Abstract
Congestive heart failure is an inexorable disease associated with unacceptably high morbidity and mortality. Preclinical results indicate that gene transfer using various proteins is a safe and effective approach for increasing function of the failing heart. In the current review, we provide a summary of cardiac gene transfer in general and summarize findings using adenylyl cyclase 6 as therapeutic gene in the failing heart. We also discuss the potential usefulness of a new treatment for congestive heart failure, paracrine-based gene transfer.
Keywords: gene transfer, congestive heart failure, adenylyl cyclase, LV contractility, Ca2+ handling
INTRODUCTION
Congestive heart failure (CHF) is a major cause of morbidity and mortality with increasing social and economic costs.1 CHF is the only cardiovascular disease that is increasing in prevalence, and there are currently 6 million people in the United States and >23 million worldwide with CHF. Patients with CHF who have symptoms with mild activity or at rest (Class III and Class IV) have a poor long-term outcome, with up to 50% of patients dying within 4 years of symptom onset despite advanced pharmacological and device therapies.2 Heart transplantation has an 80% 5-year survival rate, but fewer than 2500 cardiac transplants are performed in the United State each year. Because the prevalence of CHF is increasing and outlook remains dismal, new therapeutic approaches for CHF, including gene transfer, are warranted.
Several factors have impeded the advancement of clinical gene transfer for the treatment of CHF. These impediments include selection of (a) an effective gene; (b) suitable vectors and promoters with high efficiency and long-term expression with minimal toxicity and immunogenicity; and (c) delivery methods that are easy to deploy and result in safe and effective expression in the heart with minimal adverse effects. It is encouraging that virus-mediated gene transfer of a number of genes appears to increase cardiac function in animal models of heart failure.3–7 At present, clinical trials using virus-mediated gene transfer of sarcoendoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) and adenylyl cyclase 6 (AC6) for CHF are in progress. Other potential gene transfer proteins, with preclinical data indicating efficacy in CHF treatment, include βARKct, an inhibitor of G-protein-coupled receptor kinases, and S100A1.8
We now will review cardiac gene transfer briefly, discuss cardiac AC6 gene transfer for CHF, and introduce an alternative approach, paracrine-based gene transfer. The exciting findings from preclinical studies using other potentially therapeutic proteins to increase function of the failing heart are reviewed elsewhere in this issue of Gene Therapy.
CARDIAC GENE TRANSFER
For CHF treatment, the goal has been to deliver vectors encoding therapeutic genes safely and effectively to the heart. Although we will discuss separately the selection of therapeutic genes, vectors, promoters and delivery methods, these components ultimately must be considered collectively to select the most effective gene transfer approach.
Selection of therapeutic genes for CHF
Cyclic AMP (cAMP; 3′-5′-cyclic adenosine monophosphate) and Ca2+ handling profoundly influence left ventricular (LV) contractile function and relaxation. It is not surprising that a majority of promising therapeutic genes for CHF target defective cAMP or Ca2+ handling in cardiac myocytes. Additionally, decreasing myocardial ischemia by increasing myocardial blood flow is an attractive approach for myocardial infarction-induced CHF and also for CHF in general. Although myocardial ischemia most commonly is treated by coronary artery bypass graft surgery and percutaneous coronary interventions, there may be a role for angiogenic gene transfer.9–11 This strategy has been reviewed elsewhere12,13 and will not be discussed further here. Manipulation of RNA stability and protein synthesis by small RNAs (for example, microRNA) may provide new options for CHF gene transfer. However, concerns about specificity must first be resolved.14
β-Adrenergic receptor signaling pathway
CHF is generally associated with impaired β-adrenergic receptor (βAR) responsiveness, and elevated circulating catecholamine levels. Changes in LV contractility are associated with βAR downregulation and desensitization, βAR uncoupling, reduced expression or function of adenylyl cyclase (AC), and impaired cAMP generating capacity.15
Pharmacological attempts to increase LV function in clinical CHF by increasing cAMP signaling have failed. In animal studies, chronic infusion of isoproterenol (a βAR agonist) and cardiac-directed expression of β1AR, Gαs and cAMP-dependent protein kinase A all have resulted in cardiomyopathy. Dobutamine (a βAR agonist) and milrinone (a phosphodiesterase inhibitor that increases intracellular cAMP levels) increase cardiac contractile function in clinical CHF, but patients do not get a survival advantage, and milrinone increases mortality.16 These deleterious effects are presumed to result from sustained increases in cAMP production. Indeed, cardiac-directed expression of constitutively active human β1AR is associated with cardiomyopathy.17,18
Deletion of G-protein-coupled receptor kinase 2 (a kinase that attenuates βAR signaling) restores βAR responsiveness in the failing heart and increases cardiac function and survival.19 Inhibition of G-protein-coupled receptor kinase 2 by βARKct, a 195 amino-acid C-terminal fragment of G-protein-coupled receptor kinase 2, also increases contractile function in several animal models of CHF and in failing human cardiac myocytes.8 These effects are associated with normalization of βAR density and βAR signaling. Importantly, βARKct and βAR blockade appear to act synergistically to increase βAR signaling to normal levels,20 although βARKct also may increase cardiac function by regulating Gβγ and apoptosis signaling.21,22
It is surprising that expression of AC6, the enzyme catalyzing cAMP formation, shows beneficial effects on the failing heart. These beneficial effects reflect unique feature of AC6, not shared by other elements of the βAR–Gs–AC pathway. For example, AC6 expression does not affect basal cAMP production in the normal heart, and may increase cardiac function through cAMP-independent mechanisms. These features of AC6 will be discussed in more detail below.
Ca2+ handling
Ca2+ directly regulates cardiac contraction and relaxation. CHF is associated with defective sarcoplasmic reticulum (SR) Ca2+ uptake and Ca2+ release. Ca2+ sensitivity of the contractile apparatus is also impaired in CHF. It is therefore logical to consider strategies to improve cardiac function by correcting defective Ca2+ handling using gene transfer.
Preclinical studies in animal models of CHF have identified SERCA2a as a promising candidate for gene transfer.23,24 A study of transgenic rats with cardiac-directed SERCA2a expression showed provocation of cardiac dysrhythmias,25 but similar problems were not apparent in subsequent studies using SERCA2a gene transfer.26,27 Intracoronary delivery of adeno-associated virus (AAV) encoding SERCA2a appears to be safe in a trial of 39 subjects with CHF.27 These results indicate the potential safety of intracoronary delivery of AAV vectors in patients with CHF.
S100A1 belongs to the family of S100 EF-hand Ca2+-binding proteins. Expression of S100A1 increases SERCA2a Ca2+ uptake activity, and also stabilizes the release of Ca2+ channel ryanodine receptor in the failing heart.28 Interestingly, S100A1 expression and βAR blockade act synergistically to improve LV function.
Other Ca2+ handling regulators (for example, protein phosphatase inhibitor 1, sorcin and parvalbumin) also show favorable effects on LV function in animal models of cardiomyopathy after virus-mediated gene transfer. Further studies are warranted to establish their usefulness in clinical CHF.
Selection of vectors
The ideal vectors for cardiac gene transfer should exhibit (1) high level expression; (2) limited off-target expression; (3) long-term expression; and (4) minimal toxicity and immunogenicity. In general, virus vectors provide superior cardiac transgene expression to non-virus vectors. Regulated expression is also desirable, but the limited capacity of vectors and large size of transgenes currently used in intracoronary delivery generally preclude its use.
The commonly used virus vectors for cardiac gene transfer are adenovirus, AAV and lentivirus. More detailed information regarding commonly used vectors have been reviewed elsewhere,29 and only brief mention will be made here. AAV has less immunogenicity and may provide long-term expression. However, its small packaging capacity limits the size of gene expression cassette (including promoter, regulatory elements and transgene) to less than 5 kb. Lentivirus has larger packaging capacity and also low immunogenicity, but cannot easily move across the endothelial cell, thus lentivirus is unsuited for intracoronary delivery.30
Selection of delivery methods
Detectable gene transfer to the heart has been achieved by several delivery methods. Direct intramyocardial injection provides transgene expression along the injected area. Intracoronary delivery of adenovirus is not typically associated with myocardial inflammation, except at extremely high doses,31 but direct myocardial injection can cause inflammation.32,33 Intracoronary delivery of AAV2 is less efficient than adenovirus,34 providing a rationale for selection of adenovirus in the AC6 clinical trial. Although intravenous delivery of newer AAV vectors (AAV6, AAV8 and AAV9) appears to provide substantial cardiac gene transfer, expression in the liver and other organs is also robust.35 The use of the term ‘cardiotropic’ for these vectors is misleading because it suggests cardiac specificity, which clearly does not occur. A potential shortcoming for intravenous delivery of AAV vectors is that the high dose of AAV required to ensure adequate cardiac gene transfer, which limits its clinical application.
Indirect intracoronary delivery is required in rodents, because the small caliber of the coronary arteries in mice and rats precluded direct intraluminal insertion. In this procedure, the ascending aorta and pulmonary artery are clamped, and virus vectors injected into the LV chamber are forced into the coronary arteries. In conjunction with hypothermia, indirect intracoronary delivery enables prolonged dwell time without ischemic injury to the heart and brain. These methods have been invaluable in cardiac gene transfer in rodents, and provide superior levels of cardiac transgene expression compared with intravenous delivery, even with AAV5, AAV6 and AAV9 (unpublished data).
Pharmacological agents that promote transcytosis after vascular delivery of vector can increase gene transfer efficiency. Such pharmacological agents, including histamine, nitroprusside, serotonin, acetylcholine, sildenafil, vascular endothelial growth factor and substance P, facilitate transvascular movement of virus vectors across the endothelial border into the cardiac interstitium. Finally, a percutaneous closed-loop recirculation system has been used to increase AAV-mediated cardiac gene transfer in sheep.7
Selection of delivery method should be considered in the context of several factors, including vector type, species and organ targeted, to obtain optimal results. Because of the safety concerns, invasive methods requiring thoracotomy, cross-clamping of the aorta or cardiopulmonary bypass are not applicable in clinical settings. Refinements in methods of delivery have improved transgene expression. The vector delivery method easiest to apply, safest and most effective will ultimately be the one adopted for clinical applications.
AC6: A PROMISING CANDIDATE FOR GENE TRANSFER
AC is the enzyme that converts ATP to cAMP. In cardiac myocytes, AC6 and AC5 are the two dominant AC isoforms. AC5 and AC6 have 65% amino-acid homology, and each contribute equally to cAMP generation in cardiac myocytes and LV membranes.36,37 Deletion of AC5 mildly increases SR Ca2+ handling and LV function.36 In contrast, deletion of AC6 is associated with marked impairment of Ca2+ signaling and LV function.37 These observations suggest that modulating cardiac AC6 content would have favorable effects on the failing heart (Table 1).
Table 1.
AC6 expression and cardiac function
| Model | Findings | Ref |
|---|---|---|
| Transgenic mice | ||
| AC6 | No change in basal cAMP production and LV function; increased LV function in response to βAR stimulation | 39 |
| AC6 | Facilitation of atrioventricular nodal conduction without altering sinus node function | 68 |
| AC6 and acute MI | Reduced mortality in acute MI; improved SR Ca2+ uptake; normalized PLB phosphorylation | 50 |
| AC6 and MI | Decreased fibrosis and increased engraftment of iPS cells in the infarcted area | 51 |
| AC6 × Gαq | Restored cAMP generation capacity; increased LV function and βAR responsiveness | 44 |
| AC6 × Gαq | Decreased LV hypertrophy, increased LV function and prolonged survival in Gαq cardiomyopathy | 45 |
| AC6 × Gαq | Normalized LV SR Ca2+ uptake and PLB phosphorylation in Gαq cardiomyopathy | 48 |
| AC6 × Gαq | No alteration in heart rate regulation | 47 |
| AC6 × Gαq | Corrects prolonged action potential duration in Gαq cardiomyopathy | 49 |
| AC6-tet | Increased LV responsiveness to βAR stimulation after activation of cardiac-directed AC6 expression | 40 |
| AC6-tet in CHF | Increased LV function of failing heart (CHF induced by MI); normalized cTnI phosphorylation | 3 |
| AC6-tet and aging | Improved Ca2+ handling and increased LV function in mice aged 24 months, but not in young mice | 57 |
| AC6 deletion | Marked adverse effects on Ca2+ uptake; reduced LV function in response to βAR stimulation | 37 |
| Gene transfer in cells | ||
| NRCM | AC6 amount determines NRCM response to βAR stimulation | 38 |
| NRCM | AC6 reduces PLB expression by increasing ATF3 content through cAMP-independent pathway | 58 |
| NRCM | AC6 increases PLB phosphorylation by activating Akt (cAMP independent) | 60 |
| NRCM | AC6 regulates Akt activity in NRCM (cAMP independent) | 59 |
| NRCM | AC6 vs β1AR: differences in intracellular distribution—an important determinant of biological effects | 69 |
| ARCM | AC6 and AC6mut (catalytically inactive) have similar benefits in ARCM | 70 |
| In vivo gene transfer | ||
| C57BL/6 mouse | Indirect intracoronary delivery of Ad.AC6: increased LV function in response to βAR stimulation | 31 |
| Gαq mouse | Intracoronary delivery of Ad.AC6: increased LV function in Gαq cardiomyopathy | 46 |
| Normal pig | Intracoronary delivery of Ad.AC6: increased LV function in response to βAR stimulation | 41 |
| CHF pig | Intracoronary delivery of Ad.AC6: increased function of failing LV | 42 |
| Normal pig | Intracoronary delivery of Ad.AC6: persisted cardiac AC6 expression for 10 weeks without toxicity | —a |
Abbreviations: AC6, adenylyl cyclase 6; ARCM, adult rat cardiac myocytes; ATF3, activating transcription factor 3; βAR, β-adrenergic receptor; cAMP, 3′,5′-cyclic adenine monophosphate; CHF, congestive heart failure; cTnI, cardiac troponin I; iPS cells, induced pluripotent stem cells; LV, left ventricular; MI, myocardial infarction; NRCM, neonatal rat cardiac myocytes; PLB, phospholamban; SR, sarcoplasmic reticulum.
Food and Drug Administration (FDA) Investigational New Drug (IND) application.
AC6 Increases LV responsiveness to βAR stimulation
As previously alluded to, cAMP signaling is of pivotal importance in LV contraction and relaxation. AC6 protein content sets a limit on βAR-dependent cAMP generation in cardiac myocytes.38 Transgenic mice with cardiac-directed expression of AC6 show increased cAMP generating capacity in cardiac myocytes after βAR stimulation, but no change in cardiac βAR density or protein contents of Gαs and Gαi2.39,40 Associated with increased cAMP generating capacity is increased LV contractile function and relaxation in response to βAR stimulation. Conversely, AC6 deletion is associated with decreased LV function and βAR responsiveness.37 Moreover, AC6 gene transfer via intracoronary delivery increases LV function in response to βAR stimulation in mice and pigs.41,42 AC6 expression has no effect on basal LV function or basal cAMP production in LV membranes or cardiac myocytes.41 When expressed at high levels (10- to 20-fold increase in AC6 protein) for >20 months, no abnormalities are seen in LV size or basal function, and such mice have normal longevity.40 Unaltered basal cAMP production is unique to the AC6 transgenic mouse: it is not seen when other elements of the βAR signaling pathway are expressed in the hearts of transgenic lines (β1AR, Gsα, protein kinase A). This unique feature of cardiac AC6 expression may in part explain why cardiac-directed AC6 expression is beneficial rather than deleterious.
AC6 improves defective Ca2+ handling in the failing heart
Mimicking important aspects of clinical CHF, Gαq cardiomyopathy is associated with impaired cAMP production, defective Ca2+ handling, LV hypertrophy, dilation, and abnormal LV systolic and diastolic function.43 Expression of AC6 in this cardiomyopathic background increases LV contractile function and relaxation, attenuates LV hypertrophy and dilation, and increases survival.44–46 AC6 expression is not associated with increases in heart rate or arrythmias.47 Correction of defects in βAR-stimulated cAMP production and SR Ca2+ uptake by AC6 expression are of mechanistic importance for these functional improvements.48 In addition, patch clamping shows that AC6 expression corrects prolonged action potential associated with Gαq cardiomyopathy, suggesting a possible beneficial role of AC6 in attenuating ventricular arrythmias.49
AC6 expression also increases survival by two fold after acute myocardial infarction, although it has no effect on infarct size.50 This favorable effect of AC6 on survival may be resulted from increased LV function and reduced LV dilation. Increased LV function is associated with increased phospholamban (PLB) phosphorylation, SR Ca2+ uptake and SERCA2a affinity to Ca2+, in addition to reduced AV block. AC6 expression also increases engraftment of inducible pluripotent stem cells in the infarcted area, suggesting a novel function of AC6 in the setting of myocardial ischemia.51
In mice with severe CHF induced by myocardial infarction, activation of cardiac AC6 expression increases LV contractility and reduces LV dilation, as shown by increases in LV ejection fraction, pressure development (LV +dP/dt), cardiac output and slope of the endsystolic pressure–volume relationship. Diastolic function is also improved by the activation of cardiac AC6 expression. Similarly, AC6 gene transfer in pacing-induced CHF in pigs shows favorable effects on LV function and reduces LV chamber dilation, thereby reducing LV end-systolic wall stress.42 Associated with these favorable effects are increases in βAR-stimulated cAMP production, protein kinase A activity and cardiac troponin I phosphorylation at Ser23/24.3 No change is found in protein contents of other signaling elements important for LV function. As cardiac troponin I phosphorylation at Ser23/24 is critical for increase of off-rate for Ca2+ exchange with cTnC and also for improvement of LV function in CHF,52,53 these results suggest that increased cardiac troponin I phosphorylation is important for AC6 function in the failing heart.
A recent publication indicated that constitutive cardiac expression of AC6 has a deleterious effect on LV function in mice 4 weeks after proximal aortic constriction,54 but a subsequent paper showed beneficial effects in the same model using regulated AC6 expression.55
AC6 increases function in the aged heart
Cell senescence is associated with reduced LV function and impaired βAR responsiveness in aged myocardium.1,2 LV contractile reserve at age 80 years is less than one-half of that at age 20 years. This age-related reduction of cardiac function affects the severity and prognosis of CHF in the elderly.2 Epidemiological studies have found that CHF is a common disease of the elderly.1,2 The incidence of CHF in the United States is 1% in people aged 65 years and older and increases to over 30% at age 80 years.2
In the aging heart, reduced LV function and βAR responsiveness occur in the presence of increased plasma catecholamine levels,56 indicating an abnormality in AC signaling. Reduced cardiac AC6 expression and impaired LV cAMP generation capacity is associated with advanced age. The hypothesis that increased AC6 protein content in the aged myocardium increases LV function was tested using a transgenic mouse line with regulated AC6 expression.57
Activation of cardiac AC6 expression in aged mice (aged 20 months) improves LV +dP/dt, stroke work, end-systolic pressure–volume relationship and −dP/dt.57 These changes in contractile function and relaxation are associated with increased SR Ca2+ uptake and SERCA2a affinity for Ca2+. There is no evidence of LV hypertrophy or fibrosis after activation of AC6 expression in the aged myocardium. Activation of AC6 expression is also associated with increased cAMP generating capacity, cAMP-dependent protein kinase activity, cardiac troponin I phosphorylation and PLB phosphorylation. There are no changes in fetal gene expression, SERCA2a protein content, calsequestrin protein content and Na+–Ca2+ exchanger protein content. Interestingly, activation of AC6 expression has no effect on LV contractile function and relaxation in young mice (aged 7 months).
AC6 and cAMP-independent effects
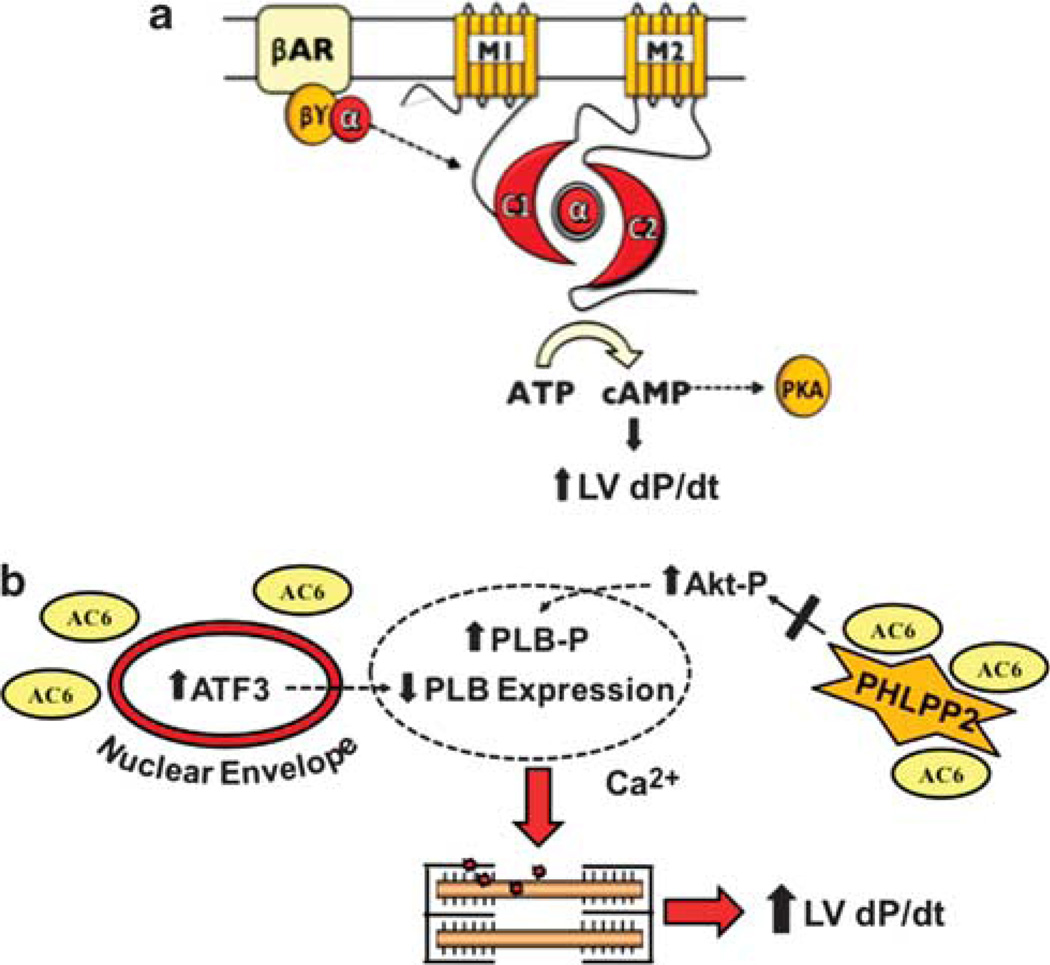
In addition to cAMP-dependent events, AC6 expression affects cell signaling through mechanism independent of cAMP generation (Figure 1). For example, AC6 gene transfer increases protein content of ATF3 (activating transcription factor 3), which in turn suppresses gene transcription of atrial natriuretic factor (ANF) and PLB. These effects of AC6 occur in the absence of catecholamines and in the presence of specific protein kinase A inhibitors, suggesting that they are independent of cAMP signaling.58–60 Decreased PLB content would be predicted to increase SR Ca2+ uptake by SERCA2a and have a favorable effect on cardiac myocyte contractile function and relaxation. AC6 also directly binds to PHLPP2 (pleckstrin homology domain leucine-rich protein phosphatase 2) and prevents PHLPP2 inhibition on Akt activity in cardiac myocytes.59 Activated Akt can phosphorylate PLB at Ser16 site, although PLB is not a good substrate for Akt.60 Like native form of AC6, an AC6 mutant (AC6mut) with marked impairment in cAMP generation, influences intracellular signaling events. AC6mut also activates Akt, increases ATF3 expression and reduces PLB expression. These data confirm that many of the effects of AC6 on cell signaling do not require increased cAMP generation. Additional studies are warranted to investigate the relative importance of cAMP-dependent versus cAMP-independent cell signaling in regulating cardiac myocyte and LV function.
Figure 1.
Hypothetical model of cAMP-independent effects of AC6 gene transfer. Cardiac AC6 expression has beneficial cardiac effects, through both (a) cAMP-dependent and (b) cAMP-independent events. The hypothetical mechanistic model summarizes data from experiments using adenovirus-mediated gene transfer of AC6 in cultured cardiac myocytes. Transgene AC6, widely distributed in the cytoplasm owing to gene transfer, has access to previously inaccessible intracellular proteins and cytoplasmic compartments. For example, AC6 is associated with the nuclear envelope and increased nuclear ATF3 expression is observed, which reduces PLB expression.58 Association of transgene AC6 with PHLPP2 (an Akt phosphatase) reduces its phosphatase activity, promoting Akt activation and subsequent PLB phosphorylation.59,60 The effects of transgene AC6 on PLB, which promote calcium handling and contractile function, are cAMP independent. α, Gαs subunit; βγ, Gβγ subunits; Akt-P, Akt phosphorylation; ATF3, activating transcription factor 3; C1, AC6 catalytic domain 1; C2, AC6 catalytic domain 2; LV, left ventricular; M1, AC6 transmembrane domain 1; M2, AC6 transmembrane domain 2; PHLPP2, pleckstrin homology domain leucine-rich repeat protein phosphatase 2; PKA, cAMP-dependent protein kinase A; PLB, phospholamban; PLB-P, phospholamban phosphorylation.
In summary, these studies demonstrate that AC6 expression has protean cardiac effects, both cAMP-dependent and cAMP-independent, and safely increases function of the failing heart (Table 1). Based on these results, a clinical trial of cardiac AC6 gene transfer in CHF patients is in progress (ClinicalTrials.gov, NCT00787059). This randomized, double-blinded, placebo-controlled trial will evaluate the safety and clinical effectiveness of ascending doses of human adenovirus-5 (E1/E3-deleted, replication incompetent) encoding human AC6 (Ad5.hAC6) by intracoronary delivery.
PARACRINE-BASED GENE TRANSFER
Gene transfer for the treatment of cardiovascular diseases is conceptually attractive, but difficulty in obtaining a high-yield transgene expression in the heart, in a manner that can be easily and safely applied, has been a chief impediment to progress. Current potential methods of gene transfer for clinical heart disease include intramuscular injection into heart muscle, intracoronary delivery or percutaneous recirculation—methods that typically provide limited expression or are cumbersome to apply. Consequently, clinical cardiac gene therapy trials have been somewhat disappointing likely because of failure to obtain adequate gene expression in the heart to attain a beneficial effect.
To circumvent this problem, we have considered the usefulness of a vector encoding a paracrine-type transgene, which affects cardiac function after being released to the circulation from distant sites. For example, systemic delivery of a long-term expression vector encoding a paracrine gene would enable sustained release of the transgene to serum, where it could exert beneficial effects on the heart ‘from a distance’. Such an approach would limit the number of candidate genes—insulin-like growth factor-I (IGF-I), growth hormone, B-type natriuretic peptide, urocortin-2 (to name a few)—but may enhance the prospects of successful clinical gene therapy for CHF. An additional advantage of this approach is that it would enable CHF patients to be treated by a simple intravenous injection during an office visit, circumventing the need for more expensive and potentially hazardous invasive procedures such as cardiac catheterization and intracoronary vector delivery.
A seminal paper in clinical gene therapy recently reports that intravenous delivery of an AAV vector increases serum levels of transgene in a sustained manner safely and effectively.61 This is demonstrated in human subjects with hemophilia B using AAV8 encoding human Factor IX, which is deficient in such patients. Although this study is by necessity limited to a few subjects (n=6), sustained increases in serum levels of Factor IX are documented, and transfusion requirements are reduced, demonstrating a clinical benefit. This paper documents proof of concept for the paracrine approach, and could be tailored easily for application to clinical CHF.
In considering such an approach, the use of a long-term expression vector such as AAV necessitates regulation of transgene expression. Integrating a regulation system (for example, tetracycline-regulated or rapamycin-regulated system)62,63 into the AAV vector would enable turning transgene expression off in the event of untoward effects. For example, if such effects occur, the subject would simply stop taking the activating compound (tetracycline or rapamycin analog). Regulated expression would also enable intermittent rather than constant transgene expression, allowing the physician and patient to tailor therapy by altering the oral dose of activating agents. Doses as low as 10–20 mg doxycycline on alternate days could be adequate to activate the more recent tetracycline-regulation systems.64
To test the feasibility of paracrine-based gene transfer in the failing heart, we injected an AAV5 vector encoding IGF-I under tetracycline-regulated expression (AAV5.IGFI-tet) into skeletal muscle of rats with myocardial infarction-induced severe CHF.63 IGF-I is a peptide with protean favorable cardiovascular effects (inotropic, angiogenic, antiapoptotic). Five weeks after gene transfer, 50% of the rats were randomized to receive doxycycline in their water supply (to activate IGF-I expression; IGF-On); the remaining rats did not receive doxycycline and served as controls (IGF-Off). IGF-On rats showed increased LV ejection fraction (P=0.02) and reduced LV end-systolic diameter (P=0.03). Furthermore, LV contractile function, assessed by the rate of pressure development (LV +dP/dt) during dobutamine infusion, is increased after initiation of IGF-I expression (P=0.001). In addition, favorable changes in cardiac output (P=0.007) and stroke work (P=0.003) are observed. Serum IGF-I is increased 5 weeks after transgene activation (IGF-Off: 164 ± 24 ng ml−1; IGF-On: 218 ± 11 ng ml−1; P=0.008; n=9 each group). These data indicate that skeletal muscle injection of AAV5.IGFI-tet enables tetracycline-activated expression, increases serum IGF-I levels and improves function of the failing heart.63
Translation of these studies to clinical trials will require selection of the optimal AAV vector (likely to be AAV8 or AAV9 rather than AAV5), and intravenous rather than intramuscular delivery. For example, clinical trials in hemophilia that employed intramuscular delivery of AAV vectors are associated with immune responses that abrogated therapeutic Factor IX levels,65,66 a problem that is subsequently circumvented by intravenous delivery.61
Paracrine-based gene transfer may be suitable for any circulating peptide with beneficial cardiovascular effects. For example, in addition to IGF-I and growth hormone, B-type natriuretic peptide is another biologically effective peptide used for the treatment of clinical CHF that could be delivered in a similar manner. Moreover, prostacyclin analogs can be effective in treating pulmonary hypertension. Current agents (epoprostenol and trepostinil) require constant systemic injection, and the treatment itself is associated with high morbidity.67 A regulated expression vector encoding prostacyclin synthase is a plausible paracrine-type gene therapy of pulmonary hypertension.
CONCLUSION
Gene transfer for the treatment of CHF is justified because there is an unmet medical need for treating CHF, and gene transfer, based on preclinical studies, has the potential to meet that need. Preclinical studies have documented the therapeutic potential in CHF of many genes, two of which have advanced to clinical trials: SERCA2a and AC6. However, the major impediment to progress has been the difficulty in obtaining a high-yield transgene expression in the heart. One approach that could potentially circumvent this problem is paracrine-based gene transfer, in which systemic injection of a long-term regulated expression vector encoding a paracrine gene with favorable cardiovascular effects. Whether this approach can overcome numerous obstacles and improve prospects for successful cardiovascular gene therapy is unknown, but preclinical studies are promising. A final note: cell-based therapy has not yet been demonstrated to be effective in double-blinded, placebo-controlled, randomized clinical trials of CHF. Gene transfer is simpler, easier to regulate, and, based upon preclinical studies just as promising as cell therapy. It will be interesting to see which of these approaches is more useful in the treatment of clinical CHF.
ACKNOWLEDGEMENTS
This work was supported by a Grant-in-Aid from American Heart Association, grants from the National Institute of Health (P01 HL66941, HL088426 and HL081741) and a Merit Review Award from the Department of Veterans Affairs.
Footnotes
CONFLICT OF INTEREST
Drs Tang and Gao declare no conflict of interest. Dr Hammond is founder, consultant and equity holder in Renova Therapeutics. Renova was not involved in any manner with the studies reviewed.
AUTHOR CONTRIBUTIONS
TT, MHG and HKH wrote and approved the final draft of the manuscript.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Lai NC, Tang T, Gao MH, Saito M, Takahashi T, Roth DM, et al. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol. 2008;51:1490–1497. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002097. 92ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, et al. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehr447. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaye DM, Preovolos A, Marshall T, Byrne M, Hoshijima M, Hajjar R, et al. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 8.Raake PW, Tscheschner H, Reinkober J, Ritterhoff J, Katus HA, Koch WJ, et al. Gene therapy targets in heart failure: the path to translation. Clin Pharmacol Ther. 2011;90:542–553. doi: 10.1038/clpt.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKirnan M, Lai N, Waldman L, Dalton N, Guo X, Roth D, et al. Intracoronary gene transfer of fibroblast growth factor-4 increases regional contractile function and responsiveness to adrenergic stimulation in heart failure. Card Vasc Regen. 2000;1:11–21. [Google Scholar]
- 10.Leotta E, Patejunas G, Murphy G, Szokol J, McGregor L, Carbray J, et al. Gene therapy with adenovirus-mediated myocardial transfer of vascular endothelial growth factor 121 improves cardiac performance in a pacing model of congestive heart failure. J Thorac Cardiovasc Surg. 2002;123:1101–1113. doi: 10.1067/mtc.2002.121044. [DOI] [PubMed] [Google Scholar]
- 11.Lynch P, Lee TC, Fallavollita JA, Canty JM, Jr, Suzuki G. Intracoronary administration of AdvFGF-5 (fibroblast growth factor-5) ameliorates left ventricular dysfunction and prevents myocyte loss in swine with developing collaterals and ischemic cardiomyopathy. Circulation. 2007;116 doi: 10.1161/CIRCULATIONAHA.106.681866. I71–176. [DOI] [PubMed] [Google Scholar]
- 12.Vincent KA, Jiang C, Boltje I, Kelly RA. Gene therapy progress and prospects: therapeutic angiogenesis for ischemic cardiovascular disease. Gene Therapy. 2007;14:781–789. doi: 10.1038/sj.gt.3302953. [DOI] [PubMed] [Google Scholar]
- 13.Hammond HK, McKirnan MD. Angiogenic gene therapy for heart disease: a review of animal studies and clinical trials. Cardiovasc Res. 2001;49:561–567. doi: 10.1016/s0008-6363(00)00257-1. [DOI] [PubMed] [Google Scholar]
- 14.Poller W, Hajjar R, Schultheiss HP, Fechner H. Cardiac-targeted delivery of regulatory RNA molecules and genes for the treatment of heart failure. Cardiovasc Res. 2011;86:353–364. doi: 10.1093/cvr/cvq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 17.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelhardt S, Grimmer Y, Fan GH, Lohse MJ. Constitutive activity of the human beta(1)-adrenergic receptor in beta(1)-receptor transgenic mice. Mol Pharmacol. 2001;60:712–717. [PubMed] [Google Scholar]
- 19.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, et al. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkers M, Weidenhammer C, Herzog N, Qiu G, Spaich K, von Wegner F, et al. The inotropic peptide betaARKct improves betaAR responsiveness in normal and failing cardiomyocytes through G(betagamma)-mediated L-type calcium current disinhibition. Circ Res. 2011;108:27–39. doi: 10.1161/CIRCRESAHA.110.225201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, et al. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res. 2010;107:1140–1149. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 2010;10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwathmey JK, Yerevanian AI, Hajjar RJ. Cardiac gene therapy with SERCA2a: from bench to bedside. J Mol Cell Cardiol. 2011;50:803–812. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Escoubet B, Prunier F, Amour J, Simonides WS, Vivien B, et al. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias. Circulation. 2004;109:1898–1903. doi: 10.1161/01.CIR.0000124230.60028.42. [DOI] [PubMed] [Google Scholar]
- 26.Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohde D, Brinks H, Ritterhoff J, Qui G, Ren S, Most P. S100A1 gene therapy for heart failure: a novel strategy on the verge of clinical trials. J Mol Cell Cardiol. 2011;50:777–784. doi: 10.1016/j.yjmcc.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Lyon AR, Sato M, Hajjar RJ, Samulski RJ, Harding SE. Gene therapy: targeting the myocardium. Heart. 2008;94:89–99. doi: 10.1136/hrt.2007.116483. [DOI] [PubMed] [Google Scholar]
- 30.Fleury S, Simeoni E, Zuppinger C, Deglon N, von Segesser LK, Kappenberger L, et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation. 2003;107:2375–2382. doi: 10.1161/01.CIR.0000065598.46411.EF. [DOI] [PubMed] [Google Scholar]
- 31.Roth DM, Lai NC, Gao MH, Drumm JD, Jimenez J, Feramisco JR, et al. Indirect intracoronary delivery of adenovirus encoding adenylyl cyclase increases left ventricular contractile function in mice. Am J Physiol Heart Circ Physiol. 2004;287:H172–H177. doi: 10.1152/ajpheart.01009.2003. [DOI] [PubMed] [Google Scholar]
- 32.Hammond HK. Intracoronary gene transfer of fibroblast growth factor in experimental and clinical myocardial ischemia. Gene Ther Regul. 2002;1:325–342. [Google Scholar]
- 33.French BA, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 34.Kaspar BK, Roth DM, Lai NC, Drumm JD, Erickson DA, McKirnan MD, et al. Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus. J Gene Med. 2005;7:316–324. doi: 10.1002/jgm.665. [DOI] [PubMed] [Google Scholar]
- 35.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang T, Lai NC, Roth DM, Drumm J, Guo T, Lee KW, et al. Adenylyl cyclase type V deletion increases basal left ventricular function and reduces left ventricular contractile responsiveness to beta-adrenergic stimulation. Basic Res Cardiol. 2006;101:117–126. doi: 10.1007/s00395-005-0559-y. [DOI] [PubMed] [Google Scholar]
- 37.Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, et al. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- 38.Gao M, Ping P, Post S, Insel PA, Tang R, Hammond HK. Increased expression of adenylylcyclase type VI proportionately increases beta-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci USA. 1998;95:1038–1043. doi: 10.1073/pnas.95.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, et al. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99:1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- 40.Gao MH, Bayat H, Roth DM, Yao Zhou J, Drumm J, Burhan J, et al. Controlled expression of cardiac-directed adenylylcyclase type VI provides increased contractile function. Cardiovasc Res. 2002;56:197–204. doi: 10.1016/s0008-6363(02)00539-4. [DOI] [PubMed] [Google Scholar]
- 41.Lai NC, Roth DM, Gao MH, Fine S, Head BP, Zhu J, et al. Intracoronary delivery of adenovirus encoding adenylyl cyclase VI increases left ventricular function and cAMP-generating capacity. Circulation. 2000;102:2396–2401. doi: 10.1161/01.cir.102.19.2396. [DOI] [PubMed] [Google Scholar]
- 42.Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, et al. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110:330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 43.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, et al. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci USA. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth DM, Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, et al. Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation. 1999;99:3099–3102. doi: 10.1161/01.cir.99.24.3099. [DOI] [PubMed] [Google Scholar]
- 45.Roth DM, Bayat H, Drumm JD, Gao MH, Swaney JS, Ander A, et al. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 46.Rebolledo B, Lai NC, Gao MH, Takahashi T, Roth DM, Baird SM, et al. Adenylylcyclase gene transfer increases function of the failing heart. Hum Gene Ther. 2006;17:1043–1048. doi: 10.1089/hum.2006.17.1043. [DOI] [PubMed] [Google Scholar]
- 47.Roth DM, Drumm JD, Bhargava V, Swaney JS, Gao MH, Hammond HK. Cardiac-directed expression of adenylyl cyclase and heart rate regulation. Basic Res Cardiol. 2003;98:380–387. doi: 10.1007/s00395-003-0429-4. [DOI] [PubMed] [Google Scholar]
- 48.Tang T, Gao MH, Roth DM, Guo T, Hammond HK. Adenylyl cyclase type VI corrects cardiac sarcoplasmic reticulum calcium uptake defects in cardiomyopathy. Am J Physiol Heart Circ Physiol. 2004;287:H1906–H1912. doi: 10.1152/ajpheart.00356.2004. [DOI] [PubMed] [Google Scholar]
- 49.Timofeyev V, He Y, Tuteja D, Zhang Q, Roth DM, Hammond HK, et al. Cardiac-directed expression of adenylyl cyclase reverses electrical remodeling in cardiomyopathy. J Mol Cell Cardiol. 2006;41:170–181. doi: 10.1016/j.yjmcc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi T, Tang T, Lai NC, Roth DM, Rebolledo B, Saito M, et al. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation. 2006;114:388–396. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- 51.Dai B, Huang W, Xu M, Millard RW, Gao MH, Hammond HK, et al. Reduced collagen deposition in infarcted myocardium facilitates induced pluripotent stem cell engraftment and angiomyogenesis for improvement of left ventricular function. J Am Coll Cardiol. 2011;58:2118–2127. doi: 10.1016/j.jacc.2011.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solaro RJ. Modulation of cardiac myofilament activity by protein phosphorylation. In: Fozzarad H, Solaro RJ, editors. Handbook of Physiology, Volume 1, The Heart. New York: Oxford University Press; 2002. pp. 264–300. [Google Scholar]
- 53.Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol. 2007;42:247–259. doi: 10.1016/j.yjmcc.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Guellich A, Gao S, Hong C, Yan L, Wagner TE, Dhar SK, et al. Effects of cardiac overexpression of type 6 adenylyl cyclase affects on the response to chronic pressure overload. Am J Physiol Heart Circ Physiol. 2010;299:H707–H712. doi: 10.1152/ajpheart.00148.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugano Y, Lai NC, Gao MH, Firth AL, Yuan JX, Lew WY, et al. Activated expression of cardiac adenylyl cyclase 6 reduces dilation and dysfunction of the pressure-overloaded heart. Biochem Biophys Res Commun. 2011;405:349–355. doi: 10.1016/j.bbrc.2010.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowe JW, Troen BR. Sympathetic nervous system and aging in man. Endocr Rev. 1980;1:167–179. doi: 10.1210/edrv-1-2-167. [DOI] [PubMed] [Google Scholar]
- 57.Tang T, Hammond HK, Firth AL, Yang Y, Gao MH, Yuan JX, et al. Adenylyl cyclase 6 improves calcium uptake and LV function in aged hearts. J Am Coll Cardiol. 2011;57:1846–1855. doi: 10.1016/j.jacc.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao MH, Tang T, Guo T, Sun SQ, Feramisco JR, Hammond HK. Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J Biol Chem. 2004;279:38797–38802. doi: 10.1074/jbc.M405701200. [DOI] [PubMed] [Google Scholar]
- 59.Gao MH, Miyanohara A, Feramisco JR, Tang T. Activation of PH-domain leucine-rich protein phosphatase 2 (PHLPP2) by agonist stimulation in cardiac myocytes expressing adenylyl cyclase type 6. Biochem Biophys Res Commun. 2009;384:193–198. doi: 10.1016/j.bbrc.2009.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao MH, Tang T, Guo T, Miyanohara A, Yajima T, Pestonjamasp K, et al. Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. J Biol Chem. 2008;283:33527–33535. doi: 10.1074/jbc.M805825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 63.Lai NC, Tang T, Gao MH, Saito M, Miyanohara A, Hammond HK. Improved function of the failing rat heart by regulated expression of insulin-like growth factor I via intramuscular gene transfer. Hum Gene Ther. 2012;23:255–261. doi: 10.1089/hum.2011.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rolain JM, Mallet MN, Raoult D. Correlation between serum doxycycline concentrations and serologic evolution in patients with Coxiella burnetii endocarditis. J Infect Dis. 2003;188:1322–1325. doi: 10.1086/379082. [DOI] [PubMed] [Google Scholar]
- 65.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 66.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 67.O’Callaghan DS, Savale L, Montani D, Jais X, Sitbon O, Simonneau G, et al. Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol. 2011;8:526–538. doi: 10.1038/nrcardio.2011.104. [DOI] [PubMed] [Google Scholar]
- 68.Sastry A, Arnold E, Gurji H, Iwasa A, Bui H, Hassankhani A, et al. Cardiac-directed expression of adenylyl cyclase VI facilitates atrioventricular nodal conduction. J Am Coll Cardiol. 2006;48:559–565. doi: 10.1016/j.jacc.2006.01.082. [DOI] [PubMed] [Google Scholar]
- 69.Gao MH, Tang T, Miyanohara A, Feramisco JR, Hammond HK. beta(1)-Adrenergic receptor vs adenylyl cyclase 6 expression in cardiac myocytes: differences in transgene localization and intracellular signaling. Cell Signal. 2010;22:584–589. doi: 10.1016/j.cellsig.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao MH, Tang T, Lai NC, Miyanohara A, Guo T, Tang R, et al. Beneficial effects of adenylyl cyclase type 6 (AC6) expression persist using a catalytically inactive AC6 mutant. Mol Pharmacol. 2011;79:381–388. doi: 10.1124/mol.110.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]