Abstract
Background
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) demonstrated similar rates of the primary composite endpoint between carotid artery stenting (CAS) and carotid endarterectomy (CEA), although the risk of stroke was higher with CAS, and the risk of myocardial infarction (MI) was higher with CEA. Given the large number of patients who are candidates for these procedures, an understanding of their relative cost and cost-effectiveness may have important implications for healthcare policy and treatment guidelines.
Methods
We performed a formal economic evaluation alongside the CREST trial. Costs were estimated from all trial participants over the first year of follow-up using a combination of resource use data and hospital billing data. Patient-level health utility scores were obtained using data from the SF-36. We then used a Markov disease-simulation model calibrated to the CREST results to project 10-year costs and quality-adjusted life expectancy for the 2 treatment groups.
Results
Although initial procedural costs were $1025/patient higher with CAS, post-procedure costs and physician costs were lower, such that total costs for the index hospitalization were similar for the CAS and CEA groups ($15,055 versus $14,816; mean difference $239/patient, 95% CI for difference, −$297 to $775). Neither follow-up costs after discharge nor total 1-year costs differed significantly. For the CREST population, model-based projections over a 10-year time horizon demonstrated that CAS would result in a mean incremental cost of $524/patient and a reduction in quality-adjusted life expectancy of 0.008 years compared with CEA. Probabilistic sensitivity analysis demonstrated that CEA was economically attractive at an incremental cost-effectiveness threshold of $50,000/quality-adjusted life-year gained in 54% of samples, whereas CAS was economically attractive in 46%.
Conclusions
Despite slightly lower in-trial costs and lower rates of stroke with CEA compared with CAS, projected 10-year outcomes from this controlled clinical trial demonstrate only trivial differences in overall healthcare costs and quality-adjusted life expectancy between the 2 strategies. If the CREST results can be replicated in clinical practice, these findings suggest that factors other than cost-effectiveness should be considered when deciding between treatment options for carotid artery stenosis in patients at standard risk for surgical complications.
Keywords: stroke care, economics, quality of life, stenting, endarterectomy, prevention
INTRODUCTION
Major stroke is associated with high medical care costs and reductions in life expectancy and quality of life.1–10 Carotid endarterectomy (CEA) has been shown in several trials to be superior to medical management for the prevention of stroke in select patients with symptomatic or asymptomatic carotid artery stenosis.11, 12 More recently, carotid artery stenting (CAS) with the use of an embolic protection device has been introduced as a less invasive approach to carotid revascularization.
In patients for whom surgery poses an increased risk, the Stenting and Angioplasty with Protection in Patients at High Risk of Endarterectomy (SAPPHIRE) trial demonstrated CAS to be clinically non-inferior to CEA, with a favorable cost-effectiveness ratio.13, 14 In patients at standard risk for surgical complications, the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) found no significant difference in the primary clinical outcome of stroke, myocardial infarction (MI) or death within the periprocedural period or any ipsilateral stroke thereafter, although there was a significantly higher rate of periprocedural stroke with CAS and a significantly higher rate of periprocedural MI with CEA.15 Given the large number of patients who are candidates for these alternative procedures, an understanding of their relative cost and cost-effectiveness may have important implications for healthcare policy and treatment guidelines. We therefore performed a prospective economic evaluation alongside the CREST trial in which we used empirical data on healthcare costs and health utilities, and clinical outcomes from the trial as inputs to a Markov state-transition model that was used as the basis for formal cost-effectiveness analysis.
METHODS
Patient Population
Between December 2000 and July 2008, 2522 patients with symptomatic or asymptomatic carotid stenosis who were judged to be clinically and anatomically suitable for both CAS and CEA were enrolled in the CREST trial (ClinicalTrials.gov number NCT00004732) at 108 U.S. and 9 Canadian centers. Full details of the inclusion and exclusion criteria as well as the complete study protocol have been published previously.15, 16
Eligible patients were randomized in a 1:1 ratio to CAS (n=1271) or CEA (n=1251). After excluding 20 patients due to suspected scientific misconduct, the primary analysis of clinical outcomes was conducted on a data set that included 2502 patients (n=1262 CAS; 1240 CEA).15 For the economic study, we defined 2 analytic populations: a modified intention-to-treat (ITT) population that included all patients except those who declined their assigned procedure after randomization (n=1213 CAS; 1193 CEA), and an Assigned Treatment Received population that included only those patients in whom the assigned treatment was actually performed without crossover treatment during the periprocedural period (n=1136 CAS; 1184 CEA; online-only Data Supplement Figure S1). The modified ITT population was considered the primary population for the cost and cost-effectiveness analyses, unless otherwise specified.
Estimation of In-Trial Costs
Medical care costs for treatment of cerebrovascular disease and its direct and indirect complications were assessed for all patients over a 1-year follow-up period using a combination of resource-based costs and hospital billing data as previously described.17 All costs were assessed in year 2008 dollars, or converted to 2008 dollars using the medical care component of the Consumer Price Index.18
Index Procedures
Detailed resource use was recorded for each procedure, and costs were calculated as the product of resource use and unit cost for each component. Acquisition costs for each item were estimated using 2008 acquisition costs from a sample of study centers (Table 1). The study stent (RX ACCULINK Carotid Stent System, Abbott Vascular, Inc., Santa Clara, CA) was assigned an acquisition cost of $2107, and the embolic protection device (RX ACCUNET Embolic Protection System, Abbott Vascular, Inc.) was assigned an acquisition cost of $1505, based on national average pricing data obtained from the IMS Hospital Supply Index as of September 2010. Ancillary costs for the catheterization laboratory or interventional radiology suite (for CAS procedures) and for the operating room (for CEA procedures) included overhead and depreciation, non-physician personnel, and general supplies required for each procedure, and were estimated based on a survey of study hospitals.
Table 1.
Index Procedural Resource Use and Costs (Assigned Treatment Received Population)
| Mean Value (Median) |
Unit Cost | |
|---|---|---|
| CAS resource use (n=1136) | ||
| Procedure duration, min | 69 ± 31 (62) | $17.24/min |
| Study stents | 1.01 ± 0.30 (1) | $2107 |
| Embolus protection devices/filters | 0.95 ± 0.38 (1) | $1505 |
| Guiding catheters | 1.06 ± 0.81 (1) | $50 |
| Interventional guidewires | 1.47 ± 1.12 (1) | $92.55 |
| IVUS catheters | 0.04 ± 0.23 (0) | $825 |
| Balloon catheters | 1.67 ± 0.69 (2) | $270 |
| Pulmonary artery catheters | 0.01 ± 0.09 (0) | $48 |
| Temporary pacing catheters | 0.002 ± 0.04 (0) | $85 |
| IABPs | 0.05 ± 0.22 (0) | $1440 |
| Contrast volume, mL | 147 ± 62 (140) | $0.13–$0.44/mL |
| CEA resource use (n=1184) | ||
| Procedure duration, minutes | 171 ± 55 (165) | $23.75/min |
| Patches/grafts | 0.62 ± 0.48 (1) | $106.50 |
| Shunts | 0.57 ± 0.48 (1) | $399 |
| CAS procedure cost | ||
| Room/overhead, supplies, nonphysician personnel | $2284 ± 541 (2172) | |
| Devices | $4510 ± 872 (4320) | |
| Total | $6794 ± 1109 (6544) | |
| CEA procedure cost | ||
| Room/overhead, supplies, nonphysician personnel | $5476 ± 1315 (5344) | |
| Devices | $293 ± 213 (399) | |
| Total | $5769 ± 1343 (5561) |
CAS indicates carotid stenting; IVUS, intravascular ultrasound; CEA, carotid endarterectomy; IABP, intra-aortic balloon pump.
Procedure-related physician fees for the primary operator and first assistant (where applicable) were calculated using national average rates from the 2008 Medicare Fee Schedule and included the cost for the revascularization procedure, plus the cost of diagnostic cerebral angiography if applicable. Anesthesiologist fees for CEA procedures were calculated based on measured procedure duration according to the Medicare fee schedule.
Post-Procedure Hospital Care
Costs for all other aspects of care during the initial hospitalization were estimated based on hospital billing data. Itemized hospital bills and UB-92 or UB-04 forms (summary billing statements) were obtained for 577 CAS patients and 637 CEA patients. After excluding any charges associated with the index procedure, post-procedure hospital costs were calculated by multiplying the remaining hospital charges by year- and hospital-specific revenue code-level cost-to-charge ratios obtained from each center’s Medicare Cost Report.17
For index hospitalizations without available billing data (n=1203), post-procedural hospital costs were estimated using a linear regression model developed from hospital admissions for which billing information was available (R2=0.51). Covariates included in the final model were treatment group, sex, length of stay (intensive care unit and non-intensive care unit), previous coronary artery bypass graft, and procedure-related complications (MI, minor stroke, major stroke, bleeding). Inclusion of additional covariates and alternative model structures did not improve model fit.
Rehospitalization Costs
Detailed resource use was collected for repeat carotid revascularization procedures, and costs were assigned to those admissions using the same approach as that developed for the estimation of index hospitalization costs. Subsequent hospitalizations associated with MI, transient ischemic attack, stroke and death events were each mapped to a diagnosis-related group by a coder blinded to treatment assignment, and costs for each hospital admission were assigned based on the corresponding national average Medicare reimbursement rates for 2008.19 Physician fees for rehospitalizations were estimated as a percentage of hospital costs according to diagnosis-related group as described previously.20, 21
Statistical Analysis
Discrete data are reported as frequencies, and continuous data are reported as mean ± SD and median. Discrete variables were compared by Fisher’s exact test. Normally distributed continuous variables were compared by Student t-test, and non-normally distributed data were compared by the Wilcoxon rank-sum test. Given the skewed distribution of length of stay and cost data, we used non-parametric bootstrapping (1000 replicates) to estimate p-values and 95% confidence intervals (CIs) for differences in length of stay and costs between the two treatment arms.22 All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc. Cary, NC). A p-value <0.05 was considered statistically significant for all comparisons.
Cost-Effectiveness Analysis
Cost-effectiveness of CAS versus CEA was assessed in terms of cost per quality-adjusted life-year (QALY) gained over a 10-year time horizon from the perspective of the U.S. healthcare system. Since resource use and cost data were collected only for the first year of follow-up in CREST, and there were differences between CAS and CEA for prognostically important events, it was necessary to extrapolate the observed results beyond the trial period to provide meaningful cost-effectiveness results. We therefore developed a state-transition (Markov) model in order to project clinical outcomes, quality of life, survival, and costs beyond the trial period. Our general approach was to base this model to the greatest extent on the observed results of the CREST trial. The model structure and the assumptions for transition probabilities, costs, and utility weights (along with their underlying distributions and data sources) are summarized in the online-only Data Supplement. The model was programmed using TreeAge Pro 2011 (TreeAge, Inc., Williamstown, MA) and structured as a microsimulation, with patients sampled from an underlying population that mirrored the age and symptomatic status distributions of the CREST trial population.
Model Calibration and Analyses
Model calibration was confirmed by comparing the projected one-month and one-year costs and clinical event rates against the observed trial results (results within 3% of observed for all major outcomes; see online-only Data Supplement Table S1). For the purposes of cost-effectiveness analysis, all future costs, life-years, and QALYs were discounted at 3% per year, consistent with current guidelines.23 The incremental cost-effectiveness ratio (ICER) for CAS versus CEA was calculated by dividing the difference in mean discounted costs by the difference in mean quality-adjusted life expectancy for the two treatment strategies. The overall impact of uncertainty was assessed by means of probabilistic sensitivity analysis in which all model parameters were sampled from their appropriate distributions over 1000 runs. The results of these analyses are displayed graphically as a joint distribution of the incremental cost and effectiveness of CAS versus CEA in the cost-effectiveness plane. In addition to the primary analysis for the overall trial population, we performed pre-specified subgroup analyses for the asymptomatic and symptomatic CREST populations.
RESULTS
Study Population
Of the 1262 patients randomized to CAS, 1213 (96.1%) underwent attempted CAS and 1136 (89.4%) had CAS performed as assigned. Of the 1240 patients randomized to CEA, 1193 (95.4%) underwent attempted CEA, and 1184 (94.6%) had CEA performed as assigned (online-only Data Supplement Figure S1). Baseline characteristics did not differ between treatment groups for either the modified ITT or the Assigned Treatment Received populations (online-only Data Supplement Table S2). The prevalence of symptomatic carotid stenosis was 53% for each treatment group for both the modified ITT and Assigned Treatment Received populations.
Index Hospitalization Resource Use and Costs
For the modified ITT population, 1136 of the 1213 patients randomized to CAS received CAS only; 8 underwent attempted CAS followed by CEA, and 40 underwent CEA only. The remaining 29 patients underwent carotid and cerebral angiography only. The main reason for crossover to CEA was anatomic unsuitability for CAS. Of the 1193 modified ITT patients randomized to CEA, 1184 underwent CEA and 9 received only angiography; no patients in the CEA group underwent CAS.
Resource use and costs for the index procedures are summarized in Table 1. A mean of 1.01 stents and 0.95 embolic protection devices were used in the CAS arm, with total device-related costs of $4510/patient. Although operating room costs (including overhead, depreciation, supplies, and non-physician personnel) were substantially higher for CEA than for CAS, after including device-related costs, total procedural costs were higher with CAS ($6794 versus $5769, p<0.001). On the other hand, physician fees were higher with CEA ($1951 versus $1514, p<0.001), mainly due to anesthesiology services.
Periprocedural event rates, post-procedural length of stay, and overall costs for the index hospitalization are summarized in Table 2. Within the modified ITT population, rates of periprocedural stroke were higher for CAS, whereas rates of MI were higher for CEA. Mean post-procedure length of stay was 0.4 days longer in the CEA arm (3.0 versus 2.6 days; p=0.002), with significant differences in both Intensive Care Unit and non-Intensive Care Unit days. Post-procedural hospital costs were $363/patient higher for CEA ($7122 versus $6759; p=0.927). Overall costs for the initial hospitalization (including procedural, post-procedural, and physician costs) were slightly but non-significantly higher with CAS than with CEA ($15,055 versus $14,816; 95% CI for difference $297 less to $775 more, p=0.185)
Table 2.
Index Hospitalization Events, Resource Use, and Costs (Modified ITT Population)
| CAS (n=1213) | CEA (n=1193) | Difference (95% CI) |
P Value |
|
|---|---|---|---|---|
| Death, % | 0.3 | 0.2 | 0.2 (−0.2 to 0.6) | 0.687 |
| Stroke, % | 3.0 | 1.3 | 1.6 (0.5–2.8) | 0.006 |
| Major | 0.5 | 0.3 | 0.2 (−0.4 to 0.7) | 0.753 |
| Minor | 2.5 | 1.0 | 1.5 (0.4–2.5) | 0.006 |
| MI, % | 1.5 | 2.9 | −1.4 (−2.6 to −0.3) | 0.016 |
| Unplanned CAS, % | 0.2 | 0.0 | 0.2 (0.0–0.5) | 0.250 |
| Unplanned CEA, % | 0.1 | 0.3 | −0.3 (−0.6 to 0.1) | 0.215 |
| Post-Procedural LOS, d | ||||
| Non-ICU | 1.9 ± 3.2 [1] | 2.2 ± 4.1[1] | 0.2 (−0.55 to 0.06) | 0.053 |
| ICU | 0.7 ± 1.1 [0] | 0.8 ± 1.4 [1] | −0.1 (−0.24 to 0.04) | 0.002 |
| Total | 2.6 ± 3.3 [1] | 3.0 ± 4.5 [1] | −0.4 (−0.70 to 0.06) | 0.011 |
| Index hospitalization costs | ||||
| Procedural | $6782 ± 1412[6554] | $5743 ± 1370[5553] | $1039(926–1148) | <0.001 |
| Postprocedural | $6759 ± 4815[5012] | $7122 ± 6895[4926] | −$363(−831 to 107) | 0.927 |
| Physician fee | $1514 ± 446[1471] | $1951 ± 693[1876] | −$437(−485 to −394) | <0.001 |
| Total index hospitalization | $15 055 ± 5539[13 347] | $14 816 ± 7709[12 777] | $239(−302 to 778) | 0.185 |
Values in brackets are medians.
ITT indicates intention-to-treat; CAS, carotid stenting; CEA, carotid endarterectomy; MI, myocardial infarction; LOS, length of stay; ICU, intensive care unit.
One-Year Clinical Outcomes and Costs
One-year rates of rehospitalization for major cardiovascular events and their associated costs are shown in Table 3. Overall rates of hospitalization for repeat carotid revascularization (either by CAS or CEA) did not differ significantly for the CAS versus CEA groups. As a result, mean follow-up costs did not differ significantly between the CAS and CEA groups ($1321 versus $1293; p=0.441). Total one-year costs including the initial revascularization procedures were $267/patient higher for CAS, but this difference was not statistically significant ($16,375 versus $16,108; 95% CI for difference $366 less to $961 more, p=0.223).
Table 3.
One-Year Follow-Up Events and Costs (Modified ITT Population)
| CAS (n=1213) |
CEA (n=1193) |
Difference (95% CI) |
P Value |
|
|---|---|---|---|---|
| Events after initial hospitalization | ||||
| Stroke, % | 3.0 | 2.1 | 0.9 (−0.5 to 2.2) | 0.173 |
| Major, % | 1.6 | 1.5 | 0.1 (−0.9,1.0) | 0.907 |
| Minor, % | 1.4 | 0.6 | 0.8 (−0.0 to–1.6) | 0.044 |
| MI,* % | 0.3 | 0.5 | −0.2 (−0.7 to 0.3) | 0.545 |
| Repeat revascularization, % | 4.2 | 5.8 | −1.6 (−3.3 to 0.2) | 0.076 |
| CAS, % | 2.1 | 1.8 | 0.3 (−0.8 to 1.4) | 0.598 |
| CEA, % | 2.1 | 3.9 | −1.8 (−3.2 to −0.4) | 0.010 |
| Death, % | 1.0 | 0.7 | 0.3 (−0.4 to 1.0) | 0.388 |
| TIA, % | 0.9 | 0.5 | 0.4 (−0.3 to 1.1) | 0.236 |
| Costs to 1 y | ||||
| Total index hospitalization | $15 055 ± 5539[13 347] | $14 816 ± 7709[12 777] | $239(−302 to 778) | 0.185 |
| Discharge to 1 y | $1321 ± 4827[0] | $1293 ± 4502[0] | $28(−335 to 396) | 0.441 |
| Cumulative to 1 y | $16 375 ± 7730[13 637] | $16 108 ± 9030[13 112] | $267(−366 to 961) | 0.223 |
Values in brackets are medians.
ITT indicates intention-to-treat; CAS, carotid stenting; CEA, carotid endarterectomy; MI, myocardial infarction; TIA, transient ischemic attack.
MI was reported only when it occurred within 30 d of index procedure.
Utility Weights and In-Trial Quality-Adjusted Life Expectancy
There were small but statistically significant differences in utility weights favoring CAS at both the 2-week and - month timepoints, but no differences at 1 year (Table 4). Quality-adjusted life expectancy over the first year of follow-up was virtually identical for the CAS and CEA groups (0.704 versus 0.708, mean difference 0.005; 95% CI for difference −0.006 to 0.153; p=0.403). Linear regression analysis demonstrated that the occurrence of major stroke was associated with a significant reduction in utilities among surviving patients, with disutility values of 0.10 at 1 month and 0.06 at 12 months. Minor stroke also had a sustained effect on follow-up utilities, with estimated disutility values of 0.02 at 1 month and 0.03 at 12 months. Neither MI nor cranial nerve injury had a detectable impact on utilities at either timepoint.
Table 4.
Utility Weights and Quality-Adjusted Life-Years Over the First Year of Follow-up
|
Time Point |
CAS (n=1213) |
CEA (n=1193) |
Mean difference (95% CI) |
P Value |
|---|---|---|---|---|
| Baseline utility | 0.697 ± 0.130 | 0.702 ± 0.126 | −0.005 (−0.015 to 0.005) | 0.347 |
| 2-wk utility | 0.710 ± 0.129 | 0.695 ± 0.120 | 0.015 (0.005– 0.025) | 0.004 |
| 1-mo utility | 0.716 ± 0.312 | 0.699 ± 0.120 | 0.017 (0.006–0.027) | 0.001 |
| 1-y utility | 0.717 ± 0.123 | 0.720 ± 0.123 | −0.003 (−0.013 to 0.007) | 0.579 |
| QALYs at 1 y | 0.704 ± 0.142 | 0.708 ± 0.125 | −0.005 (−0.015 to 0.006) | 0.403 |
CEA indicates carotid endarterectomy; CAS, carotid artery stenting; QALYs, quality-adjusted life-years.
Cost-Effectiveness Analysis
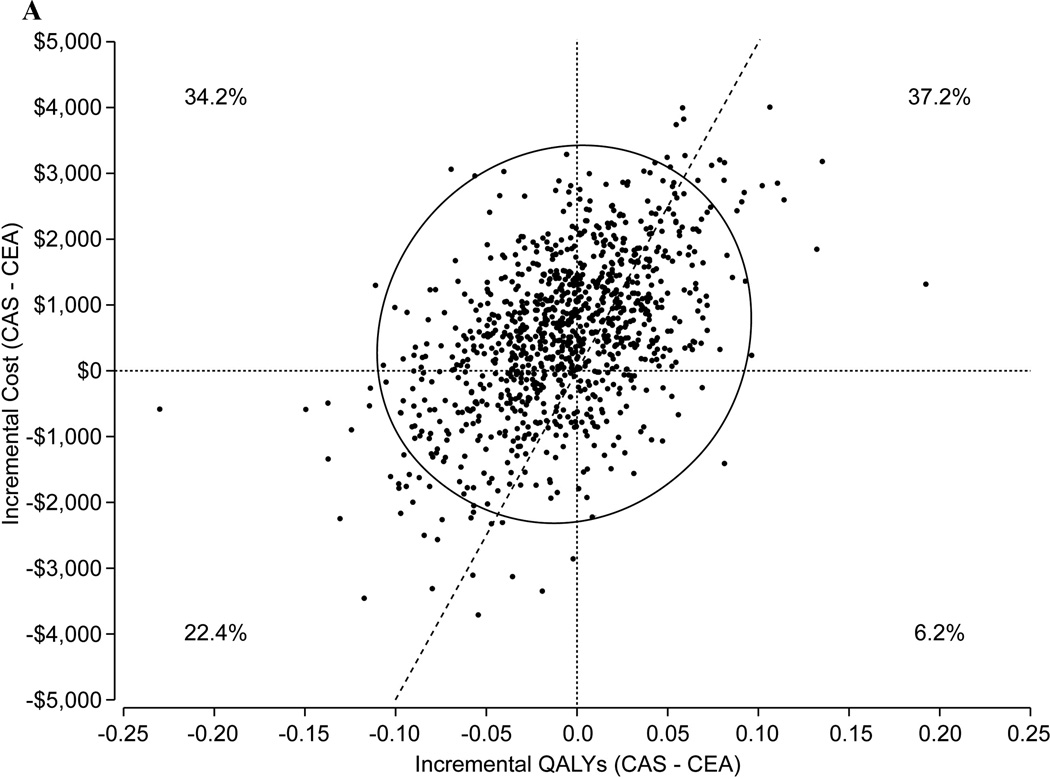
Based on the observed 1-year trial results alone, CAS was associated with a small increase in cost ($267) and a small decrease in QALYs (0.005), rendering CEA an economically dominant therapy (i.e., associated with both lower cost and greater effectiveness than CAS) in the short term. The results of our 10-year cost-effectiveness analysis based on the trial-based Markov model are summarized in Table 5 and Figures 1A–1C. For an overall population similar to that enrolled in CREST (mean age 69 years, 53% symptomatic), our model projected that over a 10-year time horizon CEA would be an economically dominant strategy with net cost savings of $524 and an increase in quality-adjusted life expectancy of 0.008 years. Probabilistic sensitivity analysis demonstrated considerable uncertainty in the long-term results, however (Figure 1A). CEA was economically dominant in 34.2% of bootstrap replicates, and CAS was economically dominant in 6.2%. The probability that CEA was economically attractive at a willingness-to-pay threshold of $50,000/QALY gained was 54.4%.
Table 5.
Projected 10-Y Costs, Quality-Adjusted Life-Years, and Cost-Effectiveness Ratios Based on the Markov Model
| Population | Cost | QALYs | Δ Cost |
Δ QALYs |
ICER (CAS versus CEA) |
CEA Dominant* |
CEA Preferred† |
CAS Dominant* |
CAS Preferred† |
|---|---|---|---|---|---|---|---|---|---|
| Overall | |||||||||
| CAS | $80141 | 4.841 | $524 | −0.008 | CEA | 38.6% | 54.9% | 16.5% | 45.1% |
| CEA | $79617 | 4.849 | … | … | Dominant | ||||
| ASX | |||||||||
| CAS | $80314 | 4.862 | $609 | 0.002 | $277 249/QALY | 37.3% | 54.3% | 17.0% | 45.7% |
| CEA | $79705 | 4.859 | … | … | Gained | ||||
| SX | |||||||||
| CAS | $79988 | 4.823 | $448 | −0.017 | CEA | 40.4% | 56.8% | 15.1% | 43.2% |
| CEA | $79540 | 4.840 | … | … | Dominant |
QALYs indicates quality-adjusted life-years; Δ, difference between CAS and CEA strategies;ICER, incremental cost-effectiveness ratio;CAS, carotid stenting; CEA, carotid endarterectomy; ASX, asymptomatic; SX, symptomatic.
Associated with both lower cost and greater effectiveness.
Economically preferred at a societal willingness-to-pay threshold of $50 000/QALY gained.
Figure 1.
A. Scatterplot of the joint distribution of cost and QALY differences between CAS and CEA for a CREST-like population (mean age 69 years, 53% symptomatic) based on probabilistic sensitivity analysis (1000 replications). The probability that the cost and QALY difference is in each of the 4 quadrants is indicated. The diagonal line through the origin represents a cost-effectiveness threshold of $50,000/QALY gained. Each point below and to the right of this line represents an estimate derived from one model-based simulation for which CAS was found to have an estimated cost-effectiveness ratio less than the threshold ratio of $50,000 per QALY gained; points above and to the left of the line correspond to model based estimates in which the incremental cost-effectiveness ratio for CAS relative to CEA was >$50,000/QALY. The probability that CEA was economically attractive at this threshold was 54.4%. Points falling within the upper left-hand quadrant correspond to model-based results for which CAS was found to be dominated by CEA associated with both increased costs and lower effectiveness. Points falling within the lower right-hand quadrant correspond to results for which CAS was found to be a dominant therapeutic approach associated with both lower costs and increased effectiveness in terms of QALYs gained. The ellipse indicates the 95% credible interval for the joint distribution.
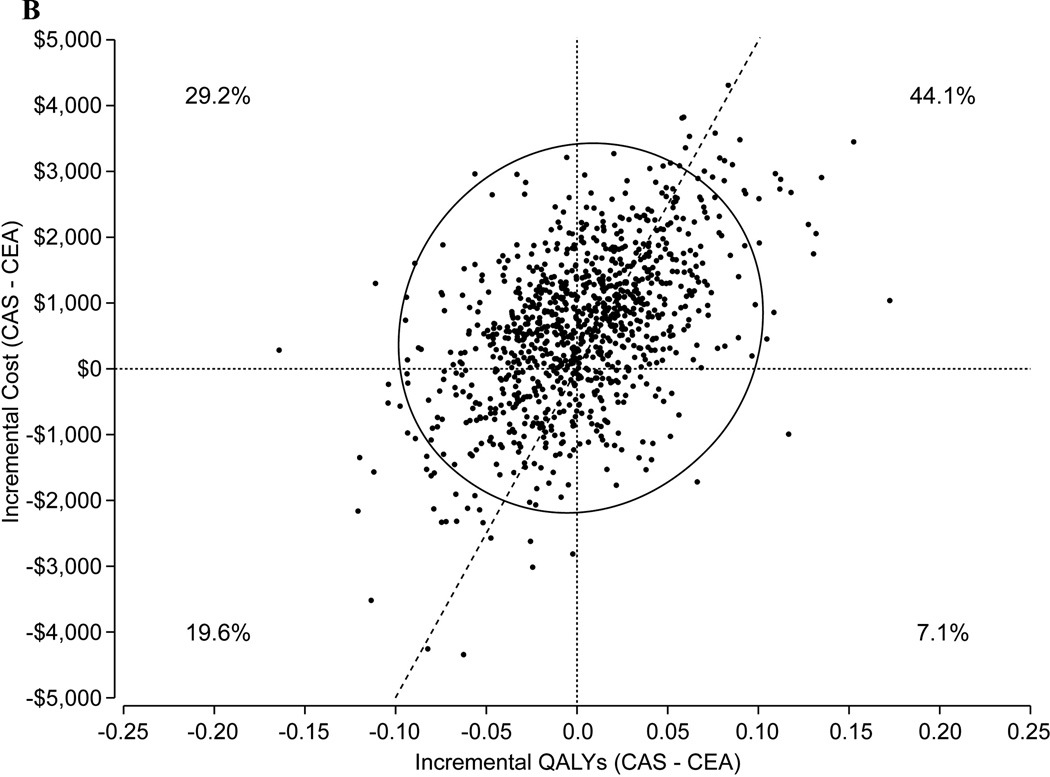
B. Scatterplot of the joint distribution of cost and QALY differences between CAS and CEA for a 100% asymptomatic population based on probabilistic sensitivity analysis (1000 replications). The probability that the cost and QALY difference is in each of the 4 quadrants is indicated. The probability that CEA was economically attractive at this threshold was 51.4%.
C. Scatterplot of the joint distribution of cost and QALY differences between CAS and CEA for a 100% symptomatic population based on probabilistic sensitivity analysis (1000 replications). The probability that the cost and QALY difference is in each of the 4 quadrants is indicated. The probability that CEA was economically attractive at this threshold was 56.3%.
CEA indicates carotid endarterectomy; CAS, carotid artery stenting; QALY, quality-adjusted life-year.
For symptomatic patients, CEA was projected to be a dominant approach with projected cost savings of $448/patient and a gain in quality-adjusted life expectancy of 0.017 years in the base case analysis. The probability that CEA was economically attractive at a willingness-to-pay threshold of $50,000/QALY gained was 56.3% (Figure 1B). For asymptomatic patients, CAS was associated with a 0.002 QALY benefit and an incremental cost of $609, yielding an estimated ICER of $277,249/QALY gained with CAS versus CEA. The probability that CAS would be economically attractive at a willingness-to-pay threshold of $50,000/QALY gained was 48.6% (Figure 1C). Analyses restricted to shorter time horizons demonstrated similar findings although the results were somewhat more robust. For example, over a 5-year time horizon, the probability that CEA would be economically attractive at a $50,000/QALY threshold was 71.3%.
DISCUSSION
Although several previous studies have compared CAS versus CEA for patients with carotid artery disease at standard surgical risk,24, 25 CREST is the first trial to incorporate a comprehensive health economic evaluation. In this study, we collected detailed medical resource use and quality-of-life data on the complete trial population and hospital billing data on a large subset. Results from analysis of these data revealed that both initial treatment costs and total 1-year costs were slightly higher for CAS versus CEA, driven largely by higher initial procedural costs. Results from the analysis of quality-of-life26 and health state use data tended to favor CAS during the first month of follow-up but revealed no differences thereafter. Based on these findings, formal cost-effectiveness analysis demonstrated that any projected cost and QALY differences between CAS and CEA were small (<0.02 QALYs and <$650), such that the resulting ICERs were unstable with ~50% probability of economically attractive results (i.e. ICER <$50,000/QALY gained) with either treatment. Similar results were observed when the analysis was restricted to symptomatic or asymptomatic patients. These findings suggest that for patients similar to those enrolled in the CREST trial, there is little reason to strongly prefer either CAS or CEA on economic grounds, and that other factors such as individual patient preferences and center-specific outcomes should be considered when choosing between these two approaches to carotid revascularization.
Comparison With Previous Studies
Several previous studies have compared initial treatment costs for CAS versus CEA.27–31 These studies have consistently shown that procedural costs are higher with CAS than with CEA, due almost entirely to the cost of disposable devices (e.g. stents, embolic protection devices, etc.). In a single-center randomized trial of 104 patients, Brooks and colleagues27 found that variable costs were approximately $600/patient higher with CAS. Similar to our results, they observed modest cost savings for post-procedural care that were insufficient to offset the substantial increase in procedural costs with CAS. In a single-center observational study of 62 patients, Pawaskar and colleagues31 found that procedural costs were nearly $5000/patient greater with CAS than CEA with only minimal reductions in other hospital costs; as a result, the cost of CAS exceeded that of CEA by >$4000/patient. Using data from their hospital’s cost accounting system, Sternberg and colleagues32 also reported hospital costs that were >$3000/patient higher with CEA than CAS. On the other hand, several other single-center studies have demonstrated substantial reductions in lengths of stay with CAS with either similar28 or lower total hospital costs.29
It is difficult to reconcile the differences between the results of previous single-center cost studies and those of the CREST trial. The single-center studies are limited by relatively small sample sizes (generally <200 patients), and lack of randomization, and limited description of costing methodology. In contrast, CREST included 2502 patients randomized at 117 sites, and incorporated costing methods based on measured resource use. The CREST costing methodology was similar to that used in a variety of other cardiovascular trials33–35 and accounted for the full costs of treatment (including allocated hospital overhead). Current guidelines generally favor this approach for informing decisions regarding societal resource allocation, since all costs are variable in the long run.36 On the other hand, studies based on hospitals’ internal accounting systems tend to focus on variable costs, which are more appropriate for short-term resource allocation decisions at the hospital level.
In the only other multicenter study to date, McDonald and colleagues17 used data from the National Inpatient Sample to estimate hospital costs for nearly 200,000 patients who underwent either CAS or CEA between 2001 and 2008. They found that hospital costs were nearly $5000/patient higher with CAS than CEA in this unselected population — a difference that was explained at least in part by much higher rates of comorbidities and complications among patients treated with CAS. In addition, they estimated patient-level costs based on whole hospital cost to charge ratios. This approach has been shown to be less precise than our approach incorporating both resource-based costs (for procedures) and department-specific cost to charge ratios (for non-procedural care) — particularly when the admission involves a high cost procedure. Finally, their study did not consider physician costs, which are substantially higher with CEA (due to anesthesiology services).
Comparison With Other Populations
Several previous studies have examined the relative cost-effectiveness of CAS versus CEA for patients at high risk of surgical complications. Maud and colleagues37 used published, aggregate data from the SAPPHIRE trial to estimate the cost-effectiveness of CAS versus CEA for high-risk surgical patients. Over a 1-year follow-up period, they estimated that the ICER for CAS versus CEA was approximately $67,000/QALY gained. Mahoney and colleagues13, using prospectively collected patient-level resource use from the SAPPHIRE trial, reported somewhat different findings. Although procedural costs were substantially higher with CAS than with CEA, there were substantial reductions in other hospital costs such that total costs for the index hospitalization were only $500/patient higher with CAS. Lifetime projections of cost and life expectancy suggested that CAS was an economically attractive strategy for high risk patients, with a lifetime ICER of approximately $65,000/QALY gained compared with CEA.
The results of the CREST economic analysis differ from those from the SAPPHIRE trial because of differences in the patient populations as well as clinical outcomes. In SAPPHIRE, patients were required to be at high risk of complications from CEA because of either anatomic challenges or extensive comorbidity. In that population, CAS resulted in substantial reductions in hospital length of stay as well as better clinical outcomes at 1-year, including reduced long-term mortality.14 Consequently, despite its higher cost, CAS was an economically attractive strategy for the high-risk population. In contrast, CREST enrolled a much lower-risk patient population and demonstrated relatively small differences between treatment groups in clinical outcomes, and no long-term differences in survival or quality of life. With only minimal differences in both long-term cost and quality-adjusted life expectancy, it is not surprising that neither CAS nor CEA was clearly preferred on economic grounds. The relative cost-effectiveness of these alternative procedures therefore appears to depend heavily on patient characteristics and, in particular, the likelihood of ischemic complications (death, stroke, or MI) with the 2 procedures.
Study Limitations
Our study should be considered in light of the following limitations. First, our analysis was performed from the perspective of the U.S. healthcare system and may not reflect the perspective of any other health system or payor whose costs may differ from those we observed. In addition, the costs we applied to resource use for our analysis included both fixed and variable costs. Although there is general agreement that this approach is appropriate when considering a long-term decision (because virtually all costs are variable in the long run), for short-term resource allocation, variable costs may be a closer representation of the true opportunity costs of each treatment strategy.36 Because disposable costs (which are variable even in the short term) represent a larger proportion of costs for the CAS group, restriction of our analysis to variable costs (which would have required access to each hospital’s internal accounting data) would likely have resulted in a greater cost difference between the 2 groups in favor of CEA.
Third, although CREST enrolled patients at more than 100 centers, the sites and operators were chosen on the basis of adequate volumes and outcomes (for CEA) as well as an extensive training and certification process (for CAS). Thus, it is uncertain whether the clinical results (and by extension, the economic outcomes) obtained in this trial would be representative of those in routine clinical practice. A recent study of carotid stenting in Medicare recipients revealed significantly higher mortality rates than those seen in CREST. 38
Finally, it is important to recognize that by design, the CREST trial and its economic analysis only compared 2 alternative revascularization procedures and did not include a parallel medical therapy group. Implicit in this analytic approach is an assumption that CEA is cost-effective relative to medical therapy for the CREST population. Although previous studies support the validity of this assumption for both symptomatic and asymptomatic patients,39 there continues to be substantial debate on this issue — particularly for asymptomatic patients.40 Only a dedicated clinical trial using both contemporary revascularization techniques and medical therapy can resolve this issue.
Conclusions
In a population of patients with carotid artery stenosis who were not at increased risk for surgical complications, CEA was associated with a slightly lower cost and slightly greater quality-adjusted life expectancy compared with CAS, but cost-effectiveness results were highly unstable. These findings, which were derived from a controlled clinical trial with careful credentialing of both surgeons and interventionalists, suggest that there is insufficient evidence at present to strongly favor one or the other treatment strategy on economic grounds.
Supplementary Material
ACKNOWLEDGMENTS
None
FUNDING SOURCES
Supported by the National Institute of Neurological Disorders and Stroke (NINDS) and the NIH (R01 NS 038384) with supplemental funding from Abbott Vascular, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Katherine Vilain: None
Elizabeth Magnuson: Research grants from Eli Lilly, Daiichi Sankyo, Sanofi-Aventis, and Bristol-Myers Squibb
Haiyan Li: None
Wayne Clark: None
Richard Begg: None
Albert Sam: None
Charles Sternbergh: None
Fred Weaver: None
William Gray: None
Jenifer Voeks: None
Thomas Brott: None
David Cohen: Research grants from Boston Scientific, Medtronic, Abbott Vascular, and Edwards Lifesciences. Consulting fees from Medtronic, Cordis, and Abbott Vascular.
REFERENCES
- 1.Caro JJ, Ishak KJ, Migliaccio-Walle K. Estimating survival for cost-effectiveness analyses: A case study in atherothrombosis. Value Health. 2004;7:627–635. doi: 10.1111/j.1524-4733.2004.75013.x. [DOI] [PubMed] [Google Scholar]
- 2.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–1836. [PubMed] [Google Scholar]
- 3.Gresham GE, Kelly-Hayes M, Wolf PA, Beiser AS, Kase CS, D'Agostino RB. Survival and functional status 20 or more years after first stroke: The Framingham Study. Stroke. 1998;29:793–797. doi: 10.1161/01.str.29.4.793. [DOI] [PubMed] [Google Scholar]
- 4.Lee WC, Christensen MC, Joshi AV, Pashos CL. Long-term cost of stroke subtypes among medicare beneficiaries. Cerebrovasc Dis. 2007;23:57–65. doi: 10.1159/000096542. [DOI] [PubMed] [Google Scholar]
- 5.Leibson CL, Hu T, Brown RD, Hass SL, O'Fallon WM, Whisnant JP. Use of acute care services in the year before and after first stroke: A population-based study. Neurology. 1996;46:861–869. [PubMed] [Google Scholar]
- 6.Matchar D, Duncan PW. The cost of stroke. Natl Stroke Assoc. 1994;5:9–12. [Google Scholar]
- 7.Matchar DB. The value of stroke prevention and treatment. Neurology. 1998;51:S31–S35. doi: 10.1212/wnl.51.3_suppl_3.s31. [DOI] [PubMed] [Google Scholar]
- 8.Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke. The Framingham Study. Stroke. 1982;13:290–295. doi: 10.1161/01.str.13.3.290. [DOI] [PubMed] [Google Scholar]
- 9.Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: A medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–349. doi: 10.1161/01.str.30.2.338. [DOI] [PubMed] [Google Scholar]
- 10.Samsa GP, Matchar DB, Goldstein L, Bonito A, Duncan PW, Lipscomb J, et al. Utilities for major stroke: Results from a survey of preferences among persons at increased risk for stroke. Am Heart J. 1998;136:703–713. doi: 10.1016/s0002-8703(98)70019-5. [DOI] [PubMed] [Google Scholar]
- 11.Endarterectomy for asymptomatic carotid artery stenosis. Executive committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 12.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial collaborators. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney EM, Greenberg D, Lavelle TA, Natarajan A, Berezin R, Ishak KJ, et al. Costs and cost-effectiveness of carotid stenting versus endarterectomy for patients at increased surgical risk: Results from the SAPPHIRE trial. Catheter Cardiovasc Interv. 2011;77:463–472. doi: 10.1002/ccd.22869. [DOI] [PubMed] [Google Scholar]
- 14.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 15.Brott TG, Hobson RW, II, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, et al. Design of the Carotid Revascularization Endarterectomy versus. Stenting Trial (CREST) Int J Stroke. 5:40–46. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taira DA, Seto TB, Siegrist R, Cosgrove R, Berezin R, Cohen DJ. Comparison of analytic approaches for the economic evaluation of new technologies alongside multicenter clinical trials. Am Heart J. 2003;145:452–458. doi: 10.1067/mhj.2003.3. [DOI] [PubMed] [Google Scholar]
- 18.Bureau of Labor Statistics. Consumer Price Index - All Urban Consumers: Medical Care. [Accessed 6/24/2011]; Available at: http://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths.
- 19.Centers for Medicare & Medicaid Services 100% MEDPAR Inpatient Hospital National Data for Fiscal Year 2008 Short Stay Inpatient Diagnosis Related Groups. [accessed Jan 22, 2010]; Available at: https://www.cms.gov/MedicareFeeforSvcPartsAB/03_MEDPAR.asp#TopOfPage.
- 20.Mahoney EM, Jurkovitz CT, Chu H, Becker ER, Culler S, Kosinski AS, et al. Cost and cost-effectiveness of an early invasive versus conservative strategy for the treatment of unstable angina and non-st-segment elevation myocardial infarction. Jama. 2002;288:1851–1858. doi: 10.1001/jama.288.15.1851. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JB, McCall NT, Burge RT, Boyce S. Health Economics Research, Inc.; 1995. Per case prospective payment for episodes of hospital care. Report #500-92-0020. [Google Scholar]
- 22.Efron B, Tibshirani RJ. An introduction to the bootstrap. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- 23.Lipscomb J, Weinstein MC, Torrance GW. Time preference. In: Gold M, Siegel J, Russell L, Weinstein M, editors. Cost-effectiveness in health and medicine. New York City, NY: Oxford University Press; 1996. pp. 216–235. [Google Scholar]
- 24.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 25.Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: A randomised non-inferiority trial. Lancet. 2006;368:1239–1247. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 26.Cohen DJ, Stolker JM, Wang K, Magnuson EA, Clark WM, Demaerschalk BM, et al. Health-related quality of life after carotid stenting versus carotid endarterectomy: Results from CREST (Carotid Revascularization Endarterectomy versus Stenting Trial) J Am Coll Cardiol. 2011;58:1557–1565. doi: 10.1016/j.jacc.2011.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy: Randomized trial in a community hospital. J Am Coll Cardiol. 2001;38:1589–1595. doi: 10.1016/s0735-1097(01)01595-9. [DOI] [PubMed] [Google Scholar]
- 28.Ecker RD, Brown RD, Jr, Nichols DA, McClelland RL, Reinalda MS, Piepgras DG, et al. Cost of treating high-risk symptomatic carotid artery stenosis: Stent insertion and angioplasty compared with endarterectomy. J Neurosurg. 2004;101:904–907. doi: 10.3171/jns.2004.101.6.0904. [DOI] [PubMed] [Google Scholar]
- 29.Gray WA, White HJ, Jr, Barrett DM, Chandran G, Turner R, Reisman M. Carotid stenting and endarterectomy: A clinical and cost comparison of revascularization strategies. Stroke. 2002;33:1063–1070. doi: 10.1161/hs0402.105304. [DOI] [PubMed] [Google Scholar]
- 30.Park B, Mavanur A, Dahn M, Menzoian J. Clinical outcomes and cost comparison of carotid artery angioplasty with stenting versus carotid endarterectomy. J Vasc Surg. 2006;44:270–276. doi: 10.1016/j.jvs.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 31.Pawaskar M, Satiani B, Balkrishnan R, Starr JE. Economic evaluation of carotid artery stenting versus carotid endarterectomy for the treatment of carotid artery stenosis. J Am Coll Surg. 2007;205:413–419. doi: 10.1016/j.jamcollsurg.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Sternbergh WC, Crenshaw GD, Bazan HA. Carotid endarterectomy is more cost-effective than carotid artery stenting. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2011.12.045. (in press). [DOI] [PubMed] [Google Scholar]
- 33.Bakhai A, Stone GW, Grines CL, Murphy SA, Githiora L, Berezin RH, et al. Cost-effectiveness of coronary stenting and abciximab for patients with acute myocardial infarction: Results from the CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) trial. Circulation. 2003;108:2857–2863. doi: 10.1161/01.CIR.0000103121.26241.FA. [DOI] [PubMed] [Google Scholar]
- 34.Cohen DJ, Bakhai A, Shi C, Githiora L, Lavelle T, Berezin RH, et al. Cost-effectiveness of sirolimus-eluting stents for treatment of complex coronary stenoses: Results from the SIRolImUS-eluting balloon expandable stent in the treatment of patients with de novo native coronary artery lesions (SIRIUS) trial. Circulation. 2004;110:508–514. doi: 10.1161/01.CIR.0000136821.99814.43. [DOI] [PubMed] [Google Scholar]
- 35.Cohen DJ, Cosgrove RS, Berezin RH, Teirstein PS, Leon MB, Kuntz RE. Cost-effectiveness of gamma radiation for treatment of in-stent restenosis: Results from the Gamma-1 trial. Circulation. 2002;106:691–697. doi: 10.1161/01.cir.0000023625.12626.29. [DOI] [PubMed] [Google Scholar]
- 36.Luce BR, Manning WG, Siegle JE, Lipscomb J. Estimating costs in cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York City, NY: Oxford University Press; 1996. pp. 176–213. [Google Scholar]
- 37.Maud A, Vazquez G, Nyman JA, Lakshminarayan K, Anderson DC, Qureshi AI. Cost-effectiveness analysis of protected carotid artery stent placement versus endarterectomy in high-risk patients. J Endovasc Ther. 2010;17:224–229. doi: 10.1583/09-2938.1. [DOI] [PubMed] [Google Scholar]
- 38.Nallamothu BK, Gurm HS, Ting HH, Goodney PP, Rogers MA, Curtis JP, et al. Operator experience and carotid stenting outcomes in Medicare beneficiaries. JAMA. 2011;306:1338–1343. doi: 10.1001/jama.2011.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuntz KM, Kent KC. Is carotid endarterectomy cost-effective? An analysis of symptomatic and asymptomatic patients. Circulation. 1996;94:194–198. [PubMed] [Google Scholar]
- 40.Chaturvedi S. Should the multicenter carotid endarterectomy trials be repeated? Arch Neurol. 2003;60:774–775. doi: 10.1001/archneur.60.5.774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.