapy is being considered; (BIII)
In a family setting, where recurrent skin infections continue despite repeated review and reinforcement of hygiene measures, and there is not known to be a high prevalence of CA-MRSA in the community; (BIII) and
To investigate an outbreak in a closed population with continuing new infections despite repeated reinforcement of hygiene practices. (BIII) When a colonization survey is performed as part of an outbreak investigation, assessing carriage sites other than the nares may be considered, in consultation with public health officials and/or other experts (51). (BIII)
7.2. Decolonization
Decolonization refers to the process of eradicating or reducing carriage of a particular organism from the skin, nose or other mucosal surfaces. In the case of staphylococcal carriage, decolonization has been attempted using topical or systemic (usually oral) therapy. The available systemic options include rifampin plus another antistaphylococcal antibiotic, such as TMP-SMX, clindamycin, fusidic acid, doxycyline or minocycline. Eradication from the skin can be attempted using topical agents, such as chlorhexidine or triclosan, whereas nasal decolonization usually requires intranasal mupirocin. Eradication from sites other than the nose usually requires systemic and topical therapy in addition to intranasal therapy. However, decolonization regimens have met with limited success. A systematic review (128) of the literature published in 2003 concluded that there is insufficient evidence to support the use of topical or systemic antimicrobial therapy for eradicating nasal or extranasal MRSA. Experience in hospitals indicates that recolonization is frequent (129,130). In the community setting, decolonization has been attempted with mixed success. Decolonization was successful in eradicating CA-MRSA carriage in a daycare facility (51); however, recurrent infection occurred despite decolonization attempts in two CA-MRSA outbreaks involving football teams (73,74).
One disadvantage of attempted decolonization is the development of resistance to the agents used. Mupirocin resistance has been documented in several studies (131–134), usually associated with prolonged use or repeated courses of mupirocin. In Canada, Mulvey et al (109) have reported mupirocin resistance rates of up to 50% in community isolates on the prairies. Limiting its use to a maximum course of five to 10 days and ensuring a minimum time period of one month between recurrent use are strategies that have been found to be effective in preventing the emergence of mupirocin resistance (133,134).
Recommendations
Decolonization is not recommended for usual management of individual CA-MRSA infections, endemic infection or outbreaks (128,131,135–138). (AI)
-
Decolonization should be considered only in exceptional circumstances, which may include the following:
recurrent CA-MRSA skin infections (two or more in six months) with no evidence of repeated re-exposures when hygiene measures have been reinforced and after discussion with an infectious disease expert; and (BIII)
as a public health strategy for ongoing transmission despite repeated review and reinforcement of appropriate hygiene interventions in outbreaks in selected closed settings. (BIII)
7.3. Guidelines for the use of decolonization regimens
In exceptional situations in which decolonization regimens are used, several options are available: intranasal mupirocin ointment, topical antiseptics applied to the skin and systemic antibiotics active against the colonizing strain, particularly those that achieve high levels in body secretions, such as rifampin and clindamycin. Some experts favour a combined approach (including intranasal mupirocin, topical antiseptics and two systemic agents) for maximum possible effect in the rare circumstances when decolonization is indicated. Other experts offer only intranasal mupirocin to patients with isolated nasal carriage of S aureus(139).
Recommendations
Decolonization regimens should be administered only to individual patients or well-defined closed cohorts, and only over a limited time interval to minimize the potential for resistance to develop. (BIII)
Selection of a decolonization regimen should take into consideration the sensitivities of the organism isolated as well as the sites colonized, and infectious disease consultation should be sought. (BIII)
The recommended regimen for nasal decolonization for mupirocin-susceptible isolates is mupirocin ointment to the nares twice daily for five to 10 days (133,134,140). (BII)
When mupirocin is used to eradicate carriage, isolate susceptibility to mupirocin should be tested. (BIII)
No recommendations can be made at this time for the use of other topical intranasal agents for decolonization. (CIII)
There is insufficient evidence to make a recommendation for or against the use of topical antiseptics for cleaning or cutaneous decolonization. (CIII)
A combined strategy of intranasal mupirocin, topical antiseptics and systemic antibiotics (active against the colonizing strain and achieving high levels in body secretions [eg, rifampin or clindamycin]) may be considered (140). (BIII)
8.0. POPULATION SURVEILLANCE
8.1. Population surveillance program for CA-MRSA
The Working Group meeting identified several possible purposes of a population surveillance program: to document the emergence of resistance to methicillin among community isolates of S aureus to inform empirical therapy; to describe the occurrence and impact of severe S aureus disease in a community, irrespective of resistance pattern; and to facilitate timely identification of potential outbreaks.
Recommendations
Surveillance for methicillin resistance among CA strains of S aureus should be considered. (CIII)
Population-based surveillance for severe CA S aureus infections (including severe SSTIs, osteomyelitis, pyomyositis, necrotizing fasciitis, sepsis and endocarditis [Table 6]), irrespective of susceptibility, should be considered. (CIII)
Intermittent or targeted surveillance of all purulent skin lesions in patients presenting to primary care may be considered to support timely identification of outbreaks, and to recognize emergence and spread of new strains in the community. (CIII)
Populations recognized to be at increased risk (eg, sports teams and Aboriginal communities) should be included in the development of a surveillance program. (BIII)
8.2. Laboratory support
Monitoring CA-MRSA in the community requires collaboration among the clinician managing individual patients, the microbiology laboratory and public health departments.
Recommendations
Clinicians and public health personnel in a given region should develop, together with microbiological laboratories, a method for rapid dissemination and timely feedback of susceptibility profiles for CA-MRSA in the region. (AIII)
Clinical microbiology laboratories should follow the current guidelines laid out by the Clinical and Laboratory Standards Institute (USA) when testing erythromycin-resistant strains of CA-MRSA for inducible clindamycin resistance (ie, D testing). (AIII)
Antimicrobials that are tested and reported back to practitioners should reflect the usual standard of care for CA-MRSA (eg, fluoroquinolone susceptibilities should not be provided). (AIII)
9.0. PREVENTION
9.1. Prevention of transmission of CA-MRSA
The goal of community control of CA-MRSA is to prevent spread of the bacteria from an infected or colonized individual to other persons in the family and the community. This requires individuals to take a proactive role to limit transmission. As a general rule, the prevention of CA-MRSA and infections with other common skin pathogens requires consistent application and reinforcement of good hygienic practices with emphasis on handwashing, not sharing potentially contaminated personal articles, and covering of draining skin lesions to prevent direct or indirect contact with infected secretions of another person. These measures are not specific to CA-MRSA, and apply to draining lesions, wounds or potentially infected sites caused by any microorganism.
9.1.1. Role of the individual
Recommendations
-
Individuals should follow basic practices for good hygiene at all times and in all settings. These include, but are not limited to the following:
regular hand hygiene to limit personal contamination and transmission; and (AIII)
regular bathing with soap and water. (AIII)
-
If skin lesions are present:
cover lesions with appropriate dressings to contain drainage or exudate, and ensure that appropriate medical care has been received; (AIII)
do not share creams, lotions, soaps, cosmetics and other personal products that are in contact with the skin; (AIII)
do not share unwashed towels; (AIII)
do not share personal items that come in contact with the skin lesions – such as razors, toothbrushes, towels, nail files, combs and brushes – without cleaning; (AIII)
discard contaminated waste, including used dressings, in a safe and timely manner to avoid exposure to other individuals; (AIII) and
wash hands with soap and water after touching any skin lesions and potentially infected materials, such as soiled dressings. (AIII)
9.1.2. Role of health care practitioners
Recommendations
-
Practitioners should use antibiotics judiciously (141). (AIII)
Treatment of viral infections with antimicrobials should be avoided; and
Patients should be encouraged to complete all courses of antibiotics as prescribed.
Public health officials should be notified if spread occurs beyond a family unit to a localized community group, such as a school or sports team (ie, if an outbreak of the disease is suspected). (AIII)
Educate patients about appropriate hygiene practices, as outlined in section 9.1.1. (AIII)
9.1.3. Role of health authorities
Recommendations
Communication strategies that inform the general public, as well as high-risk groups, about CA-MRSA and practices to limit infection need to be developed, implemented and evaluated. (AIII)
Strategies for ensuring early diagnosis and appropriate treatment of skin infections should target physicians, and should include education about risk factors, clinical features and expected treatment response time. (AIII)
Regional and local programs to review antibiotic use and resistance should be developed. (BIII)
Education programs should be developed to educate the public on the proper use of antibiotics in the community. (AIII)
9.2. Prevention in specific settings
9.2.1. Households with CA-MRSA infection
In addition to the general measures outlined in section 9.1, specific measures can be recommended within households in which one or more members have a CA-MRSA infection.
Recommendations
The household environment should be regularly cleaned with a standard household detergent. (AIII)
Clothes and linens from individuals who are MRSA-positive or have other skin lesions can be included in the regular household laundry. Usual laundry washing and drying destroys most potentially pathogenic bacteria. (AIII)
Cutlery and dishes may be washed in the usual manner with other household utensils using soap and hot water, or a dishwasher. (AIII)
Individuals who are MRSA-positive, or their family members, should be advised to notify at the time of contact with the health care system that they are either MRSA-positive or living in a household with someone who is MRSA-positive. (BIII)
9.2.2. Daycare centres and schools
Isolation of children with CA-MRSA in childcare settings or schools is not a practical solution and impacts negatively on the child’s well-being. The emphasis must be placed on the consistent application of hygienic measures within the daycare or school setting to reduce the risk of transmission. In addition to the general measures outlined in section 9.1, specific measures are recommended to prevent transmission in schools and daycare centres.
Recommendations
Educate providers, teachers, children and families on general hygiene practices (eg, hand hygiene, respiratory etiquette and staying home if ill). (AIII)
Ensure availability of products to allow hand hygiene to be performed. This includes access to liquid soap in pump dispensers, running water and paper towels to dry hands. Alcohol-based, waterless hand sanitizers can be used as an alternative as long as hands are not visibly soiled. (AIII)
Structure activities to include opportunities for hand hygiene to be practised (before eating, after outdoor play and after using the washroom). (BIII)
In situations in which open lesions cannot be kept covered, consider temporary exclusion from the daycare or school setting until the wound has healed or drainage can be contained. (BIII)
Ensure that frequently touched surfaces (eg, counters, desks and toys) are cleaned at least daily with a disinfectant solution. (AIII)
Items soiled with body fluids should be cleaned and disinfected as soon as possible and before use by another child. (AIII)
9.2.3. Sports settings
CA-MRSA transmission has been documented in several reports among athletes and contact sports participants (70–74). In addition to the general measures outlined in section 9.1, the following recommendations address infection prevention and control in this high-risk group.
Recommendations
At all times:
Use alcohol-based hand antiseptic rinse or gel when handwashing facilities are not available. (AIII)
Individuals participating in sports should shower with soap and water after every practice or tournament. (AIII)
Do not share hygiene items, such as bar soap or towels (73). (AIII)
Ensure regular cleaning of communal bathing facilities and frequently touched surfaces. (AIII)
Personal items, such as towels and supporters, should be laundered or cleaned after each use. (AIII)
Clean or launder shared athletic equipment, such as pads or helmets, at least once a week, but ideally after each use. Establish a routine cleaning schedule for nonpersonal devices, such as sensor wires used in fencing. (AIII)
Individuals with skin lesions:
Provide both verbal and written instructions describing management of skin lesions infected with CA-MRSA or other potential pathogens to coaches and/or participants. (BIII)
Individuals who have open lesions that cannot be kept covered should not participate in contact sports until the wound has healed or drainage can be contained. (AIII)
Individuals who have open skin lesions should be excluded from common whirlpools or saunas. (AIII)
Persons with skin lesions should not share athletic equipment that is in contact with the skin. (AIII)
9.2.4. Pets and other animals
Recurrent MRSA infections in household contacts of colonized companion animals (pets) have been described (91,96,102,103). Given the evidence of transmission of MRSA between humans and animals, there is concern that pets may serve as a reservoir for MRSA in the community (96). In addition to the general measures outlined in section 9.1, the following recommendations are made for owners of pets infected or colonized with MRSA.
Recommendations
Pet ownership and contact information may identify risk and should be queried as part of the standard history for any patient. Known MRSA status of pets or owners, when available, should be documented. (AIII)
Pet screening should only be considered when recurrent infections are occurring within an isolated group exposed to the pet and despite repeated reinforcement of hygiene practices. Consultation with a veterinarian, as well as a public health or infectious disease expert, is recommended. (BIII)
Treatment of colonized pets is not indicated because there is little evidence that antimicrobial-based eradication therapy is effective in colonized pets, and colonization tends to be short term. (BIII)
In exceptional circumstances, when a colonized pet is implicated as a source of infection and the infection is serious and recurrent, temporary removal of the pet from the household may be considered. While there is the potential for pets to be involved in dissemination of MRSA in the community, the beneficial effects of pet contact should be considered in any discussion about removal of the pet from the household. (BIII)
There should be increased awareness in the veterinary community about MRSA infection and colonization in pets, interspecies transmission of MRSA, appropriate testing, management of infected and colonized pets, and relevant infection control measures. (BIII)
9.2.5. Correctional facilities or shelters
Outbreaks of CA-MRSA have been documented in incarcerated populations in the US, Australia and Canada (82–84,86,87,142). In addition to the general measures outlined in section 9.1, the following recommendations address this high-risk group.
Recommendations
Educate correctional facility staff and inmates on transmission, prevention, treatment and containment of MRSA infections. (BIII)
Restrict inmates who have uncovered draining skin lesions, as well as inmates with skin lesions and poor hygiene, to prevent exposure of other inmates. (BIII)
Consider housing assignments based on the potential harm to individuals who could acquire infection. (BIII)
9.2.6. Newborn care facilities
Routine practices can be expected to limit the transmission of CA-MRSA within newborn care facilities and must be followed at all times (143).
Recommendations: Routine care
Wash hands before and after contact with the newborn. (AIII)
Use gloves until the newborn has been cleaned or bathed for the first time. (AIII)
Clean and disinfect all used equipment before it is used with another infant (eg, thermometers, weigh scales, glucose meters and stethoscopes). (AIII)
Staff should wear a gown when holding the infant against the body. (AIII)
There are several reports of outbreaks of CA-MRSA strains within the nursery setting (144–148). In the outbreak setting, strategies to interrupt transmission may be directed toward infants, health care workers or the nursery environment because all three elements play a role in the chain of transmission (Table 8).
TABLE 8.
Risk factors for neonatal infections and outbreaks
| Risk category | Factors |
|---|---|
| Staff | Lack of compliance with routine practices, including hand hygiene |
| Inadequate staff education | |
| Staff carriage of the outbreak strain | |
| Poor staff to patient ratios (90) | |
| Intensity of colonization (eg, chronic skin conditions and concurrent upper respiratory tract infections) | |
| Neonate | Prematurity |
| Prolonged neonatal intensive care unit stay | |
| Use of invasive medical devices | |
| Exposure intensity (eg, to carriers with chronic skin conditions or concurrent upper respiratory tract infections) | |
| Environment | Poor cleaning of equipment and environment |
| Patients residing in common areas (eg, large nurseries) | |
| Overcrowding and inadequate spacing of patients (90); nonadherence to facility guidelines | |
| Common newborn bath areas | |
| Lack of isolation areas during an outbreak |
When increased transmission of CA-MRSA is documented within the nursery, intensified infection control measures are necessary.
Recommendations: Outbreak setting
Measures directed at health care workers:
Enhance hand hygiene. Consider the use of antiseptic handwashing agents, such as chlorhexidine gluconate or triclosan, before each contact with the newborn (143,149). (BIII)
-
Reinforce infection prevention and control practices. (BIII)
Provide staff education sessions; and
Perform practice audits to document compliance.
-
Staff screening may be considered in selected situations (eg, if cohorting and barriers are in place and the outbreak continues, or if a staff member is epidemiologically linked to cases). (AIII)
Screen nares, examine hands for lesions, inquire about skin conditions on other areas of body (scalp and feet) and inquire about upper respiratory symptoms.
For staff epidemiologically linked to cases, perform thorough full-body skin examinations and culture any lesions.
Staff screening should be performed through the occupational health department, ensuring staff confidentiality, and education and counselling for staff should be provided.
Decolonization may be attempted for the identified staff carriers with topical and/or systemic therapy (149,150).
Exclusion of carriers from the area must be carefully considered, which depends on factors such as compliance with decolonization therapy, compliance with hand hygiene and routine practices or additional precautions, severity of illness in cases, exposure intensity (eg, chronic skin conditions, concurrent upper respiratory tract infection or allergic rhinitis) and evidence of ongoing transmission.
Cohort personnel who care for colonized and infected newborns. (BIII)
Increase the nurse to patient ratio. (AIII)
Measures directed at neonates:
There is insufficient evidence to make a recommendation for or against measures to decrease the burden of organisms through cord care, topical antiseptic baths or intranasal mupirocin (149–157). (CIII)
-
Consider screening all patients for the epidemic strain. (AIII)
Examine carefully for skin lesions and eye discharge and culture any lesions or pustules.
Culture nares, perineum, umbilicus, device exit sites and open skin lesions.
Continue weekly screening of entire cohort until the outbreak is over.
At the time of discharge:
Communicate with family physicians and pediatricians receiving newborns from the facility, bringing to their attention the risk of colonization or infection with CA-MRSA in discharged neonates. (AIII)
There is insufficient evidence to make a recommendation for or against routine screening at the time of discharge and periodically thereafter. Although colonization may not be detectable without repeated sampling, the value of screening at or after discharge is questionable if no intervention is planned for colonized infants, and this may increase parental anxiety (158). (CIII)
Advise parents of discharged infants to watch carefully for skin lesions and to report immediately whether these occur, at which time the lesions should be cultured. If a baby sees a physician for an infection or is to be readmitted to the hospital, parents should inform the physician of possible MRSA exposure. (AIII)
Measures directed at the nursery environment:
Institute contact precautions and cohorting for colonized and infected infants; avoid cohorting infants with CA-MRSA together with those with HA-MRSA. (BIII)
Reduce overcrowding, strongly encourage rooming-in and correct spacing deficiencies (90). (AIII)
Consider ward closure, balancing the risks and benefits of closure versus infection risk. (BIII)
Additional measures:
Assign a dedicated additional infection control professional to the nursery during the outbreak. (BIII)
Perform epidemiological typing of the strains. (BIII)
Consider recall of discharged infants for screening. (BIII)
Notify public health. (AIII)
Consider a case-control study. (BIII)
10.0. DIRECTIONS FOR FUTURE RESEARCH
The Working Group meeting identified numerous areas in need of further research, classified here by category.
10.1. Epidemiology
Establish the current incidence and prevalence of CA-MRSA disease in Canadian communities.
Determine modifiable risk factors for CA-MRSA infection; in particular, better define any link with antibiotic use.
Determine the impact of animals, especially household pets, on CA-MRSA infection.
Determine the role that colonized persons play in the spread of CA-MRSA in the community.
10.2. Biology of CA-MRSA: Organism versus host
Better define the virulence factors of CA-MRSA, including the role of virulence factors such as PVL and the use of immunotherapy to neutralize these virulence factors.
Pursue vaccine development.
Investigate host or pathogen factors that may be implicated in the particular virulence and rapid dissemination of CA-MRSA.
Study the effect of the pneumococcal vaccine, now in widespread use, on colonization with CA-MRSA, particularly in children.
Study the effect of influenza and influenza vaccination on CA-MRSA colonization and disease.
10.3. Clinical outcomes and management
Within the pediatric population, define the safety and efficacy of newer agents, such as daptomycin.
Investigate the use of options other than antibiotic therapy, including anticytokines, immunomodulators or intravenous immunoglobulin.
Study the effect of combination therapy (eg, addition of fusidic acid or rifampin to standard antibiotic regimens).
Define the role of novel testing modalities (eg, polymerase chain reaction for rapid diagnosis).
Define best practices in wound management that may have implications for minimizing antibiotic use.
10.4. Infection prevention and control
Investigate primary prevention initiatives.
Determine the effect of infection prevention and control practices in the community on rates of CA-MRSA and clinical outcomes.
Define appropriate infection control practices for the purulent wound, particularly the challenges of wound management outside the hospital.
Develop strategies for managing household clusters.
Determine the psychosocial impact on individuals ‘labelled’ as MRSA-positive.
Develop strategies for infection control in the physician’s office.
10.5. Knowledge translation
Determine which public health communication strategies should be used.
Investigate factors that will result in ‘cultural’ change (eg, improving hygienic practices at a population level).
Discover how target groups (eg, the community and sports teams) can be involved in the development of guidelines and best practices.
11.0. CONCLUSION
The epidemiology of MRSA is changing, as evidenced by the dramatic increase and stable high prevalence of CA-MRSA in several US communities. While the problem does not yet appear to be widespread in Canada, several early reports indicate that CA-MRSA is emerging as an important pathogen in Canada as well. Physicians and other health care providers should be aware of the increasing prevalence and potential severity of CA-MRSA infection because beta-lactam antibiotics, currently used for the treatment of presumed staphylococcal infections in the community, are ineffective. The management of presumed S aureus infection should include surgical drainage when appropriate, culture of significant draining lesions and abscess collections, and empirical antibiotic therapy, taking into account both the severity of the infection and the regional prevalence of CA-MRSA. Families, school and daycare centre personnel, and sports teams should be actively encouraged to practice meticulous handwashing, the most important measure to control or attenuate the community transmission of CA-MRSA.
Acknowledgments
The authors thank Elizabeth Uleryk, Sue Weller, Anne Matlow, Ian Kitai and Jane McIvor for their assistance in the preparation of the present document for distribution, as well as Sheldon Kaplan for his review of the manuscript.
APPENDIX. Useful resources for specific questions related to the management of health care facilities and health care workers
Patrick D, Henry B, Gamage B. Management of community-associated methicillin-resistant Staphylococcus aureus infections in primary care. BCMJ 2006;48:114–5.
Coia JE, Duckworth GJ, Edwards DI, et al; Joint Working Party of the British Society of Antimicrobial Chemotherapy; Hospital Infection Society; Infection Control Nurses Association. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect 2006;63:S1–44.
First Nations and Inuit Health Committee, Canadian Paediatric Society (CPS). Methicillin-resistant Staphylococcus aureus in First Nations communities in Canada. Paediatr Child Health 2005;10:557–9.
Gemmell CG, Edwards DI, Fraise AP, Gould FK, Ridgway GL, Warren RE; Joint Working Party of the British Society for Joint Working Party of the British Society for Antimicrobial Chemotherapy, Hospital Infection Society and Infection Control Nurses Association. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J Antimicrob Chemother 2006;57:589–608.
Gorwitz RJ, Jernigan DB, Powers JH, Jernigan JA; Participants in the CDC-Convened Experts’ Meeting on the Management of MRSA in the Community. Strategies for clinical management of MRSA in the community: Summary of an experts’ meeting convened by the Centers for Disease Control and Prevention, 2006. <http://www.cdc.gov/ncidod/dhqp/ar_mrsa_ca.html> (Version current at August 25, 2006).
Moore D; Infectious Diseases and Immunization Committee, Canadian Paediatric Society (CPS). Control and treatment of methicillin-resistant Staphylococcus aureus in Canadian paediatric healthcare institutions. Paediatr Child Health 2006;11:163–5. (Abst)
Public Health Agency of Canada. Controlling Antimicrobial Resistance: An Integrated Action Plan for Canadians. <http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/97vol23/23s7/index.html> (Version current at August 25, 2006).
Public Health Agency of Canada. Foot Care by Health Care Providers. <http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/97vol23/23s8/fcindexe.html>. (Version current at August 25, 2006).
Public Health Agency of Canada. Handwashing, Cleaning, Disinfection and Sterilization in Health Care. <http://www.phacaspc.gc.ca/publicat/ccdr-rmtc/98pdf/cdr24s8e.pdf> (Version current at August 25, 2006).
Public Health Agency of Canada. Infection prevention and control practices for personal services: Tattooing, ear/body piercing, and electrolysis. <http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/99pdf/cdr25s3e.pdf> (Version current at August 25, 2006).
Public Health Agency of Canada. Prevention and Control of Occupational Infections in Health Care. <http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/02pdf/28s1e.pdf> (Version current at August 25, 2006).
Public Health Agency of Canada. Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Health Care. <http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/99pdf/cdr25s4e.pdf> (Version current at August 25, 2006).
Simor AE, Loeb M. The management of infection and colonization due to methicillin-resistant Staphylococcus aureus: A CIDS/CAMM position paper. Can J Infect Dis 2004;15:39–48.
Footnotes
MEMBERS OF THE EXPERT COMMITTEE: Clare Barry, Phil Jackson, Upton Allen, Michelle Barton, Jim Carson, John Conly, Simon Dobson, Dorothy Moore, Joanne Embree, John Embil, E Lee Ford-Jones, Michael Hawkes, Michael Mulvey, Lindsay Nicolle, Marianna Ofner-Agostini, Susan Richardson, Liz Van Horne, Rick Walters and Richard Wray
REFERENCES
- 1.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–91. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 2.Chambers HF. Community-associated MRSA– Resistance and virulence converge. N Engl J Med. 2005;352:1485–7. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 3.Gorwitz RJ, Jernigan DB, Powers JH, Jernigan JA, Participants in the Centers for Disease Control and Prevention-Convened Experts’ Meeting on Management of MRSA in the Community Strategies for clinical management of MRSA in the community: Summary of an experts’ meeting convened by the Centers for Disease Control and Prevention. < http://www.cdc.gov/ncidod/dhqp/pdf/ar/CAMRSA_ExpMtgStrategies.pdf> (Version current at August 25, 2006)
- 4.Purcell K, Fergie JE. Exponential increase in community-acquired methicillin-resistant Staphylococcus aureus infections in South Texas children. Pediatr Infect Dis J. 2002;21:988–9. doi: 10.1097/00006454-200210000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Conly J, Gilbert M, Zhang K, et al. Rapidly progressive fatal necrotizing pneumonitis FNP 2o to Panton-Valentine Leukocidin PVL +, SCCmec type IVa community-acquired methicillin-resistant S aureus CAMRSA –A harbinger of the future?. AMMI Canada-CACMID 2005 Annual Conference; Ottawa, Ontario. April 14 to 17, 2005. [Google Scholar]
- 6.Janvier J, Elsayed S, Gregson D, et al. Necrotizing pneumonia secondary to community-associated methicillin-resistant S aureus (CA-MRSA) USA300 strain without evidence of antecedent viral respiratory tractinfection. [Plenary session]. AMMI Canada-CACMID 2006 Annual Conference; Victoria, British Columbia. March 15 to 19, 2006. [Google Scholar]
- 7.Patrick D, Henry B, Gamage B. Management of community-associated methicillin-resistant Staphylococcus aureus infections in primary care. BCMJ. 2006;48:114–5. [Google Scholar]
- 8.Carson J, First Nations and Inuit Health Committee, CPS Methicillin-resistant Staphylococcus aureus in First Nations communities in Canada. Paediatr Child Health. 2005;10:557–559. [PMC free article] [PubMed] [Google Scholar]
- 9.Kish MA. Infectious Diseases Society of America. Guide to development of practice guidelines. Clin Infect Dis. 2001;32:851–4. doi: 10.1086/319366. [DOI] [PubMed] [Google Scholar]
- 10.Morrison MA, Hageman JC, Klevens RM. Case definition for community-associated methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2006;62:241. doi: 10.1016/j.jhin.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Folden DV, Machayya JA, Sahmoun AE, et al. Estimating the proportion of community-associated methicillin-resistant Staphylococcus aureus: Two definitions used in the USA yield dramatically different estimates. J Hosp Infect. 2005;60:329–32. doi: 10.1016/j.jhin.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: A meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131–9. doi: 10.1086/345436. [DOI] [PubMed] [Google Scholar]
- 13.Hulten KG, Kaplan SL, Gonzalez BE, et al. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2006;25:349–53. doi: 10.1097/01.inf.0000207404.50143.1e. [DOI] [PubMed] [Google Scholar]
- 14.Campbell AL, Bryant KA, Stover B, Marshall GS. Epidemiology of methicillin-resistant Staphylococcus aureus at a children’s hospital. Infect Control Hosp Epidemiol. 2003;24:427–30. doi: 10.1086/502226. [DOI] [PubMed] [Google Scholar]
- 15.Donnio PY, Preney L, Gautier-Lerestif AL, Avril JL, Lafforgue N. Changes in staphylococcal cassette chromosome type and antibiotic resistance profile in methicillin-resistant Staphylococcus aureus isolates from a French hospital over an 11 year period. J Antimicrob Chemother. 2004;53:808–13. doi: 10.1093/jac/dkh185. [DOI] [PubMed] [Google Scholar]
- 16.Saiman L, O’Keefe M, Graham PL, III, et al. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin Infect Dis. 2003;37:1313–9. doi: 10.1086/379022. [DOI] [PubMed] [Google Scholar]
- 17.Kourbatova EV, Halvosa JS, King MD, Ray SM, White N, Blumberg HM. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am J Infect Control. 2005;33:385–91. doi: 10.1016/j.ajic.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Zaoutis TE, Toltzis P, Chu J, et al. Clinical and molecular epidemiology of community-acquired methicillin-resistant Staphylococcus aureus infections among children with risk factors for health care-associated infection: 2001–2003. Pediatr Infect Dis J. 2006;25:343–8. doi: 10.1097/01.inf.0000207403.67197.cc. [DOI] [PubMed] [Google Scholar]
- 19.Jevens MP. “Celbenin”-resistant Staphylococci. Brit Med J. 1961;i:124–5. [Google Scholar]
- 20.Simor AE, Ofner-Agostini M, Patin S. Surveillance for methicillin-resistant Staphylococcus aureus in Canadian hospitals – A report update from the Canadian Nosocomial Infection Surveillance Program. Can Commun Dis Rep. 2005;31:33–40. [PubMed] [Google Scholar]
- 21.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–82. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Udo EE, Pearman JW, Grubb WB. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J Hosp Infect. 1993;25:97–108. doi: 10.1016/0195-6701(93)90100-e. [DOI] [PubMed] [Google Scholar]
- 23.From the Centers for Disease Control and Prevention Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus – Minnesota and North Dakota, 1997–1999. JAMA. 1999;282:1123–5. [PubMed] [Google Scholar]
- 24.Schulz P, Allen M, Murray Q, et al. Infections due to community-acquired methicillin-resistant Staphylococcus aureus: An emergent epidemic in Kentucky. J Ky Med Assoc. 2005;103:194–203. [PubMed] [Google Scholar]
- 25.Mishaan AM, Mason EO, Jr, Martinez-Aguilar G, et al. Emergence of a predominant clone of community-acquired Staphylococcus aureus among children in Houston, Texas. Pediatr Infect Dis J. 2005;24:201–6. doi: 10.1097/01.inf.0000151107.29132.70. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics. 2004;113:e347–52. doi: 10.1542/peds.113.4.e347. [DOI] [PubMed] [Google Scholar]
- 27.Buckingham SC, McDougal LK, Cathey LD, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus at a Memphis, Tennessee Children’s Hospital. Pediatr Infect Dis J. 2004;23:619–24. doi: 10.1097/01.inf.0000131981.67342.c4. [DOI] [PubMed] [Google Scholar]
- 28.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: Worldwide emergence. Emerg Infect Dis. 2003;9:978–84. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berglund C, Molling P, Sjoberg L, Soderquist B. Predominance of staphylococcal cassette chromosome mec (SCCmec) type IV among methicillin-resistant Staphylococcus aureus (MRSA) in a Swedish county and presence of unknown SCCmec types with Panton-Valentine leukocidin genes. Clin Microbiol Infect. 2005;11:447–56. doi: 10.1111/j.1469-0691.2005.01150.x. [DOI] [PubMed] [Google Scholar]
- 30.Dufour P, Gillet Y, Bes M, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: Emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis. 2002;35:819–24. doi: 10.1086/342576. [DOI] [PubMed] [Google Scholar]
- 31.Witte W, Braulke C, Cuny C, et al. Emergence of methicillin-resistant Staphylococcus aureus with Panton-Valentine leukocidin genes in central Europe. Eur J Clin Microbiol Infect Dis. 2005;24:1–5. doi: 10.1007/s10096-004-1262-x. [DOI] [PubMed] [Google Scholar]
- 32.Huang YC, Su LH, Chen CJ, Lin TY. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without identifiable risk factors in northern taiwan. Pediatr Infect Dis J. 2005;24:276–8. doi: 10.1097/01.inf.0000154333.46032.0f. [DOI] [PubMed] [Google Scholar]
- 33.Huang YC, Su LH, Lin TY. Nasal carriage of methicillin-resistant Staphylococcus aureus in contacts of an adolescent with community-acquired disseminated disease. Pediatr Infect Dis J. 2004;23:919–22. doi: 10.1097/01.inf.0000141745.12941.ef. [DOI] [PubMed] [Google Scholar]
- 34.Chen CJ, Huang YC. Community-acquired methicillin-resistant Staphylococcus aureus in Taiwan. J Microbiol Immunol Infect. 2005;38:376–82. [PubMed] [Google Scholar]
- 35.Chen CJ, Huang YC, Chiu CH, Su LH, Lin TY. Clinical features and genotyping analysis of community-acquired methicillin-resistant Staphylococcus aureus infections in Taiwanese children. Pediatr Infect Dis J. 2005;24:40–5. doi: 10.1097/01.inf.0000148926.11227.1c. [DOI] [PubMed] [Google Scholar]
- 36.Maguire GP, Arthur AD, Boustead PJ, Dwyer B, Currie BJ. Clinical experience and outcomes of community-acquired and nosocomial methicillin-resistant Staphylococcus aureus in a northern Australian hospital. J Hosp Infect. 1998;38:273–81. doi: 10.1016/s0195-6701(98)90076-7. [DOI] [PubMed] [Google Scholar]
- 37.Maguire GP, Arthur AD, Boustead PJ, Dwyer B, Currie BJ. Emerging epidemic of community-acquired methicillin-resistant Staphylococcus aureus infection in the Northern Territory. Med J Aust. 1996;164:721–3. doi: 10.5694/j.1326-5377.1996.tb122270.x. [DOI] [PubMed] [Google Scholar]
- 38.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–84. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115:642–8. doi: 10.1542/peds.2004-2300. [DOI] [PubMed] [Google Scholar]
- 40.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 41.Kravitz GR, Dries DJ, Peterson ML, Schlievert PM. Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis. 2005;40:941–7. doi: 10.1086/428573. [DOI] [PubMed] [Google Scholar]
- 42.Chapman AL, Greig JM, Innes JA. MRSA in the community. N Engl J Med. 2005;353:530–2. doi: 10.1056/NEJM200508043530521. [DOI] [PubMed] [Google Scholar]
- 43.Fergie JE, Purcell K. Community-acquired methicillin-resistant Staphylococcus aureus infections in south Texas children. Pediatr Infect Dis J. 2001;20:860–3. doi: 10.1097/00006454-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–7. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 45.Frazee BW, Salz TO, Lambert L, Perdreau-Remington F. Fatal community-associated methicillin-resistant Staphylococcus aureus pneumonia in an immunocompetent young adult. Ann Emerg Med. 2005;46:401–4. doi: 10.1016/j.annemergmed.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Alfaro C, Fergie J, Purcell K. Emergence of community-acquired methicillin-resistant Staphylococcus aureus in complicated parapneumonic effusions. Pediatr Infect Dis J. 2005;24:274–6. doi: 10.1097/01.inf.0000154332.66085.de. [DOI] [PubMed] [Google Scholar]
- 47.Schultz KD, Fan LL, Pinsky J, et al. The changing face of pleural empyemas in children: Epidemiology and management. Pediatrics. 2004;113:1735–40. doi: 10.1542/peds.113.6.1735. [DOI] [PubMed] [Google Scholar]
- 48.Taylor G, Kirkland T, Kowalewska-Grochowska K, Wang Y. A multistrain cluster of methicillin-resistant Staphylococcus aureus based in a Native community. Can J Infect Dis. 1990;1:121–126. doi: 10.1155/1990/618630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurbis CA, Wylie JL. Community-based cluster of methicillin-resistant Staphylococcus aureus in Manitoba. Can J Infect Dis. 2001;12:149–52. doi: 10.1155/2001/136953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wylie JL, Nowicki DL. Molecular epidemiology of community- and health care-associated methicillin-resistant Staphylococcus aureus in Manitoba, Canada. J Clin Microbiol. 2005;43:2830–6. doi: 10.1128/JCM.43.6.2830-2836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahin R, Johnson IL, Jamieson F, McGeer A, Tolkin J, Ford-Jones EL. Methicillin-resistant Staphylococcus aureus carriage in a child care center following a case of disease. Toronto Child Care Center Study Group. Arch Pediatr Adolesc Med. 1999;153:864–8. doi: 10.1001/archpedi.153.8.864. [DOI] [PubMed] [Google Scholar]
- 52.McGeer A, Fleming CA, Green KA, Willey BM, Low DE. Antimicrobial resistance in Ontario hospitals: Transmission control programs begin to pay off. < www.qmpls.org/pub_resources/publications/qmpls_news/pdf/ane32.PDF> (Version current at August 25, 2006)
- 53.Gilbert M, Macdonald J, Gregson D, et al. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ. 2006;175:149–54. doi: 10.1503/cmaj.051565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deresinski S. Methicillin-resistant Staphylococcus aureus: An evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis. 2005;40:562–73. doi: 10.1086/427701. [DOI] [PubMed] [Google Scholar]
- 55.Eady EA, Cove JH. Staphylococcal resistance revisited: Community-acquired methicillin resistant Staphylococcus aureus – An emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2003;16:103–24. doi: 10.1097/00001432-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Carleton HA, Diep BA, Charlebois ED, Sensabaugh GF, Perdreau-Remington F. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): Population dynamics of an expanding community reservoir of MRSA. J Infect Dis. 2004;190:1730–8. doi: 10.1086/425019. [DOI] [PubMed] [Google Scholar]
- 57.Okuma K, Iwakawa K, Turnidge JD, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ender M, McCallum N, Adhikari R, Berger-Bachi B. Fitness cost of SCCmec and methicillin resistance levels in Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:2295–7. doi: 10.1128/AAC.48.6.2295-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 60.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci USA. 2002;99:7687–92. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 62.Vanderkooi OG, Bryce E, McGeer A, et al. Epidemiology of methicillin-resistant Staphylococcus aureus in Canadian children from 1995 to 2002. Can J Infect Dis Med Microbiol. 2005;16:123. [Google Scholar]
- 63.Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol. 2004;42:5673–80. doi: 10.1128/JCM.42.12.5673-5680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA. 2001;286:1201–5. doi: 10.1001/jama.286.10.1201. [DOI] [PubMed] [Google Scholar]
- 65.Baggett HC, Hennessy TW, Rudolph K, et al. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J Infect Dis. 2004;189:1565–73. doi: 10.1086/383247. [DOI] [PubMed] [Google Scholar]
- 66.Ochoa TJ, Mohr J, Wanger A, Murphy JR, Heresi GP. Community-associated methicillin-resistant Staphylococcus aureus in pediatric patients. Emerg Infect Dis. 2005;11:966–8. doi: 10.3201/eid1106.050142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sattler CA, Mason EO, Jr, Kaplan SL. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptible Staphylococcus aureus infection in children. Pediatr Infect Dis J. 2002;21:910–7. doi: 10.1097/00006454-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Embil J, Ramotar K, Romance L, et al. Methicillin-resistant Staphylococcus aureus in tertiary care institutions on the Canadian prairies 1990–1992. Infect Control Hosp Epidemiol. 1994;15:646–51. doi: 10.1086/646827. [DOI] [PubMed] [Google Scholar]
- 69.Ofner-Agostini M, Simor AE, Mulvey M, et al. Methicillin-resistant Staphylococcus aureus in Canadian aboriginal people. Infect Control Hosp Epidemiol. 2006;27:204–7. doi: 10.1086/500628. [DOI] [PubMed] [Google Scholar]
- 70.Begier EM, Frenette K, Barrett NL, et al. Connecticut Bioterrorism Field Epidemiology Response Team A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446–53. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 71.Cohen PR. Cutaneous community-acquired methicillin-resistant Staphylococcus aureus infection in participants of athletic activities. South Med J. 2005;98:596–602. doi: 10.1097/01.SMJ.0000163302.72469.28. [DOI] [PubMed] [Google Scholar]
- 72.Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468–75. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen DM, Mascola L, Brancoft E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg Infect Dis. 2005;11:526–32. doi: 10.3201/eid1104.041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rihn JA, Posfay-Barbe K, Harner CD, et al. Community-acquired methicillin-resistant Staphylococcus aureus outbreak in a local high school football team unsuccessful interventions. Pediatr Infect Dis J. 2005;24:841–3. doi: 10.1097/01.inf.0000177287.11971.d4. [DOI] [PubMed] [Google Scholar]
- 75.Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med. 1998;158:895–9. doi: 10.1001/archinte.158.8.895. [DOI] [PubMed] [Google Scholar]
- 76.Centers for Disease Control and Prevention (CDC) Methicillin-resistant Staphylococcus aureus infections among competitive sports participants – Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003;52:793–5. [PubMed] [Google Scholar]
- 77.Charlebois ED, Bangsberg DR, Moss NJ, et al. Population-based community prevalence of methicillin-resistant Staphylococcus aureus in the urban poor of San Francisco. Clin Infect Dis. 2002;34:425–33. doi: 10.1086/338069. [DOI] [PubMed] [Google Scholar]
- 78.Gilbert M, Siushansian J, Macdonald J. An outbreak of the USA300 strain of community-acquired methicillin-resistant Staphylococcus aureus CMRSA infections in individuals with histories of drug use, homelessness or incarceration. Can J Infect Dis. 2005;16:A108. (Abst) [Google Scholar]
- 79.Lee NE, Taylor MM, Bancroft E, et al. Risk factors for community-associated methicillin-resistant Staphylococcus aureus skin infections among HIV-positive men who have sex with men. Clin Infect Dis. 2005;40:1529–34. doi: 10.1086/429827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathews WC, Caperna JC, Barber RE, et al. Incidence of and risk factors for clinically significant methicillin-resistant Staphylococcus aureus infection in a cohort of HIV-infected adults. J Acquir Immune Defic Syndr. 2005;40:155–60. doi: 10.1097/01.qai.0000179464.40948.b9. [DOI] [PubMed] [Google Scholar]
- 81.Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39:971–9. doi: 10.1086/423965. [DOI] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention (CDC) Methicillin-resistant Staphylococcus aureus infections in correctional facilities –Georgia, California, and Texas, 2001–2003. MMWR Morb Mortal Wkly Rep. 2003;52:992–6. [PubMed] [Google Scholar]
- 83.Centers for Disease Control and Prevention (CDC) Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison – Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:919–22. [PubMed] [Google Scholar]
- 84.Baillargeon J, Kelley MF, Leach CT, Baillargeon G, Pollock BH. Methicillin-resistant Staphylococcus aureus infection in the Texas prison system. Clin Infect Dis. 2004;38:e92–5. doi: 10.1086/383146. [DOI] [PubMed] [Google Scholar]
- 85.Wootton SH, Arnold K, Hill HA, et al. Intervention to reduce the incidence of methicillin-resistant Staphylococcus aureus skin infections in a correctional facility in Georgia. Infect Control Hosp Epidemiol. 2004;25:402–7. doi: 10.1086/502413. [DOI] [PubMed] [Google Scholar]
- 86.Pan ES, Diep BA, Carleton HA, et al. Increasing prevalence of methicillin-resistant Staphylococcus aureus infection in California jails. Clin Infect Dis. 2003;37:1384–8. doi: 10.1086/379019. [DOI] [PubMed] [Google Scholar]
- 87.Main C, Jayaratne P, Haley A, Rutherford C, Smaill F, Fisman DN. Outbreaks of infection caused by community-acquired methicillin-resistant Staphylococcus aureus in a Canadian correctional facility. Can J Infect Dis. 2005;16:343–8. doi: 10.1155/2005/698181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan ES, Diep BA, Charlebois ED, et al. Population dynamics of nasal strains of methicillin-resistant Staphylococcus aureus and their relation to community-associated disease activity. J Infect Dis. 2005;192:811–8. doi: 10.1086/432072. [DOI] [PubMed] [Google Scholar]
- 89.Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg. 2004;139:947–51. doi: 10.1001/archsurg.139.9.947. [DOI] [PubMed] [Google Scholar]
- 90.Haley RW, Bregman DA. The role of understaffing and overcrowding in recurrent outbreaks of staphylococcal infection in a neonatal special-care unit. J Infect Dis. 1982;145:875–85. doi: 10.1093/infdis/145.6.875. [DOI] [PubMed] [Google Scholar]
- 91.Manian FA. Asymptomatic nasal carriage of mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MRSA) in a pet dog associated with MRSA infection in household contacts. Clin Infect Dis. 2003;36:e26–8. doi: 10.1086/344772. [DOI] [PubMed] [Google Scholar]
- 92.Weese JS, Rousseau J. Attempted eradication of methicillin-resistant Staphylococcus aureus colonisation in horses on two farms. Equine Vet J. 2005;37:510–4. doi: 10.2746/042516405775314835. [DOI] [PubMed] [Google Scholar]
- 93.Weese JS, Rousseau J, Traub-Dargatz JL, Willey BM, McGeer AJ, Low DE. Community-associated methicillin-resistant Staphylococcus aureus in horses and humans who work with horses. J Am Vet Med Assoc. 2005;226:580–3. doi: 10.2460/javma.2005.226.580. [DOI] [PubMed] [Google Scholar]
- 94.Weese JS, Rousseau J, Willey BM, Archambault M, McGeer A, Low DE. Methicillin-resistant Staphylococcus aureus in horses at a veterinary teaching hospital: Frequency, characterization, and association with clinical disease. J Vet Intern Med. 2006;20:182–6. doi: 10.1892/0891-6640(2006)20[182:msaiha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 95.O’Mahony R, Abbott Y, Leonard FC, et al. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from animals and veterinary personnel in Ireland. Vet Microbiol. 2005;109:285–96. doi: 10.1016/j.vetmic.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 96.Weese JS, Dick H, Willey BM, et al. Suspected transmission of methicillin-resistant Staphylococcus aureus between domestic pets and humans in veterinary clinics and in the household. Vet Microbiol. 2006;115:148–55. doi: 10.1016/j.vetmic.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis. 2005;11:1965–6. doi: 10.3201/eid1112.050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weese JS. Methicillin-resistant Staphylococcus aureus in horses and horse personnel. Vet Clin North Am Equine Pract. 2004;20:601–13. doi: 10.1016/j.cveq.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 99.Weese JS, Archambault M, Willey BM, et al. Methicillin-resistant Staphylococcus aureus in horses and horse personnel, 2000–2002. Emerg Infect Dis. 2005;11:430–5. doi: 10.3201/eid1103.040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weese JS, Caldwell F, Willey BM, et al. An outbreak of methicillin-resistant Staphylococcus aureus skin infections resulting from horse to human transmission in a veterinary hospital. Vet Microbiol. 2006;114:160–4. doi: 10.1016/j.vetmic.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 101.Weese JS. Methicillin-resistant Staphylococcus aureus: An emerging pathogen in small animals. J Am Anim Hosp Assoc. 2005;41:150–7. doi: 10.5326/0410150. [DOI] [PubMed] [Google Scholar]
- 102.Baptiste KE, Williams K, Willams NJ, et al. Methicillin-resistant staphylococci in companion animals. Emerg Infect Dis. 2005;11:1942–4. doi: 10.3201/eid1112.050241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duquette RA, Nuttall TJ. Methicillin-resistant Staphylococcus aureus in dogs and cats: An emerging problem? J Small Anim Pract. 2004;45:591–7. doi: 10.1111/j.1748-5827.2004.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 104.Crawford SE, Daum RS. Epidemic community-associated methicillin-resistant Staphylococcus aureus: Modern times for an ancient pathogen. Pediatr Infect Dis J. 2005;24:459–60. doi: 10.1097/01.inf.0000164170.67897.97. [DOI] [PubMed] [Google Scholar]
- 105.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 106.Reichert B, Birrell G, Bignardi G. Severe non-pneumonic necrotising infections in children caused by Panton-Valentine leukocidin producing Staphylococcus aureus strains. J Infect. 2005;50:438–42. doi: 10.1016/j.jinf.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 107.Bocchini CE, Hulten KG, Mason EO, Jr, Gonzalez BE, Hammerman WA, Kaplan SL. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117:433–40. doi: 10.1542/peds.2005-0566. [DOI] [PubMed] [Google Scholar]
- 108.Martinez-Aguilar G, Avalos-Mishaan A, Hulten K, Hammerman W, Mason EO, Jr, Kaplan SL. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J. 2004;23:701–6. doi: 10.1097/01.inf.0000133044.79130.2a. [DOI] [PubMed] [Google Scholar]
- 109.Mulvey MR, MacDougall L, Cholin B, et al. Community-associated methicillin-resistant Staphylococcus aureus, Canada. Emerg Infect Dis. 2005;11:844–50. doi: 10.3201/eid1106.041146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: Establishing a national database. J Clin Microbiol. 2003;41:5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–17. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 112.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–56. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 113.Kaplan SL. Treatment of community-associated methicillin-resistant Staphylococcus aureus infections. Pediatr Infect Dis J. 2005;24:457–8. doi: 10.1097/01.inf.0000164162.00163.9d. [DOI] [PubMed] [Google Scholar]
- 114.Lewis JS, II, Jorgensen JH. Inducible clindamycin resistance in Staphylococci: Should clinicians and microbiologists be concerned? Clin Infect Dis. 2005;40:280–5. doi: 10.1086/426894. [DOI] [PubMed] [Google Scholar]
- 115.Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis. 2003;37:1257–60. doi: 10.1086/377501. [DOI] [PubMed] [Google Scholar]
- 116.Frank AL, Marcinak JF, Mangat PD, et al. Clindamycin treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2002;21:530–4. doi: 10.1097/00006454-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 117.Simor AE, Ofner-Agostini M, Bryce E, et al. Canadian Hospital Epidemiology Committee and Canadian Nosocomial Infection Surveillance Program, Health Canada Laboratory characterization of methicillin-resistant Staphylococcus aureus in Canadian hospitals: Results of 5 years of National Surveillance, 1995–1999. J Infect Dis. 2002;186:652–60. doi: 10.1086/342292. [DOI] [PubMed] [Google Scholar]
- 118.Blumberg HM, Rimland D, Carroll DJ, Terry P, Wachsmuth IK. Rapid development of ciprofloxacin resistance in methicillin-susceptible and -resistant Staphylococcus aureus. J Infect Dis. 1991;163:1279–85. doi: 10.1093/infdis/163.6.1279. [DOI] [PubMed] [Google Scholar]
- 119.Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123–7. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 120.Fortun J, Navas E, Martinez-Beltran J, et al. Short-course therapy for right-side endocarditis due to Staphylococcus aureus in drug abusers: Cloxacillin versus glycopeptides in combination with gentamicin. Clin Infect Dis. 2001;33:120–5. doi: 10.1086/320869. [DOI] [PubMed] [Google Scholar]
- 121.Domenech A, Ribes S, Cabellos C, et al. A mouse peritonitis model for the study of glycopeptide efficacy in GISA infections. Microb Drug Resist. 2004;10:346–53. doi: 10.1089/mdr.2004.10.346. [DOI] [PubMed] [Google Scholar]
- 122.Ruhe JJ, Monson T, Bradsher RW, Menon A. Use of long-acting tetracyclines for methicillin-resistant Staphylococcus aureus infections: Case series and review of the literature. Clin Infect Dis. 2005;40:1429–34. doi: 10.1086/429628. [DOI] [PubMed] [Google Scholar]
- 123.Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: Analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–97. [PubMed] [Google Scholar]
- 124.Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother. 2002;46:1475–80. doi: 10.1128/AAC.46.5.1475-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shelburne SA, Musher DM, Hulten K, et al. In vitro killing of community-associated methicillin-resistant Staphylococcus aureus with drug combinations. Antimicrob Agents Chemother. 2004;48:4016–9. doi: 10.1128/AAC.48.10.4016-4019.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gauduchon V, Cozon G, Vandenesch F, et al. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis. 2004;189:346–53. doi: 10.1086/380909. [DOI] [PubMed] [Google Scholar]
- 127.Graham PL, III, Lin SX, Larson EL. A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med. 2006;144:318–25. doi: 10.7326/0003-4819-144-5-200603070-00006. [DOI] [PubMed] [Google Scholar]
- 128.Loeb M, Main C, Walker-Dilks C, Eady A. The Cochrane Library. 4. Chichested, UK: John Wiley & Sons, Ltd; 2003. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization (Cochrane Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Doebbeling BN, Reagan DR, Pfaller MA, Houston AK, Hollis RJ, Wenzel RP. Long-term efficacy of intranasal mupirocin ointment. A prospective cohort study of Staphylococcus aureus carriage. Arch Intern Med. 1994;154:1505–8. [PubMed] [Google Scholar]
- 130.Hudson IR. The efficacy of intranasal mupirocin in the prevention of staphylococcal infections: A review of recent experience. J Hosp Infect. 1994;27:81–98. doi: 10.1016/0195-6701(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 131.Harbarth S, Dharan S, Liassine N, Herrault P, Auckenthaler R, Pittet D. Randomized, placebo-controlled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1412–6. doi: 10.1128/aac.43.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Annigeri R, Conly J, Vas S, et al. Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int. 2001;21:554–9. [PubMed] [Google Scholar]
- 133.Riley TV, Carson CF, Bowman RA, et al. Mupirocin-resistant methicillin-resistant Staphylococcus aureus in Western Australia. Med J Aust. 1994;161:397–8. doi: 10.5694/j.1326-5377.1994.tb127503.x. [DOI] [PubMed] [Google Scholar]
- 134.Dailey L, Coombs GW, O’Brien FG, et al. Methicillin-resistant Staphylococcus aureus, Western Australia. Emerg Infect Dis. 2005;11:1584–90. doi: 10.3201/eid1110.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parras F, Guerrero MC, Bouza E, et al. Comparative study of mupirocin and oral co-trimoxazole plus topical fusidic acid in eradication of nasal carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:175–9. doi: 10.1128/aac.39.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Muder RR, Boldin M, Brennen C, et al. A controlled trial of rifampicin, minocycline, and rifampicin plus minocycline for eradication of methicillin-resistant Staphylococcus aureus in long-term care patients. J Antimicrob Chemother. 1994;34:189–90. doi: 10.1093/jac/34.1.189. [DOI] [PubMed] [Google Scholar]
- 137.Chang SC, Hsieh SM, Chen ML, Sheng WH, Chen YC. Oral fusidic acid fails to eradicate methicillin-resistant Staphylococcus aureus colonization and results in emergence of fusidic acid-resistant strains. Diagn Microbiol Infect Dis. 2000;36:131–6. doi: 10.1016/s0732-8893(99)00116-9. [DOI] [PubMed] [Google Scholar]
- 138.Walsh TJ, Standiford HC, Reboli AC, et al. Randomized double-blinded trial of rifampin with either novobiocin or trimethoprim-sulfamethoxazole against methicillin-resistant Staphylococcus aureus colonization: Prevention of antimicrobial resistance and effect of host factors on outcome. Antimicrob Agents Chemother. 1993;37:1334–42. doi: 10.1128/aac.37.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reagan DR, Doebbeling BN, Pfaller MA, et al. Elimination of coincident Staphylococcus aureus nasal and hand carriage with intranasal application of mupirocin calcium ointment. Ann Intern Med. 1991;114:101–6. doi: 10.7326/0003-4819-114-2-101. [DOI] [PubMed] [Google Scholar]
- 140.Fung SK, Louie M, Simor AE. Combined topical and oral antimicrobial therapy for the eradication of methicillin-resistant Staphylococcus aureus (MRSA) colonization in hospitalized patients. Can J Infect Dis. 2002;13:287–92. doi: 10.1155/2002/567090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Canadian Committee on Antibiotic Resistance. Optimal antibiotic use. < http://www.ccar-ccra.com/english/hh-optimal-e.shtml> (Version current at August 25, 2006)
- 142.van Hal SJ, Post JJ. Community-acquired MRSA epiduritis in an Australian prison inmate. Med J Aust. 2004;180:650–1. doi: 10.5694/j.1326-5377.2004.tb06134.x. [DOI] [PubMed] [Google Scholar]
- 143.Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1–46. doi: 10.1067/mic.2002.130391. [DOI] [PubMed] [Google Scholar]
- 144.Chuang YY, Huang YC, Lee CY, Lin TY, Lien R, Chou YH. Methicillin-resistant Staphylococcus aureus bacteraemia in neonatal intensive care units: An analysis of 90 episodes. Acta Paediatr. 2004;93:786–90. doi: 10.1080/08035250410028084. [DOI] [PubMed] [Google Scholar]
- 145.Healy CM, Hulten KG, Palazzi DL, Campbell JR, Baker CJ. Emergence of new strains of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Clin Infect Dis. 2004;39:1460–6. doi: 10.1086/425321. [DOI] [PubMed] [Google Scholar]
- 146.Sax H, Posfay-Barbe K, Harbarth S, et al. Control of a cluster of community-associated, methicillin-resistant Staphylococcus aureus in neonatology. J Hosp Infect. 2006;63:93–100. doi: 10.1016/j.jhin.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 147.Bratu S, Eramo A, Kopec R, et al. Community-associated methicillin-resistant Staphylococcus aureus in hospital nursery and maternity units. Emerg Infect Dis. 2005;11:808–13. doi: 10.3201/eid1106.040885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Centers for Disease Control and Prevention (CDC) Community-associated methicillin-resistant Staphylococcus aureus infection among healthy newborns – Chicago and Los Angeles County, 2004. MMWR Morb Mortal Wkly Rep. 2006;55:329–32. [PubMed] [Google Scholar]
- 149.Zafar AB, Butler RC, Reese DJ, Gaydos LA, Mennonna PA. Use of 0.3% triclosan (Bacti-Stat) to eradicate an outbreak of methicillin-resistant Staphylococcus aureus in a neonatal nursery. Am J Infect Control. 1995;23:200–8. doi: 10.1016/0196-6553(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 150.Saiman L, Cronquist A, Wu F, et al. An outbreak of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2003;24:317–21. doi: 10.1086/502217. [DOI] [PubMed] [Google Scholar]
- 151.Rosenfeld CR, Laptook AR, Jeffery J. Limited effectiveness of triple dye in preventing colonization with methicillin-resistant Staphylococcus aureus in a special care nursery. Pediatr Infect Dis J. 1990;9:290–1. doi: 10.1097/00006454-199004000-00013. [DOI] [PubMed] [Google Scholar]
- 152.Mullany LC, Darmstadt GL, Khatry SK, et al. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: A community-based, cluster-randomised trial. Lancet. 2006;367:910–8. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mullany LC, Darmstadt GL, Tielsch JM. Role of antimicrobial applications to the umbilical cord in neonates to prevent bacterial colonization and infection: A review of the evidence. Pediatr Infect Dis J. 2003;22:996–1002. doi: 10.1097/01.inf.0000095429.97172.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Darmstadt GL, Dinulos JG. Neonatal skin care. Pediatr Clin North Am. 2000;47:757–82. doi: 10.1016/s0031-3955(05)70239-x. [DOI] [PubMed] [Google Scholar]
- 155.Davies EA, Emmerson AM, Hogg GM, Patterson MF, Shields MD. An outbreak of infection with a methicillin-resistant Staphylococcus aureus in a special care baby unit: Value of topical mupirocin and of traditional methods of infection control. J Hosp Infect. 1987;10:120–8. doi: 10.1016/0195-6701(87)90137-x. [DOI] [PubMed] [Google Scholar]
- 156.Hitomi S, Kubota M, Mori N, et al. Control of a methicillin-resistant Staphylococcus aureus outbreak in a neonatal intensive care unit by unselective use of nasal mupirocin ointment. J Hosp Infect. 2000;46:123–9. doi: 10.1053/jhin.2000.0786. [DOI] [PubMed] [Google Scholar]
- 157.Haley RW, Cushion NB, Tenover FC, et al. Eradication of endemic methicillin-resistant Staphylococcus aureus infections from a neonatal intensive care unit. J Infect Dis. 1995;171:614–24. doi: 10.1093/infdis/171.3.614. [DOI] [PubMed] [Google Scholar]
- 158.Hicks NR, Moore EP, Williams EW. Carriage and community treatment of methicillin-resistant Staphylococcus aureus: What happens to colonized patients after discharge? J Hosp Infect. 1991;19:17–24. doi: 10.1016/0195-6701(91)90124-q. [DOI] [PubMed] [Google Scholar]
- 159.Gonzalez BE, Teruya J, Mahoney DH, Jr, et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117:1673–9. doi: 10.1542/peds.2005-2009. [DOI] [PubMed] [Google Scholar]
- 160.Kaplan SL, Afghani B, Lopez P, et al. Linezolid for the treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2003;22(Suppl 9):S178–85. doi: 10.1097/01.inf.0000087020.75886.93. [DOI] [PubMed] [Google Scholar]
- 161.Howden BP, Grayson ML. Dumb and dumber – The potential waste of a useful antistaphylococcal agent: Emerging fusidic acid resistance in Staphylococcus aureus. Clin Infect Dis. 2006;42:394–400. doi: 10.1086/499365. [DOI] [PubMed] [Google Scholar]


