Abstract
Background and Aims
This study examined the adaptive association between seed germination ecology and specialization to either forest or open habitats across a range of evolutionary lineages of seed plants, in order to test the hypotheses that (1) species' specialization to open vs. shaded habitats is consistently accompanied by specialization in their regeneration niche; and (2) species are thereby adapted to utilize different windows of opportunity in time (season) and space (habitat).
Methods
Seed germination response to temperature, light and stratification was tested for 17 congeneric pairs, each consisting of one forest species and one open-habitat species. A factorial design was used with temperature levels and diurnal temperature variation (10 °C constant, 15–5 °C fluctuating, 20 °C constant, 25–15 °C fluctuating), and two light levels (light and darkness) and a cold stratification treatment. The congeneric species pair design took phylogenetic dependence into account.
Key Results
Species from open habitats germinated better at high temperatures, whereas forest species performed equally well at low and high temperatures. Forest species tended to germinate only after a period of cold stratification that could break dormancy, while species from open habitats generally germinated without cold stratification. The empirically derived germination strategies correspond quite well with establishment opportunities for forest and open-habitat plant species in nature.
Conclusions
Annual changes in temperature and light regime in temperate forest delimit windows of opportunity for germination and establishment. Germination strategies of forest plants are adaptations to utilize such narrow windows in time. Conversely, lack of fit between germination ecology and environment may explain why species of open habitats generally fail to establish in forests. Germination strategy should be considered an important mechanism for habitat specialization in temperate herbs to forest habitats. The findings strongly suggest that phases in the plant life cycle other than the established phase should be considered important in adaptive specialization.
Keywords: Habitat specialization, germination cueing, beta niche, dormancy, forest herbs, phylogenetically independent contrasts (PICs), plant functional traits, regeneration niche, shade, monocotyledons, dicotyledons
INTRODUCTION
Environmental filtering is an important driver of plant community assembly. The biotic and abiotic environment acts upon morphological, physiological and life-history traits as a sieve, selectively filtering out certain species while allowing others to pass through (Harper, 1977; Bazzaz, 1991; Keddy, 1992; Grime, 2006). Trait filtering may be driven by resource competition and plant interactions within habitats (alpha niche diversification) and by environmental conditions at the between-habitat level (habitat diversification/beta niches) (Whittaker, 1975; Pickett and Bazzaz, 1978; Silvertown et al., 2006). In this study, we focus on the latter mechanism, by investigating the effects of interaction of environmental cues and seed traits on habitat specialization during the germination phase of plant life.
Traditionally, plant ecologists have aimed at identifying links between habitat specialization and traits of established individuals, e.g. Raunkiær's life forms (Raunkiær, 1904, 1934) and Grime's CSR strategy scheme (Grime, 1977, 2001), implicitly assuming that niches are uniform and constant throughout all life stages (e.g. MacArthur and Levins, 1967). However, niche requirements may change during the life of individuals, for example among plants establishing in environments that characteristically change during a plants' life span (Eriksson, 2002). This phenomenon, called ‘ontogenetic niche shifts’, is rarely addressed in plant ecology. The importance to habitat specialization of plant traits and requirements of the regenerative phase, i.e. their regeneration niche (Grubb, 1977), is therefore much less studied.
Plants appear to provide their seeds with mechanisms to ensure that germination takes place in a certain habitat and/or at a certain time of the year. Seed dormancy is one mechanism by which seed germination can be effectively delayed until favourable conditions for seedling establishment are likely to occur. In temperate regions, physiologically dormant seeds often require a period of chilling to break dormancy, which in practice means that dormancy is broken during winter (Baskin and Baskin, 1998). Germination can subsequently take place, usually later on during spring since temperatures required to break dormancy are often too low also to trigger germination. Light and temperature are the major environmental factors influencing germination. Species differ in temperature ranges over which germination can take place (Probert, 2000), and fluctuating temperatures have been shown to stimulate germination in many species (Knapp, 1956; Thompson and Grime, 1983; Schütz, 1999). Light serves as a cue for germination in many species (Grime and Jarvis, 1975; Thompson, 1989; Vleeshouwers et al., 1995; Pons, 2000). Milberg et al. (2000) stated that small-seeded species are more likely to have a light requirement for germination than larger seeded species, and suggested that seed mass and light requirement for germination might be coevolved plant traits.
The light environment in forests depends on the degree of filtering by the canopy. Due to absorption of red wavelengths by plant leaves, the red to far-red ratio (R:FR ratio) is reduced in canopy-filtered light. A requirement for high R:FR conditions, which are likely to indicate high radiation levels, is assumed specifically to benefit small-seeded species which would facilitate rapid seedling growth and thereby reduce mortality in the otherwise vulnerable small seedlings of these species (Jankowska-Błaszczuk and Daws, 2007).
Habitat specialization results from the requirements of all life stages of the plant, during both the regenerative (germination, seedling establishment) and vegetative (growth, survival, reproduction) phases. One may ask whether a certain life stage is more demanding in its requirements than others, and thus, for any sessile organism, effectively governs habitat specialization. However, that question is difficult to answer since for all life stages passed through by established plants found in nature, the needs have apparently been satisfied. Nevertheless, germination cueing was found to determine habitat specialization of six cold-temperate caespitose Carex species (Schütz, 1997). Species from wooded wetlands germinated at low and constant temperatures, whereas species from open wetlands germinated at higher temperatures with large diurnal fluctuations. A similar role for germination cueing in microhabitat preference was found for two Erodium species inhabiting grasslands gaps (Rice, 1985). Diurnal soil temperature fluctuation was found to promote germination response and to be much larger in gaps than in the surrounding vegetation.
In this study, we investigated the general applicability of the adaptive association between specific germination traits and habitat preference by testing across a wide range of evolutionary lineages. We adopted a comparative approach, using a large number of congeneric species pairs with contrasting habitat preference, to investigate whether germination traits of seeds co-vary predictably with habitat preference. Each pair provided an independent replicate of evolutionary divergence in habitat preference. Additionally, the shared evolutionary history until divergence within pairs would allow unequivocal exclusion of confounding effects of unmeasured traits that species might share through common descent rather than through independent evolution (Rees, 1995; Ackerly, 1999) or niche divergences deeper in the phylogeny.
The ecological contrast investigated is between species from open vs. shaded habitats, and the germination responses to environmental conditions typical for these two contrasting habitats. In open habitats, the absence of a covering tree canopy results in larger diurnal temperature fluctuations in the topsoil. The light environment in open habitats depends on the height and density of the herbaceous vegetation. The microclimate of temperate forests is characterized by a closure of the tree canopy in late spring with an accompanying reduction of the temperature and daily amplitude relative to open habitats, as well as a reduction of light quantity (photosynthetic photon flux density; PPFD) and R:FR ratio. Establishment opportunities of various forest herbs in northern temperate deciduous forests are hypothesized to depend not only on occurrence of canopy gaps. Gap size (single vs. multiple tree fall gaps) also plays a role, whereby the gap-demanding species are reported to have a light requirement whereas small-seeded species that are more shade tolerant in the adult phase do not need light for germination. Based on Ellenberg indicator values for light, (Jankowska-Błaszczuk and Grubb, 2006), we categorized forest herbs into non-gap-demanding species (Ellenberg 1–3), single tree fall gaps (4–6) and multiple tree fall gaps (7+).
The aim of this study is to test the hypotheses that (1) species' specialization to open and shaded habitats, respectively, is consistently accompanied by specialization in the regeneration niche; and (2) species are thereby adapted to utilize different windows of opportunity in time (season) and space (habitat). If species from contrasting habitats are able to germinate in each others' habitat and the seedlings are able to emerge and establish, it can be concluded that the environmental requirements and competitive ability of the mature individuals govern the habitat specialization of the species. If, on the other hand, species with contrasted habitat preference respond differently to environmental conditions at the between-habitat level already in the germination stage, the conclusion can be drawn that the regeneration niche is significant to habitat specialization.
MATERIALS AND METHODS
Species selection
When selecting species, we considered as candidates all congeneric pairs of herbaceous species occurring in southernmost Sweden and adjacent eastern Denmark and forming an open–shaded habitat match. Low availability of sufficient amounts of ripe seeds during the period of collection excluded some species, due to, for example, too sparse populations, low seed production (some common species have low but continuous seed release), heavy seed predation, etc. This constrained the number of species to a workable number, so no random selection of study species pairs from a gross list of candidates was done, as otherwise recommended by Ackerly (1999) and Westoby (2002). Species were considered to prefer shaded habitats if they predominantly occur in habitats with a tree canopy, and the opposite for open-habitat species. Judgement of habitat preference was based on field experience of the authors and their colleagues. Species tending to occur in both open and shaded habitats were excluded. Ellenberg indicator values for light are provided in Table 1. We consider the Ellenberg estimates for light for the forest species Bromus ramosus (6), Primula elatior (6) and especially Hypericum hirsutum (7) to be too high, since in Southern Scandinavia all these species are bound to shaded forest habitats at lower light conditions than the Ellenberg values suggest. A total of 34 species in 17 congeneric pairs were used in this study. Each species pair consisted of two congeners, except Hordelymus europaeus and Hordeum murinum, which belong to closely related genera (Table 1). The species included were seven pairs of grasses, two pairs of sedges (Carex) and nine pairs of forbs. Four genera (Bromus, Carex, Festuca and Stellaria) were represented with two species pairs each. The grouping into pairs within these genera was based on phylogenetic relatedness (Table 1). Nomenclature is based on Flora Europaea (Tutin et al., 1964–1980).
Table 1.
Species used in the study
| Genus | Forest | SM | SB | El | Open habitat | SM | SB | El | Seed origin |
|---|---|---|---|---|---|---|---|---|---|
| Brachypodium | sylvaticum (Huds.) P.Beauv. | 2·86 | 1 | 3 | pinnatum (L.) P.Beauv. | 2·40 | 1 | 6 | C |
| Bromus | benekenii (Lange) Trimen | 4·94 | 1 | 5 | erectus Huds. | 6·18 | 1 | 8 | RV |
| Bromus | ramosus Huds. | 4·88 | 1 | 6 | inermis Leyss. | 4·19 | 1 | 8 | G |
| Campanula | trachelium L. | 0·22 | 3 | 4 | rotundifolia L. | 0·05 | 3 | 7 | G |
| Carex | sylvatica Huds. | 0·54 | 3 | 3 | lepidocarpa Tausch | 0·80 | 3 | 9 | G |
| Carex | remota L. | 2·03 | 3 | 2 | ovalis Gooden. | 0·61 | 3 | 7 | G |
| Festuca | altissima All. | 0·94 | 1 | 3 | arundinacea Schreb. | 1·39 | 1 | 8 | CD |
| Festuca | gigantea (L.) Vill. | 2·31 | 1 | 4 | pratensis Huds. | 2·36 | 1 | 8 | C |
| Geum | urbanum L. | 2·69 | 2 | 4 | rivale L. | 1·23 | 1 | 6 | G |
| Hordelymus/Hordeum | europaeus (L.) Harz | 7·40 | 1 | 4 | murinum L. | 5·18 | 2 | 8 | U |
| Hypericum | hirsutum L. | 0·09 | 3 | 7 | perforatum L. | 0·11 | 3 | 7 | G |
| Poa | nemoralis L. | 0·17 | 1 | 5 | pratensis L. | 0·24 | 3 | 6 | G |
| Primula | elatior (L.) Hill | 0·73 | 1 | 6 | farinosa L. | 0·06 | 1 | 8 | G |
| Rumex | sanguineus L. | 0·93 | 3 | 4 | obtusifolius L. | 1·77 | 2 | 7 | RV |
| Silene | dioica (L.) Clairv. | 0·83 | 3 | 5 | latifolia Poir. ssp. alba (Mill.) Greuter & Burdet | 0·67 | 3 | 8 | CD |
| Stellaria | holostea L. | 4·31 | 1 | 5 | graminea L. | 0·21 | 3 | 6 | G |
| Stellaria | nemorum L. | 0·27 | 1 | 4 | media (L.) Vill. | 0·45 | 3 | 6 | C |
SM, seed mass (mg); SB, seed bank classification (Thompson et al., 1997). El, Ellenberg indicator value for light.
Seed origin refers to species from open habitats: C, commercial supplier; CD, coastal dune; G, grassland; RV, road verge; U, urban area. All seed of forest species were collected in deciduous forests.
Seed collection
Freshly matured seeds were collected between July and October 2004. Seed collections came from one population per species, but from several individuals per population. Special care was taken to collect only seeds of open-habitat species from open sites without any tree canopy, e.g. grasslands, road verges, ruderal sites and urban areas, and, likewise, seeds of shaded-habitat species were collected from forests only. This was done to avoid confounding effects of induced light requirement (Cresswell and Grime, 1981) and local ecological differentiation within species. Collected seeds were air-dried at room temperature and subsequently stored in paper bags at room temperature until further use. Field-collected species were complemented with seeds from commercial suppliers in the case of three species, all being open-habitat species (Table 1). These species were cultivated for one generation from seed collected in the wild and thus were not selected for specific seed characteristics.
After collection, seeds were visually checked, and only firm, filled seeds were used. Seeds of aberrant colour or shape or with signs of predation or underdevelopment were discarded.
Germination test and experimental design
In February 2005, germination tests were initiated in temperature-controlled climate rooms equipped with 400 W metal halide lamps (∼200 µmol m−2 s−1). The germination tests were performed as a full factorial design with two temperature treatment factors, temperature level and diurnal variation, each with two levels: constant-low (10 °C), constant-high (20 °C), fluctuating-low (15/5 °C) and fluctuating-high (25/15 °C) temperatures and light/darkness. The temperature conditions used correspond to daily means and diurnal fluctuations in spring (April) and summer (June) in southern Sweden. Diurnal temperature fluctuations corresponded to a light regime of 12 h (higher temperature) and 12 h of darkness (lower temperature).
For each species and treatment combination, five replicates of 50 seeds were placed in 5 or 9 cm Petri dishes on filter paper (Munktell 00K, Grycksbo, Sweden) and wetted with distilled water. A lower number of seeds per Petri dish was used due to limited availability of seeds for Hordelymus europaeus (45), Hordeum murinum (40) and Stellaria holostea (30). In the dark treatment, the Petri dishes were wrapped in a double layer of aluminium foil immediately after wetting. The Petri dishes were randomly distributed on benches in the climate rooms.
Initially, seeds were exposed to the various treatment combinations for a period of 14 d (hereafter called ‘first incubation period’). The 14 d period for germination was recommended by Baskin and Baskin (1998, p. 19). The germination criterion used was the visible protrusion of the radicle. Subsequently, the Petri dishes were put in plastic bags (to avoid desiccation) and stored at 2 °C for stratification for 3·5 months to allow the ungerminated seeds to break possible (physiological) dormancy. At the end of the stratification period, germinated seeds were counted and removed from the Petri dishes again. The stratification was followed by a second incubation period of 14 d with conditions identical to those in the first incubation period. Species which did not germinate at all during the first incubation did so after stratification. At the end of the second incubation period, the germination experiment was terminated and a final count of germinated seeds was made. In the light treatments, germinated seeds were counted regularly and removed from the Petri dish. In the dark treatment, germinated seeds were counted once after each of the three treatment periods. The counts in the dark treatment were made under dim green light. Petri dishes were kept moist continuously during the experiment. Petri dishes in light treatment periods received distilled water when needed during the incubation. The wrapping of the Petri dishes in aluminium foil (dark treatments) and additional plastic during the stratification period prevented desiccation of the seeds and filter paper. Of a total 1360 Petri dishes in the experiment, 23 were discarded due to mould contamination and hence were not included in statistical analyses (see Supplementary Data Table S1 for sample sizes).
Data analysis
The fate of seeds was analysed with generalized linear mixed modelling (GLMM), using the package lme4 (Bates and Sarkar, 2007) under the open source environment R (R Development Core Team, 2012). One model considered seed germination as a binomial response variable of germinated vs. non-germinated seeds per Petri dish. The germination timing was analysed in a second model, considering the binomial response variable of germination, taking place either pre- or post-cold stratification (germination during the cold stratification was lumped with the post-chilling germination for analysis and graphic representation). Both models used the binomial family type with the logit link function (Zuur et al., 2009). To take the paired-species design into account, genus identity was included as random factor. Further, the non-hierarchal experimental design consisted of 23 treatment combinations, which were considered by a triple crossed random effect structure for the factors light (darkness/light), warmth (cold/warm) and temperature fluctuation (constant/fluctuating). Seed mass was included as a linear explanatory variable. A required correction for overdispersion was performed by including Petri dish identity as an additional random effect, accounting for a high variance among observed values. Both models considered all six possible three-way interactions with habitat, all two-way double interactions, and the five single factors (habitat, light, temperature, fluctuation and seed mass). Based on maximum likelihood estimates and an AIC (Akaike information criterion) improvement (<2), explanatory variable combinations were sequentially eliminated from the model if not contributing explanatory power. A log transformation (ln) on the linear explanatory variable seed mass was used, because this improved the AIC value of the full model by 19·7 points. Either transformed or untransformed, the mean seed mass did not differ between open habitats and forests (paired two tailed t-tests: P = 0·12 and P = 0·13 for log-transformed and untransformed seed mass values, respectively). The final P-value approximations were based on Wald χ2 tests. Alpha was set at 0·025, since two analyses were performed on one data set (α = 0·05/2).
RESULTS
Treatment and seed mass effects on germination
Of a total 65 540 incubated seeds, 38 017 seeds germinated successfully (58 %). Open-habitat species had a higher germination percentage than forest species. All remaining main effects in the analysis (light, warmth, fluctuation and seed mass) were found to affect germination percentage significantly. High and fluctuating temperatures as well as light and high seed mass prevailed over low and constant temperatures and darkness and low seed mass, respectively. However, effects differed across species (Fig. 1). Mean germination proportions and standard deviations for each treatment combination are listed in Supplementary Data Table S1.
Fig. 1.
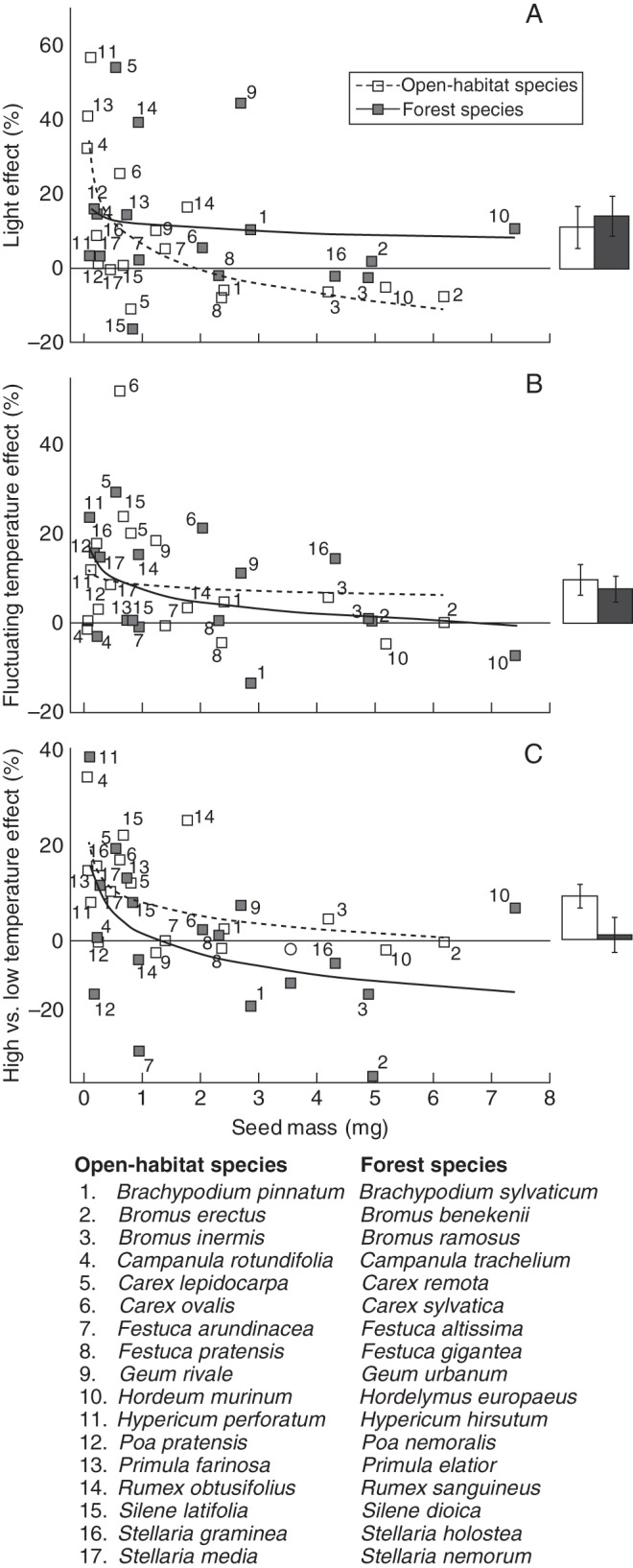
The absolute difference in germination response between light vs. darkness, fluctuating vs. constant temperature, and high vs. low temperatures are shown in relation to seed mass for all species in the experiment. The effects are calculated as percentage germination in light minus percentage germination in darkness, etc. The bars to the right of each scatter graph show means ± s.e. error for all open-habitat and forest species in that graph. Trend lines are logarithmic.
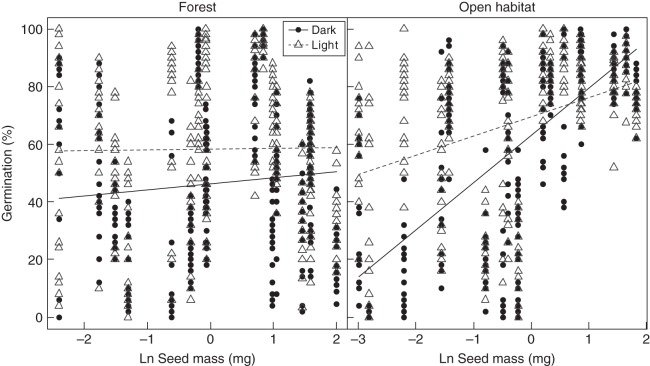
Two-way interactions of habitat with light, warmth and seed mass, respectively, were significant. Forest species responded better to light over darkness than did open-habitat species (Fig. 1A, barplot). For the forest species, light appeared to serve as a substitute for temperature fluctuations, because at constant temperature, light improved the germination response strongly compared with the dark treatments. The advantage of warmth over cold was higher among open-habitat species than in forest species, which appeared to be rather indifferent to temperature level. In general, germination of open-habitat species increased with increasing seed mass, whereas the germination of forest species was not affected by seed mass (Fig. 2). The beneficial effect of fluctuating over constant temperatures did not differ significantly across habitat types (Fig. 1B: barplot).
Fig. 2.
The three-way interaction between the explanatory factors habitat, light and seed mass on the response variable germination. Each vertical row is made up of 40 Petri dish data points for a single species (a lower number of visible data points is due to exact overlap of the germination count in some Petri dishes, and some discarded Petri dishes; see main text for details). The considerable variation in germination response within the species reflects the effects of the different temperature treatments, which are not distinguished here. The forest species show a stronger germination response in light compared with darkness, but their germination response is generally not related to the seed mass (near-horizontal lines). In contrast, the open-habitat species show a clear higher germination response with increasing seed mass (ascending lines). This effect is stronger under dark germination circumstances than in light (i.e. the lines cross).
All remaining two-way interactions between the treatment factors and seed mass, except the light–warmth interaction, were significant. Small-seeded species responded better at high or fluctuating temperatures or in light, while the larger seeded species were indifferent or showed an opposite effect (Fig. 1A, B and C: trendlines). Fluctuating temperatures had an amplifying effect on germination in light and warmth, respectively, whereas the combination of constant temperatures with low temperatures or darkness, respectively, resulted in the lowest germination.
The strong effect of seed mass on germination response in open habitats as reported above appeared to be stronger in darkness than in light, whereas germination response in forest species was indifferent to seed mass in darkness and light (significant three-way interaction; Fig. 2).
The direction of the three-way interaction of habitat, light and warmth is depicted in Fig. 3, which shows that germination in open-habitat species is stimulated by either warmth or light, while the combination of both has a slightly amplifying effect. Forest species, however, hardly respond to warmth, but do respond strongly to light over darkness.
Fig. 3.
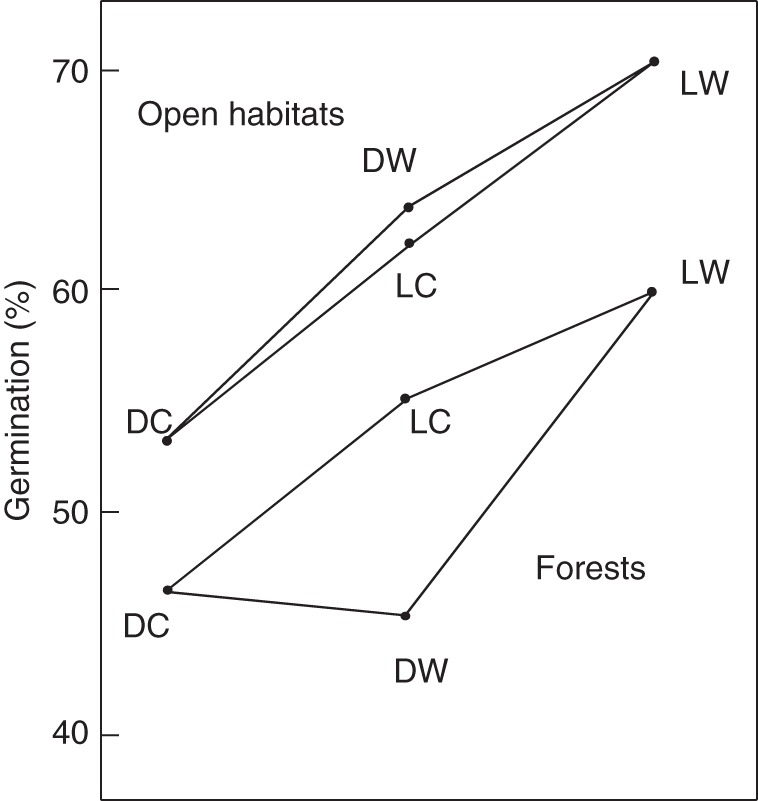
Three-way interaction of habitat, warmth and light showing germination in open habitat species (upper tetragon) to be stimulated under dark, cold circumstances by either warmth (DW) or light (LC), with the combination of both having an additive effect (LW). In contrast, the forest species (lower tetragon) do not show a positive response to warmth in darkness (DW).
Germination timing
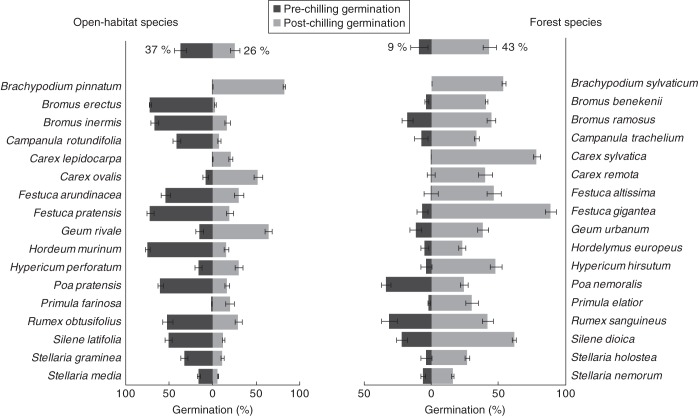
A striking difference was found in germination timing between forest and open-habitat species. Whereas the species from open habitats germinated considerably during the first incubation (59 % of total germination), the forest species germinated largely after the cold stratification period (72 %) (Fig. 4). In general, germination during the stratification period itself was low for species from both habitat types, since the stratification temperature of 2 °C is too low for most species to germinate. Considerable proportions of the seeds of some grass species, however, germinated during the stratification period (see Supplementary Data Table S1).
Fig. 4.
The pre- and post-chilling germination of open-habitat and forest species. The bars at the top of each graph represent overall mean germination values (±s.d.) over 17 species per habitat type. The remaining bars show the mean results (±s.d.) per species, for which the different treatments were pooled. The combined length of the pre- and post-chilling germination bars reflects the overall germination response, whereas the germination timing is reflected by the proportion of pre- and post-chilling germination (note different scales on x-axis).
Of the main effects in the analysis, warmth and fluctuation also significantly affected germination timing (Table 2). Low and constant temperatures delayed germination with an increased proportion of seeds germinating after cold stratification relative to high and fluctuating temperatures. The effects of these treatment factors did not vary with habitat.
Table 2.
Model results of effects of treatments and seed mass on germination and germination timing
| Germination |
Germination timing |
|||||||
|---|---|---|---|---|---|---|---|---|
| d.f. | χ2 | P | Sign | d.f. | χ2 | P | Sign | |
| Habitat | 1 | 83·6 | <0·001 | * | 1 | 596·9 | <0·001 | * |
| Light | 1 | 74·0 | <0·001 | * | 1 | 6·40 | 0·011 | * |
| Warmth | 1 | 29·6 | <0·001 | * | 1 | 307·3 | <0·001 | * |
| Fluctuation | 1 | 68·8 | <0·001 | * | 1 | 86·0 | <0·001 | * |
| Seed mass | 1 | 38·5 | <0·001 | * | 1 | 6·51 | 0·011 | * |
| Habitat × light | 1 | 6·0 | 0·01 | * | 1 | 2·24 | 0·13 | |
| Habitat × warmth | 1 | 5·9 | 0·02 | * | 1 | 0·98 | 0·32 | |
| Habitat × fluctuation | – | – | 1 | 8·11 | 0·004 | * | ||
| Habitat × seed mass | 1 | 62·4 | <0·001 | * | 1 | 28·6 | <0·001 | * |
| Light × warmth | 1 | 0·15 | 0·70 | 1 | 18·1 | <0·001 | * | |
| Light × fluctuation | 1 | 21·1 | <0·001 | * | 1 | 15·5 | <0·001 | * |
| Light × seed mass | 1 | 72·8 | <0·001 | * | 1 | 24·6 | <0·001 | * |
| Warmth × fluctuation | 1 | 8·11 | 0·004 | * | 1 | 4·63 | 0·031 | |
| Warmth × seed mass | 1 | 30·5 | <0·001 | * | 1 | 0·90 | 0·34 | |
| Fluctuation × seed mass | 1 | 13·5 | <0·001 | * | 1 | 0·03 | 0·87 | |
| Habitat × light × warmth | 1 | 6·46 | 0·011 | * | 1 | 10·1 | 0·001 | * |
| Habitat × light × fluctuation | – | – | 1 | 0·04 | 0·84 | |||
| Habitat × light × seed mass | 1 | 30·3 | <0·001 | * | – | – | ||
| Habitat × warmth × fluctuation | – | – | 1 | 1·50 | 0·22 | |||
| Habitat × warmth × seed mass | – | – | 1 | 0·19 | 0·66 | |||
| Habitat × fluctuation × seed mass | – | – | 1 | 4·86 | 0·03 | |||
All interactions without explanatory value (improvement AIC <2) have been sequentially excluded from the models (–). P-value approximations were based on Wald χ2 tests (α ≤ 0·025).
The delaying effects of constant and low temperatures were more pronounced in light than in darkness. The effect of seed mass on germination timing can be attributed mainly to open-habitat species (significant habitat–seed mass interaction), among which the proportion of seeds delaying germination until after cold stratification increased with seed mass, whereas for forest species a slight opposite effect was observed. The positive habitat–light–warmth interaction can be interpreted as advanced germination at high temperatures across habitats, while for forest this effect was only observed in light.
DISCUSSION
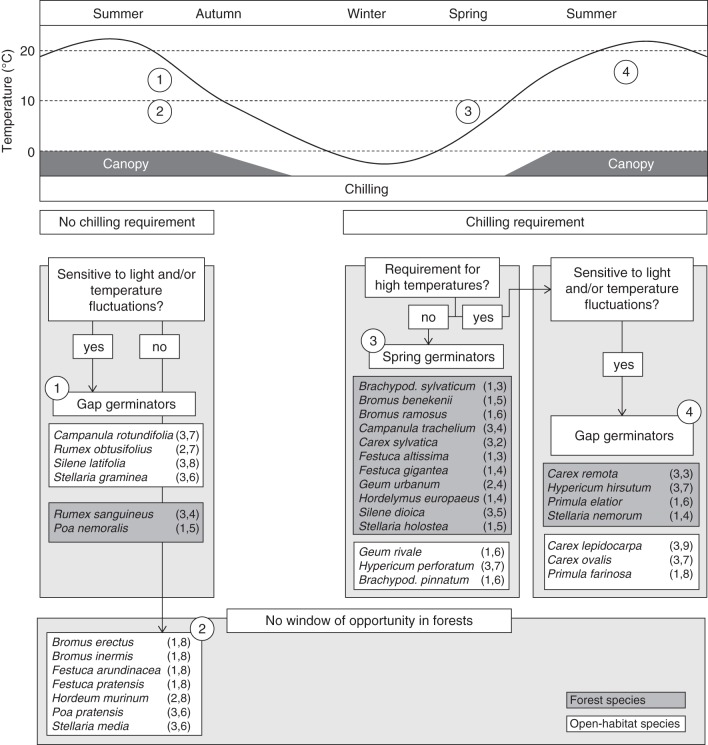
The experimental results show that specialization of plant species to contrasted habitats (open vs. shaded) is linked to specialization in the regeneration niche. A strong tendency for species from shaded habitats to depend on a period of chilling to break seed dormancy was found. Such a requirement effectively delays germination until spring. In contrast, most species from open habitats germinated readily without chilling, which in nature allows germination at any favourable point in time after seed dissemination. A conceptual model of the filtering interaction between seasonal temperature and tree canopy cover with germination strategy is shown in Fig. 5. A set of scenarios predict the success and failure of species when establishing from seed in forests habitats. Non-dormant species can germinate readily when the right set of environmental conditions is present. However, in a forest, seeds would rely on gaps in the closed tree canopy to establish during summer when the tree canopy is fully developed. Most open-habitat species fall within this scenario (Fig. 5, scenario 1). For species to be able to utilize tree canopy gaps, which by nature are unpredictable in space and time, they need to be able to sense when they are in a gap. Such a gap-sensing mechanism could consist of response to diurnal fluctuating temperatures and/or unfiltered light which characterize the forest floor below canopy gaps compared with the conditions below a closed canopy. We considered species to be able to establish in gaps when either the ‘warmth effect’ or the ‘light effect’ was >10 % (see Fig. 1).
Fig. 5.
A conceptual model of how seasonal temperature and presence of the tree canopy interact with different germination strategies, resulting in four potential scenarios explaining success and failure of species to establish in shaded habitats. Species without a chilling requirement, and thus apparent dormancy, are potentially ready to germinate when the right conditions are present. Immediately after seed dispersal they would need canopy gaps to germinate, which requires them to be sensitive to light and/or temperature fluctuations as a gap-sensing mechanism (1). In the case of late seed dispersal and a requirement for high temperatures, germination will be postponed to the next summer, and in forest species will also need a gap to germinate. For species lacking a gap-sensing mechanism, no windows of opportunities for establishment in forest are available (2). When seeds are dormant, a period a chilling is often required, delaying germination until after the winter (3, 4). Species tolerating low temperatures can germinate during early spring (3), whereas those species requiring chilling to break dormancy followed by a high temperature requirement for germination are bound to canopy gaps (4). Numbers after species names are the seed bank classification type and Ellenberg value for light, respectively. The temperature curve is based on monthly mean temperatures from the period 1961–2004 from a weather station near Lund in southern Sweden.
For species with dormant seeds, the need for a cold period to break dormancy prevents species from germinating during summer or autumn following seed dispersal. In temperate forests, rising temperature in spring is followed by canopy closure, which hampers establishment. However, the period between snowmelt and canopy closure constitutes a short window of opportunity for species able to germinate at low temperature (Fig. 5, scenario 3). Species that do have a chilling requirement, but perform poorly under subsequent low temperatures in spring, again rely on canopy gaps to be able to establish in forests (Fig. 5, scenario 4). Although species with non-dormant seeds tend to be less persistent in the soil (Thompson et al., 2003), a lack of suitable conditions might put the seed into quiescence, earlier incorrectly referred to as enforced dormancy (Harper, 1977; Thompson et al., 2003). Consequently non-dormant species of scenario 1 can also delay germination until the next summer (in which case they will also rely on canopy gaps) if seed dispersal occurs late in the season and species have a high temperature requirement for germination. This is the case for all open-habitat species in scenario 1. Our experimental results suggest that the absence of a chilling requirement, common to most open-habitat species, as well as the inability for most species to establish in gaps, contribute to explaining why those species generally do not establish in forest habitats (scenario 2). In contrast, forest species tend to conform to scenario 3, having both a chilling requirement and an ability to germinate at low temperatures. A tendency for forest species to respond to low temperatures similar to that observed in our study was found in a screening of 32 Carex species (Schütz and Rave, 1999; Schütz, 2000).
Some atypical life cycles that are not covered in the conceptual model are those of winter annuals which germinate in autumn or winter at low temperatures, live through the winter and bloom and complete their life cycle in winter or spring, and of species in forests, mainly trees, which form a seedling bank and are able to survive prolonged periods under sub-optimal conditions waiting for the light conditions to improve, e.g. following a gap-forming disturbance.
Canopy gaps in natural forests are generally temporary and occur unpredictably in space and time. Although a species might be able to sense the favourable conditions in gaps, for a seed to establish in a gap, it needs to be able to survive in the soil awaiting good conditions, e.g. be able to form a seed bank. We classified the species according to the seed bank types of Thompson et al. (1997) (Table 1). Some gap-requiring forest species (Fig. 5) are not classified as having a persistent seed bank, therefore they might only be able to establish along forest edges or utilize permanent canopy openings in forests, e.g. alongside paths or streams. Likewise, the high Ellenberg indicator values for light for open-habitat species indicate that they are probably not able to establish permanently in forests.
Jankowska-Błaszczuk and Grubb (2006) reported that small-seeded species without a gap requirement for establishment did not need light for germination. We do not have data or observations on actual gap requirements of the species used in the experiment in the field. However, in accordance with the conceptual model, the species that belong to the early spring germinators, which are considered not gap demanding, include species which significantly improve germination in light vs. darkness. A clear separation between species with or without light requirement for germination with a threshold of 1·5 mg as reported for seeds of forest species by Jankowska-Błaszczuk and Daws (2007) was not observed in our data (Fig. 1A; trendline).
The trends of shifting pre–post chilling ratios towards germination after cold stratification at low and/or constant temperatures can be explained with Vleeshouwers' (1995) concept of dormancy. The degree of dormancy is influenced by external temperature and varies on a continuous scale, whereby chilling is a common way for seeds to break dormancy. As dormancy decreases, the temperature range over which seeds can germinate widens. Seeds can be dormant to such an extent that they only respond to high and/or fluctuating temperatures (Vleeshouwers et al., 1995).
Seeds of open-habitat and forest species appeared to differ consistently in the degree of dormancy when shed from the mother plant. However, two different groups of species could be observed among the species with an apparent high degree of dormancy: (1) a group of species, exclusively monocots, which showed no or minimal germination before chilling over all treatments (Brachypodium pinnatum, B. sylvatica, Carex lepidocarpa, C. remota, C. sylvatica, Festuca altissima) and (2) a group of species that responded to fluctuating–high temperatures only before cold stratification, sometimes along with a light requirement (Bromus benekenii, Campanula trachelium, Carex ovalis, Festuca gigantea, Geum rivale, G. urbanum, Hordelymus europaeus, Hypericum hirsutum, Primula elatior, P. farinosa, Stellaria holostea, S. nemorum) (see Supplementary Data Table S1 for species-specific details). The presence of a few open-habitat species in both groups suggests that either a chilling requirement (dormancy) is shared in some congeneric species despite different habitat preferences – pointing to phylogenetic constraints – or, more probably, that some mechanism of habitat specialization other than germination cueing underlies the habitat differentiation within some congeneric species pairs.
Both open-habitat and forest species germinated more in light than in darkness, and light appeared to serve as a substitute for temperature fluctuations, especially in forest species. Light and temperature fluctuations have both been found earlier to serve as indicators of soil burial depth for seeds (Vleeshouwers et al., 1995; Pons, 2000; Fenner and Thompson, 2005).
The experimental results offer an explanation for why species adapted to conditions in open habitats often fail to establish in forests (Fig. 5). Our findings are not incompatible with studies showing forest species to have adaptive traits in the seedling or adult phase to survive in shaded habitats, such as shade avoidance or shade tolerance syndromes (Henry and Aarssen, 1997; ten Brink and Bruun, 2011). It remains, however, to be investigated to what degree germination strategy and attributes of the established phase, such as shade tolerance, are coevolved (Silvertown, 1981). However, as stated before, germination and establishment are the first critical life stages of plants, and therefore in all probability play an important role. Although some open-habitat species seem to be able to utilize small forest gaps for germination, our results show that many other open-habitat species appear not to be adapted to establish in forests. An explanation as to why forest species do not grow in open habitats cannot be derived from our results, but could be sought in a trade-off between adaptations to the shaded environment in forests and competitive ability in open habitats with abundant light, and hence in competitive exclusion of forest species in open habitats.
Our selection of 34 different herbaceous species representing different functional groups from different plant communities and the conceptual model enable us to generalize beyond particular species. When extrapolating from a laboratory germination test to the field situation, however, it should be noted that seasonal temperature development varies between years and, connected with that, the development and senescence of the tree canopy. Also, the timing of the canopy closure and the light environment on the forest floor depend on the tree species. Nevertheless, we are able to identify important causal effects of certain environmental cues on the germination response of forest and open-habitat species.
We conclude that there is a strong linkage between germination traits and habitat preference of herbs, and that germination strategies are one likely mechanism behind the coarse partitioning in open vs. shaded habitats of herbs in temperate regions. Our results may be valuable to mechanistic approaches to community assembly (Shipley et al., 2006) as well as in assessing possible effects of global warming on the distribution of plant species, if this global warming leads to a reduced chilling period and introduces unpredictable warm spells during winter.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This research was funded by the Department of Biology, Lund University. We thank Stefan Andersson for discussions of the study and the text, Per Milberg and Pål Axel Olsson for comments on the manuscript, Torbjörn Tyler for assistance in locating populations of the study species for seed collection, and Emily Martin for linguistic assistance.
LITERATURE CITED
- Ackerly DD. Comparative plant ecology and the role of phylogenetic information. In: Press MC, Scholes JD, Barker MG, editors. Physiological plant ecology. Oxford: Blackwell Science; 1999. pp. 391–413. [Google Scholar]
- Baskin CC, Baskin JM. Seeds. Ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Bates D, Sarkar D. Lme4: Linear Mixed-Effects models using S4 classes. 2007 R package version 0·999375-42. http://CRAN.R-project.org/ [Google Scholar]
- Bazzaz FA. Habitat selection in plants. American Naturalist. 1991;137:S116–S130. [Google Scholar]
- ten Brink DJ, Bruun HH. Seedling stage strategies as a means of habitat specialization in herbaceous plants. PLoS One. 2011;6:e23006. doi: 10.1371/journal.pone.0023006. http://dx.doi.org/10.1371/journal.pone.0023006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell EG, Grime JP. Induction of a light requirement during seed development and its ecological consequences. Nature. 1981;291:583–585. [Google Scholar]
- Eriksson O. Ontogenetic niche shifts and their implications for recruitment in three clonal Vaccinium shrubs: Vaccinium myrtillus, Vaccinium vitis-idaea, and Vaccinium oxycoccos. Canadian Journal of Botany. 2002;80:635–641. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Grime JP. Plant strategies, vegetation processes, and ecosystem properties. 2nd edn. Chichester, UK: John Wiley & Sons; 2001. [Google Scholar]
- Grime JP. Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. Journal of Vegetation Science. 2006;17:255–260. [Google Scholar]
- Grime JP, Jarvis BC. Shade avoidance and shade tolerance in flowering plants II. Effects of light on the germination of species of contrasted ecology. In: Evans GC, Bainbridge R, Rackham O, editors. Light as an ecological factor II: The 16th Symposium of the British Ecological Society 26–28 March 1974. Oxford: Blackwell Scientific Publications; 1975. pp. 525–532. [Google Scholar]
- Grubb PJ. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews. 1977;52:107–145. [Google Scholar]
- Harper JL. Population biology of plants. London: Academic Press; 1977. [Google Scholar]
- Henry HAL, Aarssen LW. On the relationship between shade tolerance and shade avoidance strategies in woodland plants. Oikos. 1997;80:575–582. [Google Scholar]
- Jankowska-Błaszczuk M, Grubb PJ. Changing perspectives on the role of the soil seed bank in northern temperate deciduous forests and in tropical lowland rain forests: parallels and contrasts. Perspectives in Plant Ecology, Evolution and Systematics. 2006;8:3–21. [Google Scholar]
- Jankowska-Błaszczuk M, Daws MI. Impact of red:far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Functional Ecology. 2007;21:1055–1062. [Google Scholar]
- Keddy PA. Assembly and response rules: two goals for predictive community ecology. Journal of Vegetation Science. 1992;3:157–164. [Google Scholar]
- Knapp R. Effekte wechselnder hoher und tiefer Temperaturen bei der Keimung von Sonchus arvensis L. Naturwissenschaften. 1956;43:41–42. [Google Scholar]
- MacArthur RH, Levins R. The limiting similarity, convergence, and divergence of coexisting species. American Naturalist. 1967;101:377–385. [Google Scholar]
- Milberg P, Andersson L, Thompson K. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Science Research. 2000;10:99–104. [Google Scholar]
- Pickett STA, Bazzaz FA. Organization of an assemblage of early successional species on a soil moisture gradient. Ecology. 1978;59:1248–1255. [Google Scholar]
- Pons TL. Seed responses to light. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. 2nd edn. Wallingford, UK: CABI Publishing; 2000. pp. 237–260. [Google Scholar]
- Probert RJ. The role of temperature in the regulation of seed dormancy and germination. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. 2nd edn. Wallingford, UK: CABI Publishing; 2000. pp. 261–292. [Google Scholar]
- Raunkiær C. Om biologiske Typer, med Hensyn til Planternes Tilpasninger til at overleve ugunstige Aarstider. Botanisk Tidsskrift. 1904;26 XIV. [Google Scholar]
- Raunkiær C. The life forms of plants and statistical plant geography, being the collected papers of C. Raunkiær. Oxford: Oxford University Press; 1934. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. 2012 R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ [Google Scholar]
- Rees M. EC–PC comparative analyses. Journal of Ecology. 1995;83:891–892. [Google Scholar]
- Rice KJ. Responses of Erodium to varying microsites: the role of germination cueing. Ecology. 1985;66:1651–1657. [Google Scholar]
- Schütz W. Are germination strategies important for the ability of cespitose wetland sedges (Carex) to grow in forests? Canadian Journal of Botany. 1997;75:1692–1699. [Google Scholar]
- Schütz W. Germination responses of temperate Carex-species to diurnally fluctuating temperatures – a comparative study. Flora (Jena) 1999;194:21–32. [Google Scholar]
- Schütz W. Ecology of seed dormancy and germination in sedges (Carex) Perspectives in Plant Ecology, Evolution and Systematics. 2000;3:67–89. [Google Scholar]
- Schütz W, Rave G. The effect of cold stratification and light on the seed germination of temperate sedges (Carex) from various habitats and implications for regenerative strategies. Plant Ecology. 1999;144:215–230. [Google Scholar]
- Shipley B, Vile D, Garnier E. From plant traits to plant communities: a statistical mechanistic approach to biodiversity. Science. 2006;314:812–814. doi: 10.1126/science.1131344. [DOI] [PubMed] [Google Scholar]
- Silvertown JW. Seed size, life span, and germination date as coadapted features of plant life history. American Naturalist. 1981;118:860–864. [Google Scholar]
- Silvertown JW, Dodd M, Gowing D, Lawson C, McConway K. Phylogeny and the hierarchical organization of plant diversity. Ecology. 2006;87:S39–S49. doi: 10.1890/0012-9658(2006)87[39:pathoo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Thompson K. A comparative study of germination responses to high irradiance light. Annals of Botany. 1989;63:159–162. [Google Scholar]
- Thompson K, Grime JP. A comparative study of germination responses to diurnally-fluctuating temperatures. Journal of Applied Ecology. 1983;20:141–156. [Google Scholar]
- Thompson K, Bakker JP, Bekker RM. Soil seed banks of North West Europe: methodology, density and longevity. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Thompson K, Ceriani RM, Bakker JP, Bekker RM. Are seed dormancy and persistence in soil related? Seed Science Research. 2003;13:97–100. [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, et al. Flora Europaea. Cambridge: Cambridge University Press; 1964–1980. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology. 1995;86:1031–1037. [Google Scholar]
- Westoby M. Choosing species to study. Trends in Ecology & Evolution. 2002;17:587–587. [Google Scholar]
- Whittaker RH. Communities and ecosystems. New York: Macmillan; 1975. [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. New York: Springer; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.