There are five categories in the World Health Organization's (WHO) classification of pulmonary hypertension (PH) (1): Pulmonary arterial hypertension (2): PH associated with left heart disease (3): PH associated with hypoxia or lung disease (4): chronic thromboembolic PH (CTEPH) and (5): PH of miscellaneous etiology (sarcoidosis, extrinsic compression, etc.). Group 1 PH is the only category for which there is approved, PH-specific medical therapy. These medications (phosphodiesterase 5 inhibitors, prostanoids and endothelin antagonists) improve functional class and exercise tolerance but are not curative. The only form of adult PH that is curable (other than by lung or heart-lung transplantation) is Group 4 PH (CTEPH), which can be reversed by surgical pulmonary endarterectomy (PEA).
The diagnosis of CTEPH is often delayed because the onset of symptoms is insidious and the symptoms themselves are nonspecific. CTEPH patients usually present with dyspnea, and fatigue, often of many years duration. Thus a high degree of suspicion is required to detect CTEPH. Symptoms are related to impaired cardiac output and right ventricular failure and result from obstruction of the pulmonary arteries by unresolved thrombus, compounded, in some cases, by a poorly understood vasculopathy1. The US Healthcare Cost and Utilization Project (HCUP) database suggests a 3.4% incidence of CTEPH in patients with a pulmonary embolism (reviewed in 1). CTEPH is often discovered on CT angiography or ventilation/perfusion scans conducted in the course of a systematic investigation of unexplained PH. Fully 2/3 of CTEPH patients have no clinical history of pulmonary embolism2.
The one-year untreated mortality rate in CTEPH ranges from 12-24% and is predicted by the PA pressure at diagnosis3, 4. However, with PEA, survival improves to 89% and 75% at 5-6 years, respectively5, 6. With this in mind, increased efforts should be undertaken to diagnose CTEPH and refer patients for this complex, but life-saving, surgery.
There are approximately 30 centers worldwide that offer PEA and half of the 4000 procedures performed thus far have been done at University of California, San Diego (UCSD), a program led by Dr. Stuart Jamieson 7. In addition to outstanding surgeons and anesthesiologists, a successful PEA program requires collaboration between different groups of physicians and nurses as well as consistent support from hospital administration, (Figure 1) This is particularly true because of the complex postoperative problems that can occur with PEA, including neurological impairment (related to total circulatory arrest), hemorrhage and lung reperfusion injury, often manifesting as pulmonary edema1. Assembling this team, like assembling a puzzle, requires time and patience. PEA should be reserved for dedicated centers in which the puzzle is assembled because every center starting a PEA program reports a significant learning curve. Two phases are noted in the journey toward a mature PEA program- an early phase with a higher mortality and selective case acceptance rates, and a mature phase, where operative mortality improves despite more complex cases being undertaken.
Figure 1. Component pieces of pulmonary endarterectomy program requiring intricate collaboration among many types of health care professionals.
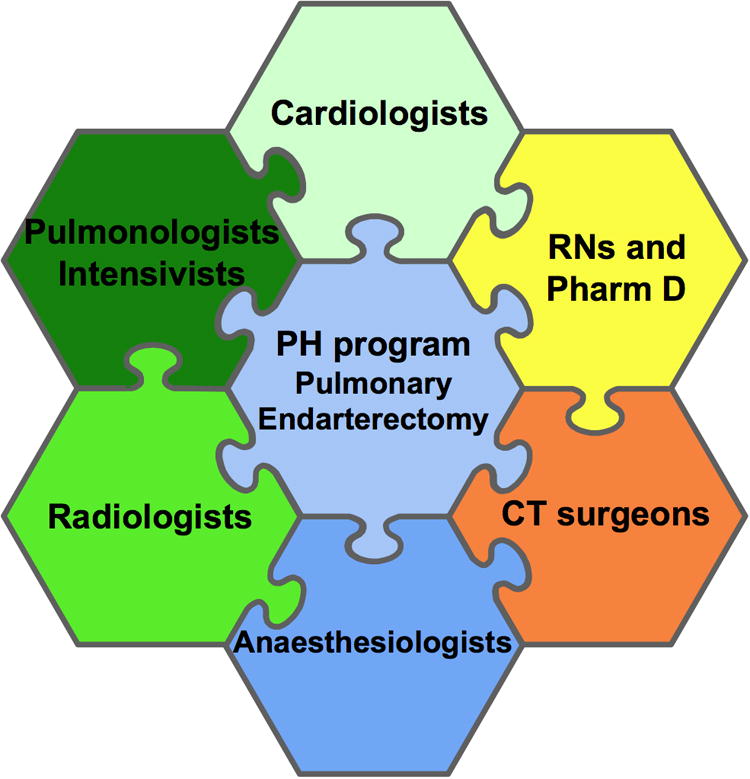
While PEA is very successful in high-volume, specialized, centers, which have all the pieces of the puzzle in place (Figure 1), there are few such centers and patients find distant travel, particularly across borders, onerous. Thus, regional centers of excellence are needed. De Perrot et al at Toronto General Hospital (TGH) describe such a program in this edition of The Canadian Journal of Cardiology8.
There are several important aspects to consider when starting a PEA program, beginning with the importance of making an accurate diagnosis. A diagnosis of CTEPH requires a mean PA pressure >25mmHg, PVR >3 Wood units, angiographic or scintigraphic evidence of obstruction of the pulmonary arterial tree despite 3 months of anticoagulation and exclusion of other cause of the PH1. Next, an extensive evaluation is required to exclude other causes of PH and exclude comorbidities, the bulk of which can be done by referring physicians. This includes an electrocardiogram, posterior-anterior and lateral chest x-ray, arterial blood gas testing, pulmonary function tests, ventilation/perfusion scans and/or high resolution chest CT scans, right heart catheterization and invasive pulmonary angiography. The angiography is usually performed at the PEA center because of local expertise available and the center-specific criteria for the preferred angiographic views. Pulmonary angiography provides valuable information regarding the proximal origins and extent of the thrombus burden and can help predict post-operative right ventricle (RV) performance 9.
The surgical technique used to perform PEA is described by de Perrot et al and has been previously reported10. However, there are certain aspects that deserve particular mention. Circulatory arrest with cooling to 20°C is essential to protect the brain while obtaining the bloodless field that is required to identify and dissect the PA/thrombus tissue plane so that the entire thromboembolic burden can be extracted. While fresh mural thrombus may be found, the pathology of CTEPH more commonly involves a fibrous incorporation of the clot into the vessel wall (explaining why the procedure is an endarterectomy and not an embolectomy). Residual disease and post-operative PH predict a poor outcome 11.
Although the role of surgical expertise in a successful PEA program cannot be overemphasized, the success of any PEA program depends heavily on the post-operative care which requires skilled management and close monitoring. In de Perrot's report, the majority of patients were extubated within 2 days. The median time to discharge post-surgery was 13 days. This results are very good and comparable to prior published series12. Obtaining excellent results, such as these, requires experienced anesthesiologists, intensive care physicians and nurses, who have an adequate PEA case volume to maintain competency. This team needs to be comfortable managing the profound hypoxemia and right heart failure that are common in the early postoperative days. Mares et al identified two such management guidelines that were beneficial. They showed that restricting inotropes to those with overt right heart failure and using low tidal volume ventilation reduced mortality post PEA by 50% 13.
The spontaneous improvement in post-operative right ventricle performance is a well-known benefit of PEA. In Stuart Jamieson's landmark paper, detailing the first 1500 cases at UCSD, he noted, that “improvement in right ventricular ejection fraction is consistent no matter what the original preoperative calculation is.…. thus…no degree of … right ventricular failure contraindicates surgery” 12. This confirms earlier work by Moser et al14. Referring physicians should heed this advice and avoid classifying patients as “too sick” for surgery because of RV dysfunction. A successful PEA results in 93% of patients becoming WHO/NYHA 1-2 and >62% of patients desiring employment and returning to work (summarized in 1). Thus, diagnosing and referring a CTEPH patient is potentially transformative to the patient, and cases should be rejected only after careful consideration by an expert. It is worthwhile to consider all patients with CTEPH as potential surgical candidates.
Referring patients to established PEA centers in San Diego, Germany 15 or Japan 16 is often difficult because of the patient's poor health status (which makes air travel difficult), social issues and/or health insurance issues. Thus there is a role for development of regional PEA centers of excellence. In this paper, de Perrot et al review the TGH experience8. PEA was performed on 52 of the 84 referred patients. The authors detail the reasons for excluding the 32 patients and note that fewer cases were declined over time. De Perrot et al report an acceptable operative mortality of 1.9% for elective PEA. This compares favorably to results at large centers where operative mortality is <5%1, 12, albeit this latter figure includes all cases, not just the lower risk elective cases. The TGH series also reports the expected postoperative decreases in mPAP and PVR and increases in cardiac index. In longer term follow-up, these hemodynamic benefits translated into improved functional class and 6-minute walk distance.
However, the learning curve is also evident in their series, most particularly in the number of CTEPH patients in whom PEA was not offered. Of the patients with distal disease, the number of patients rejected decreased from 26% before 2008 (7 out of 26 patients) to 5% after 2008 (3 out of 58 patients) (p=0.004). In a high volume program fewer patients tend to be deemed inoperable.
It is noteworthy that PEA leads to greater increases in exercise capacity as compared to those achieved by vasodilator therapies, such as bosentan 17. Patients post PEA improved to WHO class 1.5 and achieved a 6-minute walk distance of ∼ 500m (Figure 2B). This improvement is consistent with results from Matusuba et al, who noted that PEA increased the 6-minute walk distance from 358 m to 490 m 18. In contrast, retrospective analysis of the use of PAH-directed vasodilators has not been shown to produce significant benefit in either a pre-operative or post-operative setting 19. A recent Scientific Statement from the American Heart Association on CTEPH cautions against first-line use of such therapies for CTEPH, noting surgery is always the optimal technique if it is feasible (Table 1)1.
Table 1. Recommendations for Medical Therapy and Pulmonary Endarterectomy in Patients With CTEPH (from 1).
| Recommendation | Class and Level of Evidence |
|---|---|
| Patients with objectively proven CTEPH should be promptly evaluated for pulmonary endarterectomy, even if symptoms are mild | Class I; Level of Evidence B |
| Patients with objectively proven CTEPH should receive indefinite therapeutic anticoagulation in the absence of contraindications | Class I; Level of Evidence C |
| PAH (WHO Group I)-specific medical therapy maybe considered for patients with CTEPH who are not surgical candidates (because of comorbidities or patient choice) or who have residual pulmonary hypertension after operation not amenable to repeat pulmonary endarterectomy at an experienced center | Class IIb; Level of Evidence B |
| PAH (WHO Group I)-specific medical therapy should not be used in lieu of pulmonary Endarterectomy or delay evaluation for pulmonary endarterectomy for patients with objectively proven CTEPH who are or may be surgical candidates at an experienced center | Class III; Level of Evidence B |
There are limitations in this report, which need to be addressed. The authors did not use pulmonary angiograms pre-operatively or place inferior vena cava filters, both of which are considered standard care in major PEA centers. Ineffective cava filtration has been identified as a risk factor for PEA re-operation20. Although, departure from this protocol is not without precedent 21, de Perrot el al have a higher than optimal rate of denial of surgery and their mortality, while low, still exceeds that in the largest PEA centers. Pre-operative pulmonary angiography and the use of caval filters might be worthy of consideration for inclusion in the TGH algorithm.
Neurological outcomes are important determinants of long term benefit in PEA and merit discussion in any PEA series12, 22 as they can occur commonly in the setting of total circulatory arrest and hypothermia. de Perrot et al detail neurological injury in 5% of their patients, including delayed extubation in two patients because of agitation or somnolence and one patient experiencing a seizure. These results are consistent with prior results series12, 22.
The economic cost of establishing a PEA program and performing PEA has not been extensively published, which makes the financial comparisons with cardiac transplantation difficult. Traditionally patients with pulmonary hypertension have had a lower status on heart and lung transplant listings because of the belief that PH patients have poorer outcomes with transplantation than other types of patients, such as those with pulmonary fibrosis. Lung transplantation also comes at a cost of immunosuppression. Thus the surgical procedure of choice for CTEPH is PEA.
As more regional centers offer PEA the onus will be on them to document that they are achieving acceptable outcomes, a standard achieved by the TGH group. The recent establishment of the first international registry on CTEPH documented the ability of 1 Canadian and 26 European centers to perform PEA on 386 CTEPH patients (56.8% of all referrals) with low mortality rates (in-hospital mortality <5%)23. Interestingly, only 36.5% underwent operations in centers performing more than 50 PEAs per year. In centers doing 1-10 PEAs per year the in-hospital and 1-year mortality were 7.4 and 11.1%, whereas in those centers doing more than 50 PEA it was 3.5% and 5.0%, more in line with results from reference centers. Although the authors conclude that the “number of PEAs performed per year was not a risk factor for mortality”23, we believe that their data show that volume matters. Incidentally, de Perrot et al were part of this registry and thus their patients contribute to this literature.
In summary, de Perrot et al have developed an exemplary PEA program and, despite the limitations in their study, should be commended for their success and rigour. Now that they have built it, hopefully more CTEPH patients will be referred and treated.
Acknowledgments
Sources of Support: This work is supported by NIH-RO1-HL071115, 1RC1HL099462-01, and the American Heart Association (AHA).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- 1.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the american heart association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 2.Tapson VF, Humbert M. Incidence and prevalence of chronic thromboembolic pulmonary hypertension: From acute to chronic pulmonary embolism. Proc Am Thorac Soc. 2006;3:564–567. doi: 10.1513/pats.200605-112LR. [DOI] [PubMed] [Google Scholar]
- 3.Egermayer P, Peacock AJ. Is pulmonary embolism a common cause of chronic pulmonary hypertension? Limitations of the embolic hypothesis. Eur Respir J. 2000;15:440–448. doi: 10.1034/j.1399-3003.2000.15.03.x. [DOI] [PubMed] [Google Scholar]
- 4.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 5.Archibald CJ, Auger WR, Fedullo PF, Channick RN, Kerr KM, Jamieson SW, Kapelanski DP, Watt CN, Moser KM. Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med. 1999;160:523–528. doi: 10.1164/ajrccm.160.2.9808109. [DOI] [PubMed] [Google Scholar]
- 6.Saouti N, Morshuis WJ, Heijmen RH, Snijder RJ. Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: A single institution experience. Eur J Cardiothorac Surg. 2009;35:947–952. doi: 10.1016/j.ejcts.2009.01.023. discussion 952. [DOI] [PubMed] [Google Scholar]
- 7.Lang IM. Chronic thromboembolic pulmonary hypertension--not so rare after all. N Engl J Med. 2004;350:2236–2238. doi: 10.1056/NEJMp048088. [DOI] [PubMed] [Google Scholar]
- 8.de Perrot M, McRae K, Shargall Y, Pletsch L, Tan K, Slinger P, Ma M, Paul N, Moric J, Thenganatt J, Mak S, Granton J. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. The toronto experience. Can J Cardiol. 2011 doi: 10.1016/j.cjca.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Thistlethwaite PA, Madani M, Jamieson SW. Pulmonary thromboendarterectomy surgery. Cardiol Clin. 2004;22:467–478. doi: 10.1016/j.ccl.2004.04.009. vii. [DOI] [PubMed] [Google Scholar]
- 10.Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Cardiovasc Surg. 2008;14:274–282. [PubMed] [Google Scholar]
- 11.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345:1465–1472. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, Channick RN, Fedullo PF, Auger WR. Pulmonary endarterectomy: Experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–1462. doi: 10.1016/s0003-4975(03)00828-2. discussion 1462-1454. [DOI] [PubMed] [Google Scholar]
- 13.Mares P, Gilbert TB, Tschernko EM, Hiesmayr M, Muhm M, Herneth A, Taghavi S, Klepetko W, Lang I, Haider W. Pulmonary artery thromboendarterectomy: A comparison of two different postoperative treatment strategies. Anesth Analg. 2000;90:267–273. doi: 10.1097/00000539-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Dittrich HC, Nicod PH, Chow LC, Chappuis FP, Moser KM, Peterson KL. Early changes of right heart geometry after pulmonary thromboendarterectomy. J Am Coll Cardiol. 1988;11:937–943. doi: 10.1016/s0735-1097(98)90049-3. [DOI] [PubMed] [Google Scholar]
- 15.Mayer E, Klepetko W. Techniques and outcomes of pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3:589–593. doi: 10.1513/pats.200605-120LR. [DOI] [PubMed] [Google Scholar]
- 16.Ogino H, Ando M, Matsuda H, Minatoya K, Sasaki H, Nakanishi N, Kyotani S, Imanaka H, Kitamura S. Japanese single-center experience of surgery for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2006;82:630–636. doi: 10.1016/j.athoracsur.2006.03.121. [DOI] [PubMed] [Google Scholar]
- 17.Jais X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, Lang IM, Mayer E, Pepke-Zaba J, Perchenet L, Morganti A, Simonneau G, Rubin LJ. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: Benefit (bosentan effects in inoperable forms of chronic thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:2127–2134. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda H, Ogino H, Minatoya K, Sasaki H, Nakanishi N, Kyotani S, Kobayashi J, Yagihara T, Kitamura S. Long-term recovery of exercise ability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2006;82:1338–1343. doi: 10.1016/j.athoracsur.2006.03.105. discussion 1343. [DOI] [PubMed] [Google Scholar]
- 19.Jensen KW, Kerr KM, Fedullo PF, Kim NH, Test VJ, Ben-Yehuda O, Auger WR. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009;120:1248–1254. doi: 10.1161/CIRCULATIONAHA.109.865881. [DOI] [PubMed] [Google Scholar]
- 20.Mo M, Kapelanski DP, Mitruka SN, Auger WR, Fedullo PF, Channick RN, Kerr K, Archibald C, Jamieson SW. Reoperative pulmonary thromboendarterectomy. Ann Thorac Surg. 1999;68:1770–1776. doi: 10.1016/s0003-4975(99)01043-7. discussion 1776-1777. [DOI] [PubMed] [Google Scholar]
- 21.Coulden R. State-of-the-art imaging techniques in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3:577–583. doi: 10.1513/pats.200605-119LR. [DOI] [PubMed] [Google Scholar]
- 22.Macchiarini P, Kamiya H, Hagl C, Winterhalter M, Barbera J, Karck M, Pomar J, Haverich A. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: Is deep hypothermia required? Eur J Cardiothorac Surg. 2006;30:237–241. doi: 10.1016/j.ejcts.2006.02.071. discussion 241-233. [DOI] [PubMed] [Google Scholar]
- 23.Mayer E, Jenkins D, Lindner J, D'Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, Simonneau G, Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]


