Fig. 3.
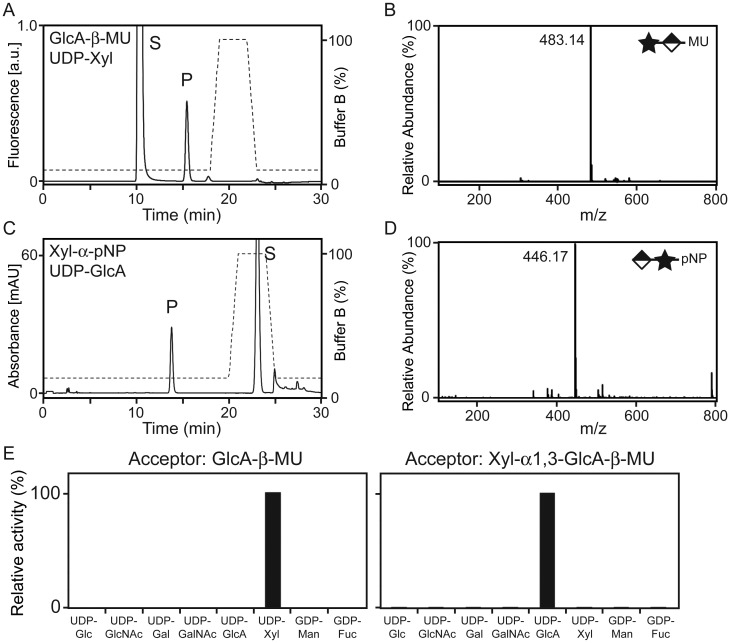
LARGE2 is a bifunctional glycosyltransferase with Xyl-T and GlcA-T activities. (A) HPLC elution profile from the LC-18 column of the products obtained from the reaction of LARGE2dTM with GlcA-β-MU and UDP-Xyl. S, unreacted substrate. P, product. Dashed line, %Buffer B. (B) Q/TOF-MS analysis of the product peak detected in (A). Star and diamond indicate Xyl and GlcA, respectively. The MS/MS fragmentation pattern (Supplementary data, Figure S3A) confirmed that the ion with an m/z of 483.14 [M–H]− is GlcA-β-MU with an added Xyl. (C) HPLC elution profile from the LC-18 column of the products obtained from the reaction of LARGE2dTM with Xyl-α-pNP and UDP-GlcA. (D) Q/TOF-MS analysis as in (B), for the product isolated from the reaction analyzed in (C). The MS/MS fragmentation pattern (Supplementary data, Figure S3B) confirmed that the ion with an m/z of 446.17 [M–H]− is Xyl-α-pNP with an added GlcA. (E) Donor substrate specificity of LARGE2dTM. Representative data from two independent assays, showing relative activity (%) of Xyl-T toward GlcA-β-MU and of GlcA-T toward Xyl-α1,3-GlcA-β-MU. No other sugars were transferred to the acceptors.