Summary
Aim
Peri-implant mucositis affects 39.4–80% of patients restored with dental implants. If left untreated it evolves in peri-implantitis. Thus far no predictable successful treatment has been reported for peri-implantitis, resulting in implant failure. Proper diagnosis and treatment of peri-implant mucositis is of crucial importance. This study aims to provide a comprehensive review of the available data regarding the effectiveness of peri-implant mucositis treatments in humans, parameters used for the diagnosis and treatment effect evaluation.
Materials and methods
A literature search for RCT and observational studies on peri-implant mucositis treatments in humans was conducted on Pubmed up to January 2012. CONSORT/STROBE and PRISMA checklists guided the evaluation of studies found and the writing of this review, respectively.
Results
Only 5 studies fulfilled the selection criteria. Few possibly effective treatments were studied. Diagnostic parameters reported were clinical only, while treatment effect evaluation was based on clinical and microbiological changes, except for one study reporting biochemical analysis. An evident heterogeneity characterized the follow-up intervals and methods used for reporting parameters changes.
Conclusions
Neither of studied treatments gave complete resolution of peri-implant mucositis. Different treatment strategies need to be studied. Authors suggest guidelines for a protocol of parameters used for determining the sample size, diagnosis and treatment effect, as well as follow-up periods, in order to permit evidence and comparison of different treatments effectiveness.
Keywords: peri-implant mucositis, non-surgical treatment, diagnosis parameters, effectiveness evaluation parameters, study in humans
Introduction
Peri-implant mucositis by definition is the inflammation confined to peri-implant soft tissues only and is caused by dental plaque accumulation (1–3). It is similar to its counterpart gingivitis (4), but with a stronger response (3), although soft tissues appear to be identical around implants as well as around natural teeth (5). It is present in 39.4–80% of patients restored with dental implants (6–8). Only in the USA, counting for 30% of the global market, 1.3–2 million implants are being placed each year (9). Peri-implant mucositis is reversible (3) but if left untreated it may lead to peri-implantitis. Inflammation progressively and rapidly (10) extends into tissues with weaker defence mechanisms than periodontal tissues due to lack of periodontal ligament and a reduced number of fibroblasts and blood vessels (11). Peri-implantitis does not have any predictable successful treatment yet (12), ultimately causing implant failure (5–11%) (13). The clinical importance of peri-implant mucositis early diagnosis and effective treatment is evident.
The main objective of the present study is to provide a systematic review and collect data on the quality and quantity of studies dealing with peri-implant mucositis, the parameters used for both the diagnosis and the evaluation of treatment effects. This information may contribute in designing a protocol for the diagnosis, treatment effect evaluation and guide future studies on data missing on this topic.
We chose to enrol only RCTs and observational studies on humans because: (i) these types of studies provide the most reliable data; (ii) the real benefits of medical intervention can only be ascertained in human studies (14). More specifically, the predictability of the implant treatment and treatment of peri-implant inflammation is related to predisposing factors as well, such as smoking (15), diabetes mellitus (15), oral hygiene and other, factors that differ between humans and animals (i.e. animals do not smoke or perform domiciliary oral hygiene); (iii) animal studies are proposed to evaluate side effects of a treatment/substance that if not harmful will be further tested in a human population. Peri-implant mucositis treatments are mainly of local non-surgical type and previously tested for their effectiveness on periodontal inflammation or inflammatory systemic diseases/disorders. Thus it can be considered safe and more appropriate to study peri-implant mucositis treatment in humans.
Review of the current literature
Strategies for literature searching and identification of studies
Firstly, we searched for the most recent review/metaanalysis on peri-implant mucositis treatment. We found one article that compares the outcomes of peri-implant inflammation treatments in humans with treatments in animals (14), showing no statistically significant differences between the two. This article was considered for three purposes: (i) to follow similar steps, when possible, in order to complete this previous work with more recently published evidence; (ii) to guide the aim of our work in making a more detailed review and meta-analysis, if possible, not only focused on the treatment efficacy, but also on the diagnostic criteria, the influence of predisposing factors on the efficacy of the treatment etc. and (iii) to see if the remarks regarding the small sample size, the lack of power report and study design heterogeneity have been corrected in the articles published afterwards.
In a second step a further literature search was conducted in Pubmed up to January 2012 using the key words: peri-implant mucositis treatment, peri-implant mucositis AND laser therapy, peri-implant mucositis AND antimicrobial therapy, peri-implant mucositis AND antibiotic therapy, peri-implant mucositis AND photodynamic therapy, peri-implant mucositis AND mechanical therapy, peri-implant mucositis AND vector therapy, peri-implant mucositis AND xylitol, peri-implant mucositis AND cranberry juice therapy/phytotherapy, peri-implant mucositis AND probiotics.
Selection limits
Only RCT and observational studies were included. These studies were conducted in adult human patients with at least one dental implant presenting signs of peri-implant mucositis (bleeding on probing with absence of peri-implant bone loss) who were treated non-surgically and followed- up for at least 3 months. They reported data on the modification of the parameters used for the evaluation of the effect of the treatment applied.
Selection procedure
Study selection process was divided in three steps: (i) find articles based on the keywords used; (ii) read abstracts and exclude articles on animals, in vitro studies, narrative studies, reviews and studies on peri-implantitis; (iii) read full text articles excluding those which included an incipient loss of peri-implant bone in the diagnostic criteria, those which did not report appropriately and fully the follow-up results and those that did not specify the diagnostic criteria for the periimplant mucositis. Each selected article was controlled according to the STROBE and CONSORT checklist for observational studies and RCT, respectively. Jadad scale was used for the quantification of the RCTs quality.
Data selection
From the enrolled articles there were selected data with regards to the diagnosis (diagnostic parameters) and treatment of peri-implant mucositis (type of treatment, parameter used for the evaluation, follow-up periods and intervals of evaluation, treatment effectiveness). Patients selection criteria and the evaluation of the influence of the predisposing factors (smoking, prosthetic restoration margins, etc.) on the effectiveness of the treatment were considered as well.
Results and discussion
Quantity, quality, impossibility to conduct a meta-analysis
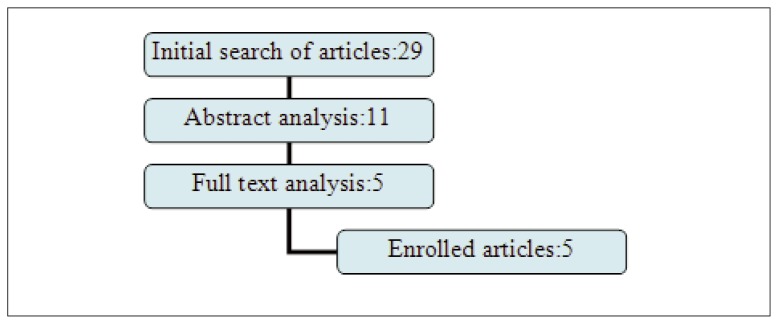
Peri-implant inflammation has become a great challenge to periodontology and implantology worldwide. Actually, the only manageable stage of peri-implant inflammation is peri-implant mucositis. In the era of evidence-based medicine the clinician takes best decisions for patient management based on the best scientific evidence collected in systematic reviews. Randomized clinical trials are considered the best scientific evidence. The key word “peri-implant mucositis treatment” corresponds to 56 available scientifically trusted studies, compared to a list of 5553 studies on gingivitis treatment. In this scarce literature even fewer (only 5) were the RCT and observational studies in humans reported according the CONSORT/STROBE checklist (Fig. 1). It was noticed that both observational studies were conducted from the same group of study in populations with approximately the same characteristics. According to Jadad scale the selected RCTs were of high quality (Tab. 1). We decided to limit the evaluation of the observation studies to the STROBE checklist only, as Newcastle-Ottawa-scale validity assessment is still under development (16).
Figure 1.
Diagram of the article selection process. The articles included in the review were only 5.
Table 1.
Jadad scale for quality assessment of RCT (High quality RCT presenting ≥ 3 points).
| Jadad scale | Heitz-Mayfield et al., 2011 | Thöne-Mühling et al., 2010 | Ramberg et al., 2009 |
|---|---|---|---|
| 1. Randomisation | |||
| Reported | 1 | 1 | 1 |
| Adequately | 1 | 1 | |
| Inadequately | |||
| Not reported | |||
|
| |||
| 2. Double-blinding | |||
| Reported | 1 | 1 | |
| Adequately | 1 | 1 | |
| Inadequately | |||
| Not reported | 0 | ||
|
| |||
| 3. Withdrawal/Dropouts | |||
| Reported | 1 | 1 | 1 |
| Not reported | |||
|
| |||
| Quality point | 5 | 3 | 4 |
allocation concealment was reported in all the three studies
Our first intention was to conduct a meta-analysis to evaluate the effectiveness of peri-implant mucositis treatment. This was not possible mainly because of the heterogeneity characterizing: (i) the type of parameters used for the diagnosis and treatment effect evaluation; (ii) the number of sites chosen for parameters measurement; (iii) the way the parameters were expressed, and (iv) the different follow-up periods and intervals.
Authors emphasize the need for an increase in quantity of studies on peri-implant mucositis treatment in humans following a similar protocol for the sample size determination, diagnosis, treatment effect evaluation and follow-up intervals in order to help comparison of the different treatments. The quality of future studies should be maintained high. RCTs in periodontics have a median report quality score of 2 (17). Authors suggest a more updated evaluation of the report quality of researches in periodontics.
Treatment
Peri-implant mucositis has an infective nature as such its therapy should be anti-infective. Mechanical removal of dental plaque has been proven to be a successful treatment but not completely resolving the inflammation (18). Consequently adjunctive treatments are proposed. In the selected studies (Tabs. 2, 3) it was reported that the use of CHX as a gel (19) or mouthrinse in the FMD (20) resulted in improved inflammation signs but not statistically significant to be considered part of the treatment protocol as adjunctive therapy. On the other hand, sodium carbonate abrasive air powder was demonstrated to be an effective treatment (21,22). Randomized controlled trials are required on this treatment. Finally, the everyday use of triclosan vs fluoride dentifrice was suggested for the reduction of signs and symptoms of peri-implant inflammation (23). In this regard, further studies are needed on the side effects of the regular use of triclosan dentifrice. Table 5 summaries the parameters used for the evaluation of the effectiveness. Authors noticed that no evidence- based explanation was provided in any of the studies for the different instruments selected for the mechanical debridement.
Table 2.
Description of the included studies.
| Study | Type | Power | Treatment | No of patients/implants | Follow-up |
|---|---|---|---|---|---|
| Heitz-Mayfield et al., 2011 | RCT | 80% For a mean difference of 1.1mm in PPD between groups |
mechanical treatment +CHX gel vs mechanical treatment alone | 15/15 vs 14/14 | 3 months |
| Thöne-Mühling M et al., 2010 | RCT | 78% Effect size 1.4 |
full mouth disinfection vs full mouth mechanical treatment | 6/22 vs 5/14 | 8 months |
| Ramberg P. et al., 2009 | RCT | 80% for a difference between groups of 12% for the BOP | 0.3% triclosan dentifrice vs sodium fluoride dentifrice | 30 vs 29 | 6 months |
| Màximo MB et al., 2009 | Obs | P< 0.05 | mechanical treatment + abrasive sodium corbonate air-powder | 12p/16i | 3 months |
| Duarte PM et al., 2009 | Obs | P< 0.05 | mechanical treatment + abrasive sodium corbonate air-powder | 10p/10i | 3 months |
Table 3.
Demographic data.
| Study | Age (mean ± SD) | Gender F:M | Periodontal history exclusions | Smoking | General health | |||
|---|---|---|---|---|---|---|---|---|
| Heitz-Mayfield et al., 2011 | T/57 | C/53 | T/6:8 | C/9:6 | Untreated periodontitis, FMNS>25% | T/2 | C/2 | healthy |
| Thöne-Mühling M et al., 2010 | T/46.3 ±10.1 | C/53.3 ±11.3 | T/2:4 | C/1:4 | Untreated periodontitis | T/3 | C/2 | healthy, no antibiotics within the last 6 month |
| Ramberg P. et al., 2009 | T/57 ± 7 | C/58 ± 8 | T/20:10 | C/19:10 | Untreated periodontitis | not reported | healthy, no antibiotics within the last 1 month | |
| Màximo MB et al., 2009 | 55.8±17 | 8F:4M | Untreated periodontitis | non smokers | healthy, non antibiotics within the last 6 months | |||
| Duarte PM et al., 2009 | 55.8±17 | 6F:4M | Untreated periodontitis | non smokers | healthy, no antibiotics within the last 6 month | |||
Table 5.
Parameters for the evaluation of the effect of treatments applied.
| Study | Intervals | PPD | BOP | MB | PI | MO analysis | BCH analysis CAL |
|---|---|---|---|---|---|---|---|
| Form | Form | ||||||
| Heitz-Mayfield et al., 2011 | Baseline/1 month 3 months |
Sum of PPD(mm) in 4 sites | Mean number of sites with BOP | Socransky et al 1998 + S. aureus | |||
| Thöne-Mühling M et al., 2010 | Baseline/1/2/4/8 months | Mean PPD(mm) In 4 sites |
mBI | Sillnes & Loe, 1964 | PCR | ||
| Ramberg P. et al., 2009 | Baseline/6 months | Mean PPD (mm) In 4 sites |
Mean % of sites with BOP | Mean % of impl surf harbouring plaque | |||
| Màximo MB et al., 2009 | Baseline/3 months | Mean PPD (mm) In 6 sites |
Mean % of sites with BOP | Mean % of sites with MB | Ainamo & Bay, 1975 | Socransky et al, 1998 DNA-DNA hybridization |
|
| Duarte PM et al., 2009 | Baseline/3 moths | Mean PPD (mm) In 6 sites |
Mean % of sites with BOP | Mean % of sites with MB | Ainamo & Bay, 1975 | IL-4, -10, -12, TNF-α, OPG, RANKL |
Further treatments such as photodynamic therapy, local chemical therapy, phytotherapy, xylitol therapy, probiotics therapy previously tested or being studied currently for their effectiveness in gingivitis/periodontitis need to be evaluated in peri-implant mucositis treatment. Surprisingly some of these therapies have been studied as treatments of peri-implantitis (antibiotic and photodynamic therapy) rather than peri-implant mucositis.
The review provided here suggests that the effectiveness of a treatment depends on the type of anti-infective treatment itself, as well as on: (i) the sample size, (ii) parameters selected for the evaluation of the effectiveness, (iii) proper diagnosis, and (iv) considerations and elimination of the local predisposing factors. Thus, further research is needed on already studied treatments as well as anti-infective treatments hypothesized to be effective for the resolution of peri-implant mucositis.
Sample size, diagnosis and treatment effect evaluation parameters
Sample size is an important factor when it comes to assessing the validity of a study. Small sized samples may give the impression that treatments compared are equally effective as long as statistically significant difference is missing (14).
Sample size is determined based on: (i) the parameter wanted to be positively influenced by the applied treatment (main parameter chosen for the evaluation of the effect), (ii) the difference of this parameter wanted to be seen between groups after treatment application, and (iii) the power of the study. In the selected studies the parameters chosen where periodontal probing depth (Heitz-Mayfield et al., 2011; 80% of power for a 1.1 mm PPD difference between control and test) and bleeding on probing [Ramberg et al., 2009 (80% power for a12% difference of BoP between groups); Thöne-Mühling et al., 2009 (78% power for and effect size of 1.4)], both parameters of periodontitis, counterpart of peri-implantitis (Table 5).
Peri-implant mucositis presents a stage of inflammation not necessarily associated with increased PPD (10). Determining the sample size on a parameter that could not be present at all among selected patients could effect the final results of the treatment. On the other hand, provoked bleeding is always present and it is worldwide accepted even as part of the definition of peri-implant mucositis (10). To author’s knowledge, marginal bleeding is considered more appropriate than bleeding on probing when dealing with inflammation confined to soft tissues only. Marginal bleeding bears the advantages of probing depth when compared to other signs of inflammation. It is an objective sign of inflammation preceding discoloration and swelling, easily measured by inserting an inexpensive instrument (periodontal probe) in the peri-implant/gingival sulcus in an angulation of approximately 60° with the long axis of the tooth/implant and running it along the gingival margin. This angulation facilitates the measurement of the parameter despite the quality of the prosthesis. Marginal bleeding is a more sensitive indicator of gingival inflammation and is less likely to elicit false-positive bleeding than probing to the bottom of the pocket (25). Marginal bleeding can be used as the main clinical parameter when determining the sample size, treatment effect and peri-implant mucositis diagnosis. Authors suggest: (i) a four grade marginal bleeding index (Newbrun 1997 reporting Mombelli et al., 1967 classification), (ii) measured in six sites per implant, (iii) reported as mean value of the implant, (iv) calculating the number of sites presenting each grade of BoP (19), to evaluate the reversibility of mucositis (% of sites completely recovered).
Furthermore, dealing with patients that smoke, studies should consider that smoking interferes with the bleeding response of soft tissues, consequently smokers and nonsmokers when included in the protocol should be analysed separately (24). None of the studies (except Duarte et al., 2009 and Maximo et al., 2009 that excluded smokers) made such a separation or mentioned smoking cessation counciling as part of the therapy.
Proper diagnosis influences the effect of treatment. Non surgical treatment is effective on mucositis but not on peri-implantitis. The lack of peri-implant bone loss and suppuration were unanimously chosen as differential diagnosis of peri-implant mucositis from peri-implantitis. The reported diagnostic parameters (Tab. 4), clinical only, were PPD ≥ 4mm (23), BoP (all selected studies) and GI (20). In the enrolled studies no treatment gave a complete resolution of the inflammation on the entire sample chosen. 38% (19) and 56.3% patients showed absence of BoP at 3 months after treatment with CHX gel and abrasive sodium carbonate air-powder respectively. This could be related to various factors, such as improper sample size, ineffective treatment, persistence of predisposing factors etc. Authors speculate that even within peri-implant mucositis different stages could be distinguished. Perhaps if the inflammation reaches a specific stage, it does not respond equally positively if compared to a more incipient stage. Biochemical changes appear earlier than clinical changes in an inflammatory process. Further-more, based on the latest reports peri-implant mucositis reversibility after 3 weeks of treatment is only biochemical indicating that clinical recovery takes a longer time to be established (3). Authors speculate that the identification of inflammatory mediators would be important in the diagnosis of incipient stages of peri-implant mucositis and treatment effect evaluation. Only one study reported biochemical changes after treatment applied (23), concluding that the levels of TNF-α and the OPG/RANKL ratio may be modulated by the treatment. Further studies are needed on this regard and on the type of inflammatory mediators that could be the more appropriate for this purpose (see existing studies on AST, IL-1, etc.) (Tab. 5).
Table 4.
Diagnosis of the peri-implant mucositis in the included studies. MB: Marginal bleeding: presence (score 1) or absence (score 0) of bleeding obtained by running a probe along the soft tissue margin without probe penetration inside the sulcus or pocket. Dichotomous: presence(1)/absence(0). (2) Absence of bone loss during the last 2 years before baseline of the study. (1) Peri-apical intraoral radiographs were obtained for each implant baseline using the paralleling technique and a radiographic positioner. The radiographs were analyzed for peri-implant bone loss by the same examiner (PMD) using abutments and the threads of the implants as reference points.
| Study | Bleeding on probing | Marginal bleeding | Gingival index | Periodontal probing depth | Suppuration | Bone loss |
|---|---|---|---|---|---|---|
| Heitz-Mayfield et al., 2011 | Dichotomous | absence | ||||
| Thöne-Mühling M et al., 2010 | Dichotomous at least at one site | GI≥ 1 at least at one site at baseline | absence2 | |||
| Ramberg P. et al., 2009 | Dichotomous | PPD≥4mm | absence | |||
| Màximo MB et al., 2009 | Dichotomous | Dichotomous | absence | absence | ||
| Duarte PM et al., 2009 | Bop: 15 seconds after gentle probing (Dichotomous) | Dichotomous | absence | absence1 |
Predisposing etiologic factors
Dental plaque is worldwide accepted as the etiologic factor of peri-implant mucositis. The quantity of plaque, neither its dichotomous presence, are necessarily related with the presence of inflammation (25). The total bacterial count or proportion of different complexes, or even specific types of microorganism can be analysed, but the presence of putative periodontal pathogens around teeth does not necessarily lead to periodontal tissue breakdown (26).
Only three studies reported microbiological effect evaluation (19–21). After treatment, a microbiological equilibrium compared to the baseline was noticed (19, 20). One study evaluated the influence of local predisposing factors on treatment effect (19) while only the observational studies (21, 22) explicitly included the elimination of this factors as treatment plan.
Once it is professionally removed the continuing presence of dental plaque depends on patient’s compliance, and persistence of local predisposing factors such as overcontoured restoration margins and other plaque retentive factors. Local treatment is suggested to include the elimination or modification of local predisposing factors. The further presence of plaque suggests lack of compliance. Authors suggest the use of mPIl, measured in 6 sites, to reinforce oral hygiene in specific sites where more needed. Dental plaque indexed or microbiological measurements seems to be more appropriate for patient compliance monitoring and identification of local retentive factors than for the diagnosis or treatment effect evaluation of the presence of inflammation around implants.
Follow-up period and intervals
Follow-up periods varied from 3 months (19, 21,22) to 6 months (23) and 8 months (20), emphasizing that in none of the studies the interval or the follow-up selected was explicitly justified. Follow-ups differed not only in length but evaluation intervals as well. Actually, the 1st month results were reported only in two studies (19,20), 2nd, 4th and 8th month only in the Thöne-Mühling et al., 2010 study, the 6th month only in the Ramberg et al., 2009 and the 3rd month from four studies (19,21–23).
The clinical relevance of the follow-up period is to determine in terms of time: (i) the evidence of the treatment effect, (ii) the duration of this effect, and (iii) the frequencies of the follow-up visits in the maintenance phase. It has been demonstrated that 3 weeks after plaque removal only biochemical reversibility could be detected (3). Furthermore, re-evaluation after non-surgical therapy is suggested 6–8 weeks after the last step of initial phase of treatment to permit tissue healing almost completed at 3 months (27). In the enrolled studies it was noticed that statistically important improvements could be detected in the first month after treatment, not always followed by similar improvement in the subsequent evaluations. Authors suggest that when evaluating the treatment effect prior to 6–8 weeks, the least invasive methods, such as biochemical evaluation, should be chosen. For one single application treatments, authors find it reasonable to be followed up for 3 months. A longer follow-up period would reflect more the quality of the domiciliary oral hygiene rather than to the direct effect of the treatment applied.
Conclusion
Peri-implant mucositis is accepted as a reversible and treatable stage of inflammation, worldwide. From the few high quality studies found on this topic, none reported complete treatment of the patient. Authors emphasized the need for a stronger evidence base, encouraging future studies on peri-implant mucositis treatment following basically a similar evidence-based protocol regarding study design, sample size, diagnostic and effectiveness parameters and follow-ups. The heterogeneity characterizing the existing studies was previously reported by Faggioni et al., 2010. Some of the enrolled studies on the present review were published afterwards, but we confirm the same observation.
Acknowledgement
This study had no financial support. Authors report no conflict of interest.
Footnotes
Clinical relevance
Peri-implant mucositis treatment is the only way to prevent the establishment of peri-implantitis and consequent implant loss. This review provided a summary of the available data on peri-implant mucositis treatment. It was noticed little evidence on this matter and a lack of studies on various possibly effective treatments. The comparison of the existing studies showed an evident heterogeneity. This observations were used to guide a protocol on future studies on peri-implant mucositis treatment.
References
- 1.Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri-implant mucositis. Aclinical study in humans. Clin Oral Imlants Res. 1994;5:254–259. doi: 10.1034/j.1600-0501.1994.050409.x. [DOI] [PubMed] [Google Scholar]
- 2.Zitzmann NU, Berglundh T, Marinello CP, Lindhe J. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28:517–523. doi: 10.1034/j.1600-051x.2001.028006517.x. [DOI] [PubMed] [Google Scholar]
- 3.Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182–190. doi: 10.1111/j.1600-0501.2011.02220.x. [DOI] [PubMed] [Google Scholar]
- 4.Lang NP, Bosshardt DD, Lulic M. Do mucositis lesions around implants differ from gingivitis lesions around teeth? J Clin Periodontol. 2011;38:182–187. doi: 10.1111/j.1600-051X.2010.01667.x. [DOI] [PubMed] [Google Scholar]
- 5.Schüpbach P, Glauser R. The defence architecture of the human peri-implant mucosa: a histological study. J Prosthet Dent. 2007;97:S15–25. doi: 10.1016/S0022-3913(07)60004-3. [DOI] [PubMed] [Google Scholar]
- 6.Rinke S, Ohl S, Ziebolz D, Lange K, Eickholz P. Prevalence of peri-implant disease in partially edentulous patients: a practice-based cross-sectional study. Clin Oral Implants Res. 2011;22:826–33. doi: 10.1111/j.1600-0501.2010.02061.x. [DOI] [PubMed] [Google Scholar]
- 7.Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;81:231–8. doi: 10.1902/jop.2009.090269. [DOI] [PubMed] [Google Scholar]
- 8.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35:286–91. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 9.Goszkowski R. Down but not out: Implant makers navigate economic storm. This week in Perio. 2011 Oct 3 [Google Scholar]
- 10.Lang NP, Berglundh T. Peri-implant diseases: where are we now? J Clin Periodontol. 2011;38:178–181. [Google Scholar]
- 11.Berglundh T, Abrahamsson I, Welander M, Niklaus PL, Lindhe J. Morphorgenesis of the peri-implant mucosa: an experimental study in dogs. Clin Oral Implants Res. 2007;18:1–8. doi: 10.1111/j.1600-0501.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- 12.Faggion CM, Jr, Listl S, Tu YK. Assessment of endpoints in studies on peri implantitis treatment- a systemic review. J Dent. 2010;38:443–450. doi: 10.1016/j.jdent.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Norowski PA, Bumgardner JD., Jr Biomaterial and antibiotic strategies for peri-implantitis. J Biomed Materials Res Part B: Applied Biomaterials. 2010;88:530–543. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 14.Faggion CM, Jr, Chambrone L, Gondim V, Schmitter M, Tu YK. Comparison of the effects of treatment of peri-implant infection in animal and human studies: systematic review and meta-analysis. Clin Oral Implants Res. 2010;21:137–47. doi: 10.1111/j.1600-0501.2009.01753.x. [DOI] [PubMed] [Google Scholar]
- 15.Anner R, Grossmann Y, Anner Y, Levin L. Smoking, diabetes mellitus, periodontitis, and supportive periodontal treatment as factors associated with dental implant survival. A long-term retrospective evaluation of patients following up to 10 years. Implant Dent. 2010;19:56–64. doi: 10.1097/ID.0b013e3181bb8f6c. [DOI] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Sjögren P, Halling A. Quality of reporting randomised clinical trials in dental and medical research. Br Dent J. 2002;192:100–103. doi: 10.1038/sj.bdj.4801304. [DOI] [PubMed] [Google Scholar]
- 18.Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35:305–15. doi: 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 19.Heitz-Mayfield LJ, Salvi GE, Botticelli D, Mombelli A, Faddy M, Lang NP. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22:237–41. doi: 10.1111/j.1600-0501.2010.02078.x. [DOI] [PubMed] [Google Scholar]
- 20.Thöne-Mühling M, Swierkot K, Nonnenmacher C, Mutters R, Flores-de-Jacoby L, Mengel R. Comparison of two full-mouth approaches in the treatment of peri-implant mucositis: a pilot study. Clin Oral Implants Res. 2010;21:504–12. doi: 10.1111/j.1600-0501.2009.01861.x. [DOI] [PubMed] [Google Scholar]
- 21.Máximo MB, de Mendonça AC, Renata SV, Figueiredo LC, Feres M, Duarte PM. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res. 2009;20:99–108. doi: 10.1111/j.1600-0501.2008.01618.x. [DOI] [PubMed] [Google Scholar]
- 22.Duarte PM, de Mendonça AC, Máximo MB, Santos VR, Bastos MF, Nociti FH. Effect of anti-infective mechanical therapy on clinical parameters and cytokine levels in human peri-implant diseases. J Periodontol. 2009;80:234–43. doi: 10.1902/jop.2009.070672. [DOI] [PubMed] [Google Scholar]
- 23.Ramberg P, Lindhe J, Botticelli D, Botticelli A. The effect of a triclosan dentifrice on mucositis in subjects with dental implants: a six-month clinical study. J Clin Dent. 2009;20:103–107. [PubMed] [Google Scholar]
- 24.Newbrun E. Indices to measure gingival bleeding. J Periodontol. 1996;67:555–561. doi: 10.1902/jop.1996.67.6.555. [DOI] [PubMed] [Google Scholar]
- 25.Trombelli L, Scapoli C, Calura G, Tatakis DN. Time as a factor in the identification of subjects with different susceptibility to plaque-induced gingivitis. J Clin Periodontol. 2006;33:324–328. doi: 10.1111/j.1600-051X.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 26.Quirynen M, Vogels R, Peeters W, van Steenberghe D, Naert I, Haffajee A. Dynamics of initial subingival colonization of “pristine” peri-implant pocket. Clin Oral Implants Res. 2006;17:25–37. doi: 10.1111/j.1600-0501.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindhe J, Lang NP, Karring K. Clinical Periodontology and Implant Dentistry. 5th edition. Oxford Blackwell; Munksgaard: 2008. p. 688.p. 774. [Google Scholar]