Abstract
LARGE-dependent modification enables α-dystroglycan (α-DG) to bind to its extracellular matrix ligands. Mutations in the LARGE gene and several others involved in O-mannosyl glycan synthesis have been identified in congenital and limb-girdle muscular dystrophies that are characterized by perturbed glycosylation and reduced ligand-binding affinity of α-DG. LARGE is a bifunctional glycosyltransferase that alternately transfers xylose and glucuronic acid, thereby generating the heteropolysaccharides on α-DG that confer its ligand binding. Although the LARGE paralog LARGE2 (also referred to as GYLTL1B) has likewise been shown to enhance the functional modification of α-DG in cultured cells, its enzymatic activities have not been identified. Here, we report that LARGE2 is also a bifunctional glycosyltransferase and compare its properties with those of LARGE. By means of a high-performance liquid chromatography-based enzymatic assay, we demonstrate that like LARGE, LARGE2 has xylosyltransferase (Xyl-T) and glucuronyltransferase (GlcA-T) activities, as well as polymerizing activity. Notably, however, the pH optima of the Xyl-T and GlcA-T of LARGE2 are distinct from one another and also from those of LARGE. Our results suggest that LARGE and LARGE2 catalyze the same glycosylation reactions for the functional modification of α-DG, but that they have different biochemical properties.
Keywords: dystroglycan, glucuronyltransferase, LARGE, muscular dystrophy, xylosyltransferase
Introduction
Dystroglycan (DG) is a basement membrane receptor composed of an extracellular α subunit and a transmembrane β subunit, and is involved in maintenance of the integrity of the skeletal muscle membrane, as well as the structure and the function of the central nervous system (Barresi and Campbell 2006). α-dystroglycan (α-DG) is highly decorated with O-glycans, and modification with a specific, unusual O-mannosyl glycan is essential for its binding to laminin-G domain-containing extracellular matrix (ECM) ligands, such as laminin, perlecan and agrin in muscle (Ibraghimov-Beskrovnaya et al. 1992; Gee et al. 1994; Talts et al. 1999), neurexin in the brain (Sugita et al. 2001) and pikachurin in the retina (Sato et al. 2008). Notably, the same modification is required for the binding of α-DG to Old World arenaviruses (Cao et al. 1998).
Several lines of evidence have demonstrated that defects in O-mannosyl glycan synthesis cause congenital muscular dystrophies with brain abnormalities that are characterized by perturbed α-DG glycosylation and a reduction in the ability of α-DG to bind ligands (referred to as the dystroglycanopathies) (Godfrey et al. 2011). First, mutations in genes encoding known and putative glycosyltransferases involved in O-mannosyl glycan synthesis have been identified in dystroglycanopathy patients (Barresi and Campbell 2006). The affected proteins include: The like-acetylglucosaminyltransferase (LARGE), protein O-mannosyltransferase 1 (POMT1), POMT2, protein O-mannosyl β-1,2-N-acetylglucosaminyltransferase 1 (POMGnT1), fukutin and fukutin-related protein (FKRP). In addition, it has been recently found that mutations in ISPD (isoprenoid synthase domain containing) cause Walker–Warburg syndrome, a type of dystroglycanopathy, and defective O-mannosylation of α-DG (Roscioli et al. 2012; Willer et al. 2012). Secondly, the LARGE mutation present in the Largemyd mouse model of muscular dystrophy results in defective α-DG glycosylation (Grewal et al. 2001). Thirdly, laminin-binding of α-DG requires phosphorylation of O-linked mannose, and LARGE, fukutin and FKRP have all been implicated in the modification pathway that assembles the laminin-binding moiety on this phosphorylated O-mannosyl glycan (Yoshida-Moriguchi et al. 2010).
We recently reported that LARGE is a bifunctional glycosyltransferase that alternately transfers xylose (Xyl) and glucuronic acid (GlcA) to α-DG, to form a polysaccharide that confers the ability to bind ligands (Inamori et al. 2012). Notably, overexpression of LARGE enhances the functional modification of α-DG in cultured cells (Barresi et al. 2004; Kanagawa et al. 2004). Overexpression of LARGE2, a paralog of LARGE, has been shown to enhance the modification of α-DG to a similar degree (Brockington et al. 2005; Fujimura et al. 2005; Grewal et al. 2005). LARGE2 has been cloned from human kidney, mouse kidney and mouse testis RNA, and the two paralogs have different tissue expression profiles: LARGE is widely expressed, with levels highest in the brain, heart and skeletal muscle; and LARGE2 is highly expressed in the kidney and the placenta, but not in the brain (Fujimura et al. 2005; Grewal et al. 2005). In spite of this characterization of LARGE2 expression, neither its contribution to the functional modification of α-DG in vivo nor its enzymatic activities have been assessed.
Here, we demonstrate that LARGE2 has xylosyltransferase (Xyl-T) and glucuronyltransferase (GlcA-T) activities as well as polymerizing activity, and in these respects it resembles LARGE. However, we also show that the pH optima for the Xyl-T and GlcA-T activities are distinct and that they differ significantly from those of LARGE. These results suggest that both LARGE paralogs are involved in the functional modification of α-DG, but that they may be fine-tuned to function optimally in different physiological contexts.
Results
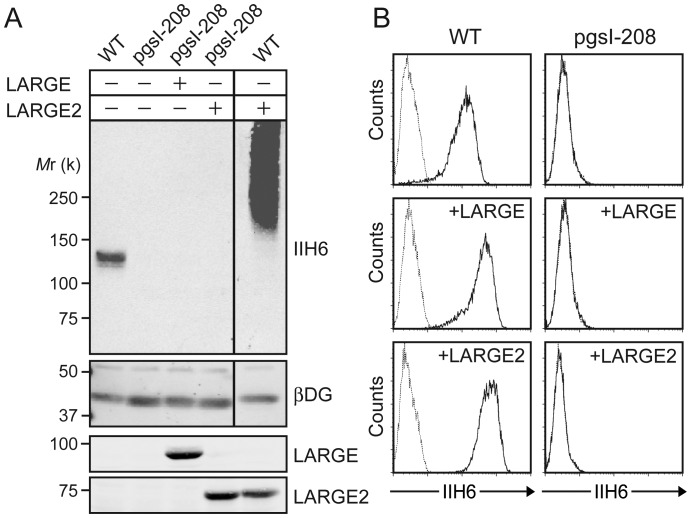
Cellular xylosylation is essential for the functional modification of α-DG as it has been shown that uridine 5′-diphosphate (UDP)-xylose synthase 1 (UXS1)-deficient Chinese hamster ovary (CHO) cells (line pgsI-208) that lack UDP-Xyl (Bakker et al. 2009) have a defect in the modification of α-DG, and overexpression of LARGE in the cells did not rescue the defect (Inamori et al. 2012). We examined whether overexpression of LARGE2 could rescue the defect or not, by immunoblotting of glycoproteins and flow cytometry for cell surface staining with IIH6 (an antibody that recognizes the laminin-binding form of α-DG). When LARGE2 was overexpressed in the wild-type (WT) CHO cells, a robust increase in the functional modification was observed (Figure 1A and Supplementary data, Figure S1), as has been reported previously (Brockington et al. 2005; Fujimura et al. 2005; Grewal et al. 2005). In contrast, overexpression of LARGE2 did not overcome the defect in pgsI-208 cells as is also the case with LARGE overexpression (Figure 1A and B), which is consistent with our previous observation. Thus, cellular xylosylation is required for the LARGE2-dependent functional modification of α-DG, as shown for LARGE.
Fig. 1.
Overexpression of the LARGE paralog does not overcome the glycosylation defect in UXS1-deficient (pgsI-208) CHO cells. (A) Immunoblotting of WGA-enriched glycoproteins for functionally modified α-DG (IIH6) and β-DG, or of cell lysates for LARGE and LARGE2, from WT or pgsI-208 cells with or without overexpression of either LARGE or LARGE2. Mr, relative molecular mass. (B) Flow cytometry of the cells for surface staining with IIH6. Dotted line, secondary antibody alone.
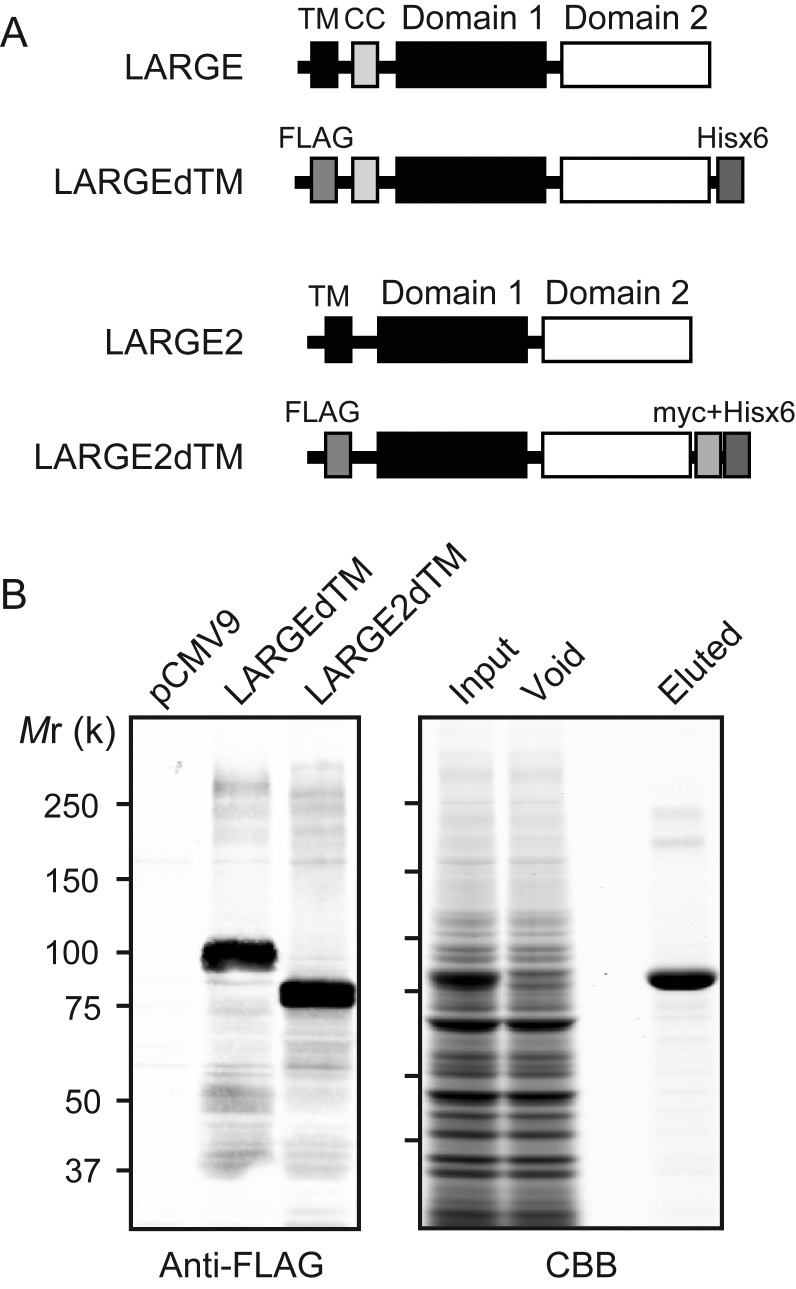
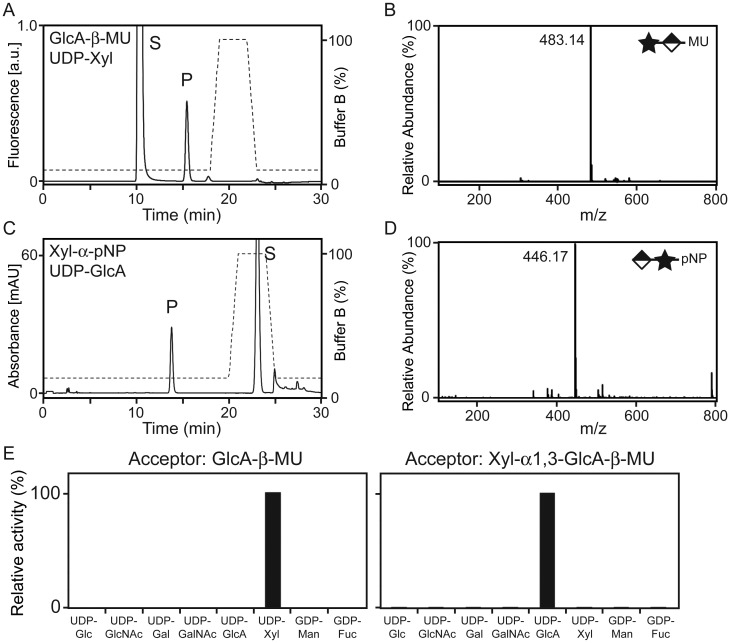
LARGE and LARGE2 share two conserved glycosyltransferase-like domains: One that belongs to glycosyltransferase family 8 (GT8) proteins (Coutinho et al. 2003) and one that is shared with β1,3-N-acetylglucosaminyltransferase 1, an enzyme required for poly-N-acetyllactosamine synthesis (Sasaki et al. 1997; Peyrard et al. 1999). In addition, the DXD motifs required for the LARGE function are conserved in LARGE2 (Brockington et al. 2005; Inamori et al. 2012). It has been demonstrated that all the three DXD motifs are required for LARGE to be functional (Brockington et al. 2005), and single DXD mutants in different domains do not complement one another biochemically (Aguilan et al. 2009). To investigate the glycosyltransferase activities of LARGE2, we generated a secreted form that lacks the transmembrane domain (LARGE2dTM) and includes both an N-terminal FLAG and C-terminal myc + Hisx6 tags (Figure 2A). Purified LARGE2dTM (Figure 2B) was tested for in vitro enzymatic assay using 4-methylumbelliferyl-β-d-glucuronide (GlcA-β-MU) or p-nitrophenyl-α-d-xyloside (Xyl-α-pNP) as acceptors, and the products were analyzed by high-performance liquid chromatography (HPLC) using an LC-18 column as described previously (Inamori et al. 2012). When GlcA-β-MU was used as the acceptor and UDP-Xyl as the donor, a unique peak was generated by adding LARGE2dTM (Figure 3A). This peak was eluted at the same retention time as the product resulting from catalysis by LARGEdTM (Supplementary data, Figure S2A). When this product was purified and analyzed by mass spectrometry (MS), the MS/MS fragmentation pattern of a parent ion at a mass/charge ratio (m/z) of 483.14 [M–H]− confirmed that Xyl was attached to GlcA-β-MU (Figure 3B and Supplementary data, Figure S3A). When Xyl-α-pNP was used as the acceptor and UDP-GlcA as the donor (Figure 3C), a unique peak appeared and the retention time was the same as that of the LARGEdTM product (Supplementary data, Figure S2B). MS analysis confirmed that GlcA was attached to Xyl-α-pNP (Figure 3D and Supplementary data, Figure S3B).
Fig. 2.
Purification of LARGE2dTM. (A) Schematic representation of LARGE and the LARGEdTM construct as described previously (Inamori et al. 2012), and LARGE2 and the LARGE2dTM construct used in the enzymatic activity assay. The transmembrane (TM) sequence of LARGE2 was replaced with a FLAG (3x) tag sequence, and the C-terminus was modified with myc and Hisx6 tags. CC, coiled-coil domain. (B) Expression and purification of test proteins. (Left) Immunoblotting with anti-FLAG antibody. For each stable clone, 50 μL of the culture medium was analyzed. pCMV9, cell clone obtained by transfection of empty vector. (Right) Purification of recombinant LARGE2dTM protein expressed in the culture medium. Proteins were run over the Talon metal affinity resin and eluted, and the fractions were analyzed by Coomassie brilliant blue (CBB) staining.
Fig. 3.
LARGE2 is a bifunctional glycosyltransferase with Xyl-T and GlcA-T activities. (A) HPLC elution profile from the LC-18 column of the products obtained from the reaction of LARGE2dTM with GlcA-β-MU and UDP-Xyl. S, unreacted substrate. P, product. Dashed line, %Buffer B. (B) Q/TOF-MS analysis of the product peak detected in (A). Star and diamond indicate Xyl and GlcA, respectively. The MS/MS fragmentation pattern (Supplementary data, Figure S3A) confirmed that the ion with an m/z of 483.14 [M–H]− is GlcA-β-MU with an added Xyl. (C) HPLC elution profile from the LC-18 column of the products obtained from the reaction of LARGE2dTM with Xyl-α-pNP and UDP-GlcA. (D) Q/TOF-MS analysis as in (B), for the product isolated from the reaction analyzed in (C). The MS/MS fragmentation pattern (Supplementary data, Figure S3B) confirmed that the ion with an m/z of 446.17 [M–H]− is Xyl-α-pNP with an added GlcA. (E) Donor substrate specificity of LARGE2dTM. Representative data from two independent assays, showing relative activity (%) of Xyl-T toward GlcA-β-MU and of GlcA-T toward Xyl-α1,3-GlcA-β-MU. No other sugars were transferred to the acceptors.
We next tested the ability of LARGE2 to modify substrate with other sugars. However, the acceptor substrate pair used for the experiments described above were not ideal because the nonfluorescent Xyl-α-pNP acceptor (a fluorescent α-linked xyloside was not available at the time of the study) used to analyze GlcA-T activity was not as sensitive as the fluorescent acceptor GlcA-β-MU used for Xyl-T activity. Thus, we instead used Xyl-α1,3-GlcA-β-MU, the product of the reaction of LARGEdTM with GlcA-β-MU and UDP-Xyl (Inamori et al. 2012), as the acceptor in this analysis. Testing of various donor sugars for LARGE2dTM activity in conjunction with the acceptors GlcA-β-MU and Xyl-α1,3-GlcA-β-MU confirmed that LARGE2 transfers Xyl and GlcA, respectively, but no other sugars (Figure 3E). Thus, LARGE2 is a bifunctional glycosyltransferase that, like LARGE, has both Xyl-T and GlcA-T activities.
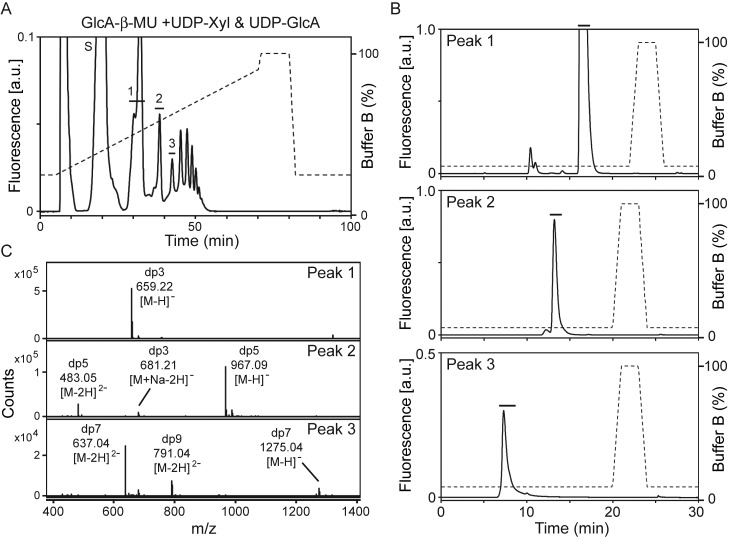
We also assessed LARGE2 for the polymerizing activity necessary to generate polysaccharide on the acceptor. To this end, we performed the LARGE2dTM reaction with GlcA-β-MU, using both UDP-Xyl and UDP-GlcA as donors, and then separated the products using an amide column. Multiple product peaks were generated (Figure 4A) as has been observed with LARGEdTM (Inamori et al. 2012). We isolated the product peaks (peaks 1–3) and further purified them by running them over an LC-18 column (Figure 4B). MS analysis of the peaks showed m/z values corresponding to those of the products expected to be modified by Xyl and GlcA (Figure 4C); thus, LARGE2 is also a polymerizing enzyme capable of synthesizing Xyl-GlcA repeats.
Fig. 4.
LARGE2dTM has polymerizing activity. (A) HPLC profile of the reaction products generated when LARGE2dTM was added to GlcA-β-MU in the presence of both donors, UDP-Xyl and UDP-GlcA, on the amide column GlycoSep N. Peaks labeled 1–3 were subjected to further purification in (B). S, unreacted substrate. Dashed line, %Buffer B. (B) Purification of GlcA-β-MU derivatives on an LC-18 column. Each peak isolated in (A) was purified and the main peak was collected (solid bar). (C) Q/TOF-MS analysis of the peaks isolated in (B). Assigned mass spectra in each peak confirmed that LARGE2dTM added repeating units of Xyl and GlcA onto the acceptor, GlcA-β-MU. dp, degree of polymerization.
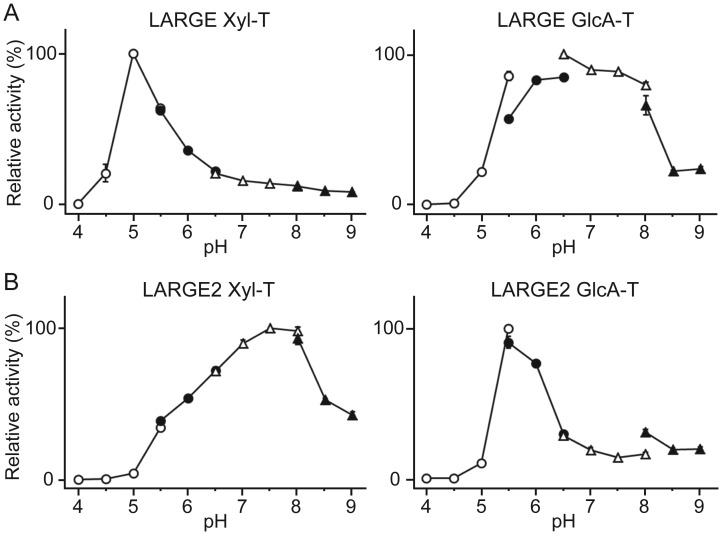
We assessed both LARGEdTM and LARGE2dTM for the pH optima of the Xyl-T and GlcA-T activities. Interestingly, in the case of LARGEdTM, Xyl-T activity was optimal at pH 5.0 but GlcA-T activity was efficient across a broad pH range (5.5–pH 8.0; Figure 5A). LARGE2dTM showed a distinctly different pattern, with a relatively broad effective pH range for Xyl-T activity (5.5–pH 9.0) but a sharp optimum with respect to the GlcA-T activity (pH 5.5; Figure 5B). Thus, each LARGE paralog has distinct enzymatic characteristics in spite of catalyzing the same glycosylation reactions.
Fig. 5.
LARGEdTM and LARGE2dTM have distinct pH optima for the Xyl-T and GlcA-T activities. Data from two independent experiments are shown as relative activity (%). The highest activity in the dataset was arbitrarily set at 100%. The Xyl-T and the GlcA-T assays were carried out using GlcA-β-MU and Xyl-α1,3-GlcA-β-MU as the acceptors, respectively. The buffers used were: Acetate for pH 4.0–5.5 (empty circles), MES for pH 5.5–6.5 (closed circles), MOPS for pH 6.5–8.0 (empty triangles) and Tris–HCl for pH 8.0–9.0 (closed triangles). The details of the conditions are presented in the Materials and methods section.
Discussion
The high degree of similarity between LARGE and LARGE2 with respect to sequence and domain structure, as well as the fact that both proteins enhance the functional modification of α-DG, suggested that LARGE2 likely has the same glycosyltransferase activities as LARGE. In this study, we have demonstrated that LARGE2 indeed has Xyl-T and GlcA-T activities and polymerizing activity in vitro, using an enzymatic assay with recombinant LARGE2dTM protein.
The Xyl-T activities of both LARGE and LARGE2 require UDP-Xyl as the donor substrate, consistent with our finding that the UXS1-deficient cell line pgsI-208, which lacks UDP-Xyl, shows defective glycosylation of α-DG. Our current result that overexpression of LARGE2 did not rescue the functional modification in pgsI-208 cells, as previously shown for LARGE, is consistent with this explanation.
Our results reveal that, in contrast to O-mannosylation of α-DG, which requires coexpression of the paralogs POMT1 and POMT2 in vitro and in vivo (Manya et al. 2004; Willer et al. 2004), functional glycosylation involves LARGE paralogs that can act independently. This is consistent with the previous observation that the kidneys (in which the LARGE2 mRNA is highly expressed) of Largemyd mice retain substantial functional glycosylation of α-DG (Grewal et al. 2005), which suggests that LARGE2 is enzymatically active in the absence of LARGE in vivo. Our results are consistent with our previous study revealing that the heteropolysaccharide generated by the polymerizing activity of LARGE conferred ligand-binding ability on α-DG (Inamori et al. 2012); here, we have demonstrated that LARGE2 has polymerizing activity. This is probably the feature that makes it possible for α-DG to bind ECM ligands as reported in previous studies involving the overexpression of LARGE2 in cultured cells (Brockington et al. 2005; Fujimura et al. 2005; Grewal et al. 2005).
Molecular recognition of the α-DG N-terminus by LARGE is an essential step for the production of functional α-DG (Kanagawa et al. 2004). Moreover, a recent study has shown that the LARGE-dependent modification required for laminin- and Old World arenavirus-binding occurs on specific Thr residues located at the extreme N-terminus of the α-DG mucin-like domain (Hara et al. 2011). Since this domain contains a number of potential O-glycosylation sites and some other sites have also been shown to be O-mannosylated in human and rabbit skeletal muscle α-DG (Nilsson et al. 2010; Stalnaker et al. 2010), it will be interesting to test whether LARGE2 has the same α-DG glycosylation-site preference as LARGE.
Most notably, we found that the LARGE paralogs have different pH optima for their enzymatic activities. In the case of LARGE2, the Xyl-T reaction was productive across the broad pH range 5.5–9.0, whereas the GlcA-T counterpart was most productive at a pH 5.5, and LARGE had almost the opposite characteristics (pH optimum for the Xyl-T reaction at pH 5.0, and that for GlcA-T across the range of pH 5.5–8.0; Figure 5). Although the physiological significance of the different pH optima is unclear, it suggests that the LARGE paralogs may be fine-tuned to function optimally under different physiological conditions in the intracellular milieu. Different types of cells and tissues may have different sets of the possible cofactors for the functional modification of α-DG, and even in the same tissues there might be possibly differences in relative expression levels and compartmentalization of the LARGE paralogs in the Golgi apparatus. Those differences could affect the formation of a functional complex or the interaction of each LARGE paralog with its cofactors resulting in more efficient modification of α-DG.
With regard to the mechanisms whereby LARGE and LARGE2 may complement one another, it is interesting to consider the biology of the bifunctional glycosyltransferases EXT1 and EXT2, which are associated with the development of hereditary multiple exostoses (Ahn et al. 1995; Stickens et al. 1996). These enzymes have GlcA-T and N-acetylglucosaminyltransferase (GlcNAc-T) activities that are required for the synthesis of heparan sulfate (Lind et al. 1998; McCormick et al. 1998), and recombinant forms of EXT1 and EXT2 have both GlcA-T and GlcNAc-T activities in vitro. Notably, their coexpression yields hetero-oligomeric complexes with augmented glycosyltransferase activities (Senay et al. 2000). Although we currently have no evidence to indicate that LARGE and LARGE2 form a heteromeric complex, such a mechanism could potentially account for complementary activity with respect to α-DG, when one or the other glycosyltransferase is inefficient at a certain pH. On the basis of the fact that the overexpression of LARGE can restore functional modification of α-DG in cells from dystroglycanopathy patients who have mutations in other genes, as well as laminin-binding, it has been suggested that LARGE is a potential target in treating dystroglycanopathies (Barresi et al. 2004; Godfrey et al. 2011). Our current results support this notion and further suggest that LARGE2 could be considered for such a role. Therefore, all efforts should be made to better understand the mechanisms behind and requirements for its enzymatic activities.
Materials and methods
Antibodies
Antibodies to glycosylated α-DG (IIH6), β-DG (7D11) and LARGE have been described previously (Ervasti and Campbell 1991; Pereboev et al. 2001; Kanagawa et al. 2004). Antibodies against mouse LARGE2 were generated by injecting a keyhole limpet hemocyanin (KLH)-conjugated synthetic peptide corresponding to the C-terminal sequence of mouse LARGE2 (C-SAALKYLTALQQARSRA) into rabbits (Rbt340). The polyclonal anti-LARGE2 antibodies were affinity-purified using a bovine serum albumin (BSA)-conjugated C-terminal peptide as described (Sharp and Campbell 1989). The above-described monoclonal antibodies, as well as the other antibody that recognizes glycosylated α-DG (VIA4-1) are available from the Developmental Studies Hybridoma Bank at the University of Iowa.
Complementary DNAs (cDNAs)
The full-length cDNAs of mouse Large (IMAGE 30725031) and mouse Large2 (IMAGE 4980759) were purchased from Thermo Scientific Open Biosystems. To generate myc + Hisx6-tagged LARGE2 at the C-terminus, the full-length open–reading frame was amplified with a primer pair #3793: 5′-TTGCGGCCGCTCATGCTGCCCCGAGGTCGC-3′/#3798: 5′-CCCTCGAGGGCCCGACTTCGGGCCTGCTG-3′. The PCR product was digested with NotI and XhoI, and then subcloned into a NotI/XhoI-digested pcDNA4/myc-His-A vector (Invitrogen), designated as pcDNA4-mLarge2/myc-His.
The lentiviral constructs and establishing stable cell lines expressing LARGE or LARGE2
For generating lentiviral constructs for stable expression of LARGE and LARGE2, a 3.7 kb SalI/MluI mouse Large cDNA fragment and a 2.4 kb MluI mouse Large2 cDNA fragment were subcloned into the pLEX-MCS vector (Thermo Scientific Open Biosystems), respectively. The cytomegalovirus (CMV) promoter region in the vector was replaced with the hEF-1α promoter. The hEF-1α promoter region in the pEF4/Myc-His vector (Invitrogen) was amplified with a primer pair 5′-CAGGCCATGGTGGCCTCGTGAGGCTCCG-3′ and 5′-GCACTAGTACCAAGCTAATTCC-3′, and the polymerase chain reaction (PCR) product was digested with SfiI and SpeI, and then subcloned into a SfiI/SpeI-digested vector. Lentiviruses were generated by the Gene Transfer Vector Core at the University of Iowa using Trans-Lentiviral ORF Packaging System (Open Biosystems). WT or pgsI-208 CHO cells were infected with multiplicity of infection of 1 and selected in medium containing 1 μg/mL puromycin (InvivoGen). Single colonies were isolated and analyzed for expression of the LARGE or LARGE2 protein by immunoblotting.
The LARGE2dTM construct and establishing a stable cell line
The construct from which mouse LARGE2 is expressed without its transmembrane region was generated by amplifying the cDNA fragments of pcDNA4-Large2/myc-His. Primer pair #7599: 5′-AAAGCGGCCGCTCGGGACCCGGATTATGGACTG—3′/#7600: 5′-GGCCAGAGTAGTGTTTGTTGG-3′ was used to produce the left fragment, which was then digested with NotI and BamHI. The right fragment was generated by digesting pcDNA4-LARGE2/myc-His with BamHI and PmeI. The two fragments were then subcloned into a NotI/EcoRV-digested p3xFLAG-CMV-9 vector (Sigma-Aldrich) to generate construct p3xFLAG-CMV-9-LARGE2dTM. This construct encodes a LARGE2 fusion protein (amino acids 30–690) tagged with a 3xFLAG at its N-terminus and a myc-Hisx6 tag at its C-terminus. A HEK293 cell line stably expressing LARGE2dTM protein was established, and LARGE2dTM secreted into the medium was purified using a metal affinity resin as described for LARGEdTM previously (Inamori et al. 2012).
Enzymatic assay
The HPLC-based enzymatic assay for LARGE2dTM was performed using Xyl-α-pNP or Xyl-α1,3-GlcA-β-MU as the acceptor to test for GlcA-T activity and GlcA-β-MU to test for Xyl-T activity. The methods for the assay and preparation of Xyl-α1,3-GlcA-β-MU were described previously (Inamori et al. 2012). For initial characterization of the Xyl-T activity of LARGE2 (Figure 3A), LARGE2dTM was incubated with 2 mM GlcA-β-MU and 1 mM UDP-Xyl at 37°C for 20 min in 0.1 M MOPS buffer, pH 7.5, at 5 mM MnCl2, 5 mM MgCl2 and 0.5% Triton X-100. For the GlcA-T assay (Figure 3C), the reaction was carried out at 37°C overnight, with 2.5 mM Xyl-α-pNP and 5 mM UDP-GlcA in 50 mM MOPS, pH 6.0, at 10 mM MnCl2, 10 mM MgCl2 and 0.5% Triton X-100. For the assessment of donor specificity, the reaction was carried out at 37°C for 20 min, with 2 mM GlcA-β-MU in 0.1 M MOPS, pH 7.5 or 0.05 mM Xyl-α1,3-GlcA-β-MU in 0.1 M sodium acetate, pH 5.5, at 5 mM MnCl2, 5 mM MgCl2 and 0.5% Triton X-100 in the presence of 1 mM each donor sugar. The specific activities toward GlcA-β-MU and Xyl-α1,3-GlcA-β-MU were 31.7 pmol/min/μg and 5.1 pmol/min/μg, respectively, and are represented as 100% (Figure 3E). For the assessment of optimal pH, the following buffers were used: 0.1 M sodium acetate buffer for pH 4.0–5.5; 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES) buffer for pH 5.5–6.5; 0.1 M 3-(N-morpholino)propanesulfonic acid (MOPS) buffer for pH 6.5–8.0 and 0.1 M Tris–HCl buffer for pH 8.0–9.0. The reaction was carried out at 37°C for 1 h, with 2 mM GlcA-β-MU or 0.1 mM Xyl-α1,3-GlcA-β-MU added to each of the buffers described above, at 10 mM MnCl2, 10 mM MgCl2 and 0.5% Triton X-100. The specific activity that represents 100% in each assay is: 13.7 pmol/min/μg for LARGE Xyl-T, 26.5 pmol/min/μg for LARGE GlcA-T, 20.9 pmol/min/μg for LARGE2 Xyl-T and 6.6 pmol/min/μg for LARGE2 GlcA-T. For the assessment of polymerizing activity, LARGE2dTM was incubated with 2 mM GlcA-β-MU, 5 mM UDP-Xyl and 5 mM UDP-GlcA at 37°C for 16 h, in 0.1 M MES, pH 6.5, at 10 mM MnCl2, 10 mM MgCl2 and 0.5% Triton X-100.
MS analysis
MS and MS/MS analyses were conducted on an Agilent 6520A Quadrupole Time of Flight (Q/TOF) mass spectrometer configured for flow-injection analysis. Borosilicate nano-capillary vials were used. The glycan samples were diluted in 50% acetonitrile and 0.1% formic acid, and loaded into the distal end of the nano vial. The capillary was fixed in a grounded stainless steel union and positioned within 8 mm of the inlet capillary to the mass spectrometer, which was maintained at −1200 V. Opposite to the inlet, the counter electrode was held at −1700 V. The Q/TOF was configured to run at high resolution (16–22 K), collecting at 4 GHz over a range from 25 to 1700 m/z.
Immunoblotting and flow cytometry
WT or pgsI-208 CHO cells with or without overexpression of LARGE or LARGE2 were analyzed by immunoblotting of glycoproteins enriched by wheat germ agglutinin (WGA)-agarose beads and flow cytometry for cell surface staining, as described previously (Inamori et al. 2012).
Supplementary data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported in part by a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant (1U54NS053672).
Conflict of interest
Kevin P. Campbell is a scientific advisory board member and consultant for ARMGO Pharma Inc. He also has a patent on increasing the functional glycosylation of α-dystroglycan in the treatment of muscle degeneration.
Abbreviations
α-DG, dystroglycan; BSA, bovine serum albumin; CBB, Coomassie brilliant blue; CHO, Chinese hamster ovary; CMV, cytomegalovirus; DG, dystroglycan; ECM, extracellular matrix; FKRP, fukutin-related protein; GlcA-β-MU, 4-methylumbelliferyl-β-d-glucuronide; GlcA, glucuronic acid; GlcA-T, glucuronyltransferase; GlcNAc-T, N-acetylglucosaminyltransferase; GT8, glycosyltransferase family 8; HPLC, high-performance liquid chromatography; KLH, keyhole limpet hemocyanin; m/z, mass/charge ratio; MES, 2-(N-morpholino)ethanesulfonic acid; MOPS, 3-(N-morpholino)propanesulfonic acid; MS, mass spectrometry; PCR, Polymerase chain reaction; POMGnT1, protein O-mannosyl β-1,2-N-acetylglucosaminyltransferase 1; POMT, protein O-mannosyltransferase; Q/TOF, quadrupole time of flight; TM, transmembrane; UDP, uridine 5′-diphosphate; UXS, UDP-xylose synthase; WGA, wheat germ agglutinin; WT, wild-type; Xyl-α-pNP, p-nitrophenyl-α-d-xyloside; Xyl, xylose; Xyl-T, xylosyltransferase.
Supplementary Material
Acknowledgements
We thank Drs. Daniel Beltrán-Valero de Bernabé and Matthew M. Goddeeris for fruitful discussions, David Venzke and all the members of the Campbell laboratory for technical support, Dr. Harry Schachter for valuable comments on the manuscript, Dr. Jeffrey D. Esko for providing pgsI-208 cells and the University of Iowa Proteomics Facility, Gene Transfer Vector Core, Developmental Studies Hybridoma Bank and Flow Cytometry Facility for their services. K.P.C. is an Investigator of the Howard Hughes Medical Institute.
References
- Aguilan JT, Sundaram S, Nieves E, Stanley P. Mutational and functional analysis of Large in a novel CHO glycosylation mutant. Glycobiology. 2009;19:971–986. doi: 10.1093/glycob/cwp074. doi:10.1093/glycob/cwp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Ludecke HJ, Lindow S, Horton WA, Lee B, Wagner MJ, Horsthemke B, Wells DE. Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1) Nat Genet. 1995;11:137–143. doi: 10.1038/ng1095-137. doi:10.1038/ng1095-137. [DOI] [PubMed] [Google Scholar]
- Bakker H, Oka T, Ashikov A, Yadav A, Berger M, Rana NA, Bai X, Jigami Y, Haltiwanger RS, Esko JD, et al. Functional UDP-xylose transport across the endoplasmic reticulum/Golgi membrane in a Chinese hamster ovary cell mutant defective in UDP-xylose Synthase. J Biol Chem. 2009;284:2576–2583. doi: 10.1074/jbc.M804394200. doi:10.1074/jbc.M804394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi R, Campbell KP. Dystroglycan: From biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. doi:10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang W, Schachter H, Dumanski JP, et al. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 2004;10:696–703. doi: 10.1038/nm1059. doi:10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- Brockington M, Torelli S, Prandini P, Boito C, Dolatshad NF, Longman C, Brown SC, Muntoni F. Localization and functional analysis of the LARGE family of glycosyltransferases: Significance for muscular dystrophy. Hum Mol Genet. 2005;14:657–665. doi: 10.1093/hmg/ddi062. doi:10.1093/hmg/ddi062. [DOI] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. doi:10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Coutinho PM, Deleury E, Davies GJ, Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J Mol Biol. 2003;328:307–317. doi: 10.1016/s0022-2836(03)00307-3. doi:10.1016/S0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. doi:10.1016/0092-8674(91)90035-W. [DOI] [PubMed] [Google Scholar]
- Fujimura K, Sawaki H, Sakai T, Hiruma T, Nakanishi N, Sato T, Ohkura T, Narimatsu H. LARGE2 facilitates the maturation of alpha-dystroglycan more effectively than LARGE. Biochem Biophys Res Commun. 2005;329:1162–1171. doi: 10.1016/j.bbrc.2005.02.082. doi:10.1016/j.bbrc.2005.02.082. [DOI] [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. doi:10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Godfrey C, Foley AR, Clement E, Muntoni F. Dystroglycanopathies: Coming into focus. Curr Opin Genet Dev. 2011;21:278–285. doi: 10.1016/j.gde.2011.02.001. doi:10.1016/j.gde.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE. Mutant glycosyltransferase and altered glycosylation of alpha-dystroglycan in the myodystrophy mouse. Nat Genet. 2001;28:151–154. doi: 10.1038/88865. doi:10.1038/88865. [DOI] [PubMed] [Google Scholar]
- Grewal PK, McLaughlan JM, Moore CJ, Browning CA, Hewitt JE. Characterization of the LARGE family of putative glycosyltransferases associated with dystroglycanopathies. Glycobiology. 2005;15:912–923. doi: 10.1093/glycob/cwi094. doi:10.1093/glycob/cwi094. [DOI] [PubMed] [Google Scholar]
- Hara Y, Kanagawa M, Kunz S, Yoshida-Moriguchi T, Satz JS, Kobayashi YM, Zhu Z, Burden SJ, Oldstone MB, Campbell KP. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc Natl Acad Sci USA. 2011;108:17426–17431. doi: 10.1073/pnas.1114836108. doi:10.1073/pnas.1114836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. doi:10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 2012;335:93–96. doi: 10.1126/science.1214115. doi:10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB, et al. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 2004;117:953–964. doi: 10.1016/j.cell.2004.06.003. doi:10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Lind T, Tufaro F, McCormick C, Lindahl U, Lidholt K. The putative tumor suppressors EXT1 and EXT2 are glycosyltransferases required for the biosynthesis of heparan sulfate. J Biol Chem. 1998;273:26265–26268. doi: 10.1074/jbc.273.41.26265. doi:10.1074/jbc.273.41.26265. [DOI] [PubMed] [Google Scholar]
- Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci USA. 2004;101:500–505. doi: 10.1073/pnas.0307228101. doi:10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Leduc Y, Martindale D, Mattison K, Esford LE, Dyer AP, Tufaro F. The putative tumour suppressor EXT1 alters the expression of cell-surface heparan sulfate. Nat Genet. 1998;19:158–161. doi: 10.1038/514. doi:10.1038/514. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Larson G, Grahn A. Characterization of site-specific O-glycan structures within the mucin-like domain of alpha-dystroglycan from human skeletal muscle. Glycobiology. 2010;20:1160–1169. doi: 10.1093/glycob/cwq082. doi:10.1093/glycob/cwq082. [DOI] [PubMed] [Google Scholar]
- Pereboev AV, Ahmed N, thi Man N, Morris GE. Epitopes in the interacting regions of beta-dystroglycan (PPxY motif) and dystrophin (WW domain) Biochim Biophys Acta. 2001;1527:54–60. doi: 10.1016/s0304-4165(01)00147-7. doi:10.1016/S0304-4165(01)00147-7. [DOI] [PubMed] [Google Scholar]
- Peyrard M, Seroussi E, Sandberg-Nordqvist AC, Xie YG, Han FY, Fransson I, Collins J, Dunham I, Kost-Alimova M, Imreh S, et al. The human LARGE gene from 22q12.3-q13.1 is a new, distinct member of the glycosyltransferase gene family. Proc Natl Acad Sci USA. 1999;96:598–603. doi: 10.1073/pnas.96.2.598. doi:10.1073/pnas.96.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscioli T, Kamsteeg EJ, Buysse K, Maystadt I, van Reeuwijk J, van den Elzen C, van Beusekom E, Riemersma M, Pfundt R, Vissers LE, et al. Mutations in ISPD cause Walker-Warburg syndrome and defective glycosylation of alpha-dystroglycan. Nat Genet. 2012;44:581–585. doi: 10.1038/ng.2253. doi:10.1038/ng.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Kurata-Miura K, Ujita M, Angata K, Nakagawa S, Sekine S, Nishi T, Fukuda M. Expression cloning of cDNA encoding a human beta-1,3-N-acetylglucosaminyltransferase that is essential for poly-N-acetyllactosamine synthesis. Proc Natl Acad Sci USA. 1997;94:14294–14299. doi: 10.1073/pnas.94.26.14294. doi:10.1073/pnas.94.26.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, et al. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. doi:10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Senay C, Lind T, Muguruma K, Tone Y, Kitagawa H, Sugahara K, Lidholt K, Lindahl U, Kusche-Gullberg M. The EXT1/EXT2 tumor suppressors: Catalytic activities and role in heparan sulfate biosynthesis. EMBO Rep. 2000;1:282–286. doi: 10.1093/embo-reports/kvd045. doi:10.1093/embo-reports/kvd045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AH, Campbell KP. Characterization of the 1,4-dihydropyridine receptor using subunit-specific polyclonal antibodies. Evidence for a 32,000-Da subunit. J Biol Chem. 1989;264:2816–2825. [PubMed] [Google Scholar]
- Stalnaker SH, Hashmi S, Lim JM, Aoki K, Porterfield M, Gutierrez-Sanchez G, Wheeler J, Ervasti JM, Bergmann C, Tiemeyer M, et al. Site mapping and characterization of O-glycan structures on alpha-dystroglycan isolated from rabbit skeletal muscle. J Biol Chem. 2010;285:24882–24891. doi: 10.1074/jbc.M110.126474. doi:10.1074/jbc.M110.126474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D, Clines G, Burbee D, Ramos P, Thomas S, Hogue D, Hecht JT, Lovett M, Evans GA. The EXT2 multiple exostoses gene defines a family of putative tumour suppressor genes. Nat Genet. 1996;14:25–32. doi: 10.1038/ng0996-25. doi:10.1038/ng0996-25. [DOI] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. doi:10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. Embo J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. doi:10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer T, Lee H, Lommel M, Yoshida-Moriguchi T, de Bernabe DB, Venzke D, Cirak S, Schachter H, Vajsar J, Voit T, et al. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker–Warburg syndrome. Nat Genet. 2012;44:575–580. doi: 10.1038/ng.2252. doi:10.1038/ng.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer T, Prados B, Falcon-Perez JM, Renner-Muller I, Przemeck GK, Lommel M, Coloma A, Valero MC, de Angelis MH, Tanner W, et al. Targeted disruption of the Walker–Warburg syndrome gene Pomt1 in mouse results in embryonic lethality. Proc Natl Acad Sci USA. 2004;101:14126–14131. doi: 10.1073/pnas.0405899101. doi:10.1073/pnas.0405899101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 2010;327:88–92. doi: 10.1126/science.1180512. doi:10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.