Abstract
Background and Aims Trithuria
is the sole genus of Hydatellaceae, a family of the early-divergent angiosperm lineage Nymphaeales (water-lilies). In this study different arabinogalactan protein (AGP) epitopes in T. submersa were evaluated in order to understand the diversity of these proteins and their functions in flowering plants.
Methods
Immunolabelling of different AGPs and pectin epitopes in reproductive structures of T. submersa at the stage of early seed development was achieved by immunofluorescence of specific antibodies.
Key Results
AGPs in Trithuria pistil tissues could be important as structural proteins and also as possible signalling molecules. Intense labelling was obtained with anti-AGP antibodies both in the anthers and in the intine wall, the latter associated with pollen tube emergence.
Conclusions
AGPs could play a significant role in Trithuria reproduction, due to their specific presence in the pollen tube pathway. The results agree with labellings obtained for Arabidopsis and confirms the importance of AGPs in angiosperm reproductive structures as essential structural components and probably important signalling molecules.
Keywords: Trithuria, Hydatellaceae, arabinogalactan proteins, monoclonal antibodies, reproductive units, starch grains
INTRODUCTION
Arabinogalactan proteins (AGPs) belong to the hydroxyproline-rich glycoprotein superfamily with a very high level of type II arabinogalactan glycosylation (Showalter, 2001). AGPs are ubiquitous in plants and particularly abundant in cell walls, plasma membranes and extracellular secretions. Such ubiquitous presence implies that AGPs are vital components of the plant cell. Indeed, many studies have implicated AGPs in important biological phenomena such as cell expansion, cell division, cell death, seed germination, pollen-tube growth and guidance, and resistance to infection (Seifert and Roberts, 2007). However, the means by which these molecules function or interact with other cell components remain undetermined (Coimbra and Pereira, 2012). Pectins are other important and complex wall macromolecules consisting of homogalacturonans that can be methylesterified and acetylesterified, rhamnogalacturonans I, rhamnogalacturonans II and xylogalacturonans (Carpita and McCann, 2000).
The cell-wall composition of early-divergent flowering plants is of great interest for understanding early evolution of angiosperm cell-wall structures. However, existing research in this field is limited. Among non-flowering land plants, AGPs were shown to be important for apical cell extension in the moss Physcomitrella patens (Lee et al., 2005). There have also been studies on green algae; for example, AGPs have been detected in Micrasterias denticulata (Eder et al., 2008), and a detailed study revealed several cell-wall polymers during antheridium development and spermatogenesis in Chara corallina (Domozych et al., 2009).
Localization of AGPs in tissues and cells can be demonstrated using specific monoclonal antibodies that bind to the structurally complex carbohydrate epitopes. The selective labelling obtained using these monoclonal antibodies in Arabidopsis and other plant species has shown that some AGPs are present as molecular markers during development and that they are probably related to important steps of the plant sexual reproductive cycle (Coimbra et al., 2007). The regulated expression and abundance of particular AGPs in the stigma, stylar transmitting tissue and pollen have led to the hypothesis that AGPs are important for plant pollen–pistil interaction (Coimbra and Pereira, 2012). AGPs are strong candidates for mediators of cell − cell communication, although the precise mechanism of AGP-mediated cell-wall signalling is unclear. Most, if not all, AGPs are anchored to the plasma membrane by a glycosylphosphatidylinositol (GPI) anchor (Borner et al., 2003). AGPs can be released by cleavage of the GPI anchor using a specific phospholipase, in response to cellular signals. The high sugar content of AGPs, together with the presence of the GPI anchor, makes these proteins major candidates for having key roles in reproductive processes, as for other eukaryotic systems (Coimbra and Pereira, 2012).
However, to date, most studies of AGPs have focused on model organisms such as Arabidopsis. Comparative studies of a phylogenetically broad range of taxa are required to understand the diversity of these proteins and their functions. The differences observed regarding the specific presence of selected epitopes during reproductive development will help us to address some fundamental questions related to the evolution and genetics of flowering. In the present work, we evaluate different AGP epitopes in the reproductive units of an early-divergent angiosperm, Trithuria submersa (Hydatellaceae), which is the closest extant relative of the water-lilies (Saarela et al., 2007). Trithuria species represent useful models for comparative studies of this type, because (uniquely for early-divergent angiosperms) they are mostly rapidly growing annual plants that are readily cultivated in vitro. Due to its ready availability, recent studies have provided detailed morphological and molecular characterization of Trithuria, including studies on morphology and systematics (Hamann, 1998; Rudall et al., 2007, 2009a; Sokoloff et al., 2008a, b, 2011; Tratt et al., 2009; Iles et al., 2012), reproductive biology (Taylor et al., 2010; Prychid et al., 2011; Taylor and Williams, 2012), embryology, pollen morphology and seed development (Friedman, 2008; Remizowa et al., 2008; Rudall et al., 2008, 2009b; Tuckett et al., 2010a, b; Friedman et al., 2012). The present study will complement these studies of this phylogenetically significant taxon and broaden our knowledge of AGP structure and function in angiosperms.
MATERIALS AND METHODS
Plant material and light microscopy
Reproductive units of Trithuria submersa grown at the Royal Botanic Gardens, Kew, were fixed in 2 % paraformaldehyde and 2·5 % glutaraldehyde in phosphate buffer (0·025 m, pH 7, with one micro drop of Tween 80), placed under vacuum for 1 h and then at 4 °C overnight. After dehydration in a graded ethanol series, the material was embedded in LR White embedding resin (London Resin Company Ltd, London, UK). Thick sections (0·5 µm) were obtained with a Leica Reichert Supernova microtome placed on glass slides, and stained with a solution of 1 % methylene blue for light microscopy, and 1 % Lugol solution (Sigma-Aldrich 62650; St Louis, MO, USA) for starch staining. Some slides were preserved unstained for immunolocalization of AGPs and pectin epitopes with monoclonal antibodies.
Immunolocalization of AGPs and pectins
A collection of monoclonal antibodies directed against glycosyl moieties specific to AGPs and pectins was provided by Prof. Paul Knox from the Centre for Plant Sciences, Faculty of Biological Sciences and University of Leeds, UK. The monoclonal antibodies recognizing AGP epitopes used were JIM8 (Pennell et al., 1991), JIM13 (Knox et al., 1991) and MAC207 (Pennell et al., 1989), and those recognizing pectin epitopes were JIM7 and JIM5 (Knox et al., 1990). LM6 (Willats et al., 1998) antibody can detect epitopes in AGPs and in pectins. The secondary antibody used was fluorescein isothiocyanate (FITC)-conjugated anti-rat IgG (Sigma-Aldrich F-1763).
Slides prepared for immunolocalization were circled with a Pap pen (Sigma-Aldrich Z672548), and treated as follows: 5 min in phosphate-buffered saline (PBS), pH 7·4, containing 5 % non-fat dried milk (blocking solution), followed by incubation with primary antibody (diluted 1 : 5 in blocking solution), overnight and at 4 °C. After washing with PBS, the sections were incubated with secondary antibody (diluted 1 : 100 in blocking solution) for 4 h in the dark, and then finally washed with PBS followed by distilled water. Slides were further stained with 0·01 % calcofluor white (Fluorescent Brightener 28; Sigma-Aldrich F3543, which is not removed) and mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA).
Bright-field and fluorescence observations were made on a Leica DMLB epifluorescence microscope (objectives were Leica N-Plan, and filters were 365/445 nm for calcofluor and 470/525 nm for fluorescein stain). Images were captured with a ProgRes® MF cool (Jenoptik, Jena, Germany) in automatic exposure mode, and processed with ProgRes® CapturePro 2·8·8 software. Control experiments were performed omitting the incubation with the primary antibody (incubation with blocking solution only) and showed no unspecific staining. Confidence in specific antibody binding was reinforced by the different patterns of labelling obtained with the different monoclonal antibodies used.
RESULTS
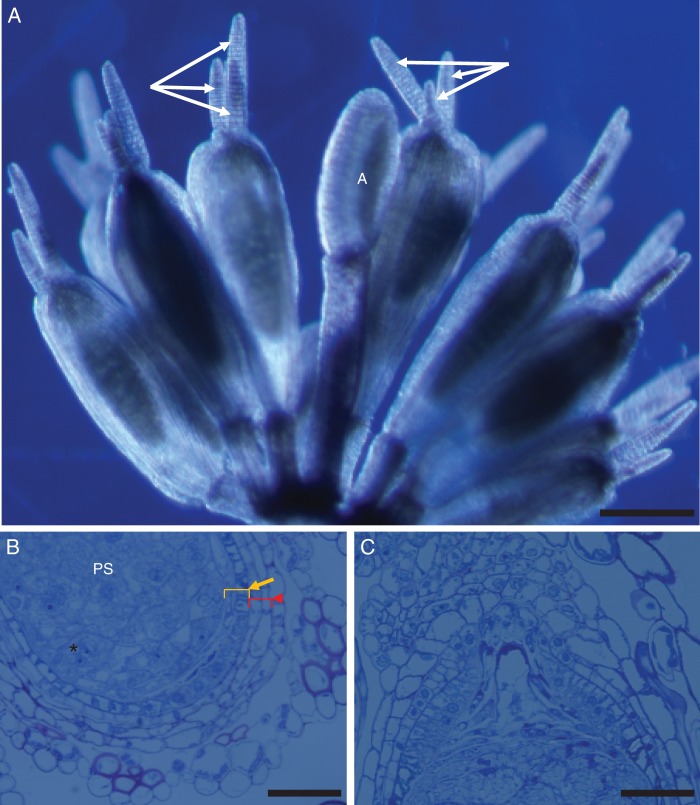
Reproductive units (RUs) of Trithuria submersa typically consist of numerous pistils surrounding one or two central stamens (Fig. 1A), all the fertile organs being enclosed by an involucre of bract-like phyllomes that resemble perianth organs. Pre-fertilized carpels were characterized here, which already contain the main seed storage tissue, the perisperm (e.g. Rudall et al., 2008). The ovule is bitegmic and the two cell-wall layers of each integument are clearly visible (Fig. 1B). At this stage of development, the micropyle is enclosed by the outer integument (Fig. 1C). A nucellar beak is also evident (Fig. 1C). For our study, the perisperm is the main tissue under investigation. As reported by Rudall et al. (2009b), the starchy perisperm develops precociously; the perisperm cells are arranged in groups whose walls start to break down and the nuclei clump together, resulting in a multinucleate appearance (Fig. 1B).
Fig. 1.
(A) Trithuria submersa reproductive unit, with a single stamen in the centre (labelled ‘A’) surrounded by several pistils, each with several long, multicellular uniseriate stigmatic hairs (arrows); scale bar = 200 µm. (B) Detail of lower part of ovule and carpel wall showing perisperm (PS) (*) with aggregated nuclei and two integuments, each with two cell layers (arrow, inner integument; arrowhead, outer integument); scale bar = 50 µm. (C) Detail of upper part of ovule and carpel wall showing the nucellar beak; scale bar = 50 µm.
Immunolocalization of AGPs and pectins in developing seeds of T. submersa
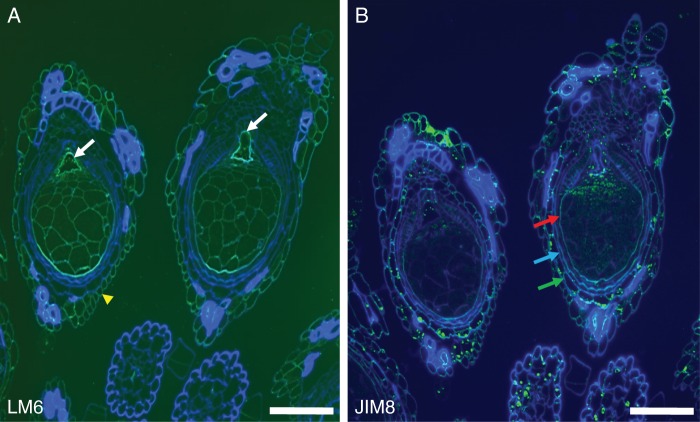
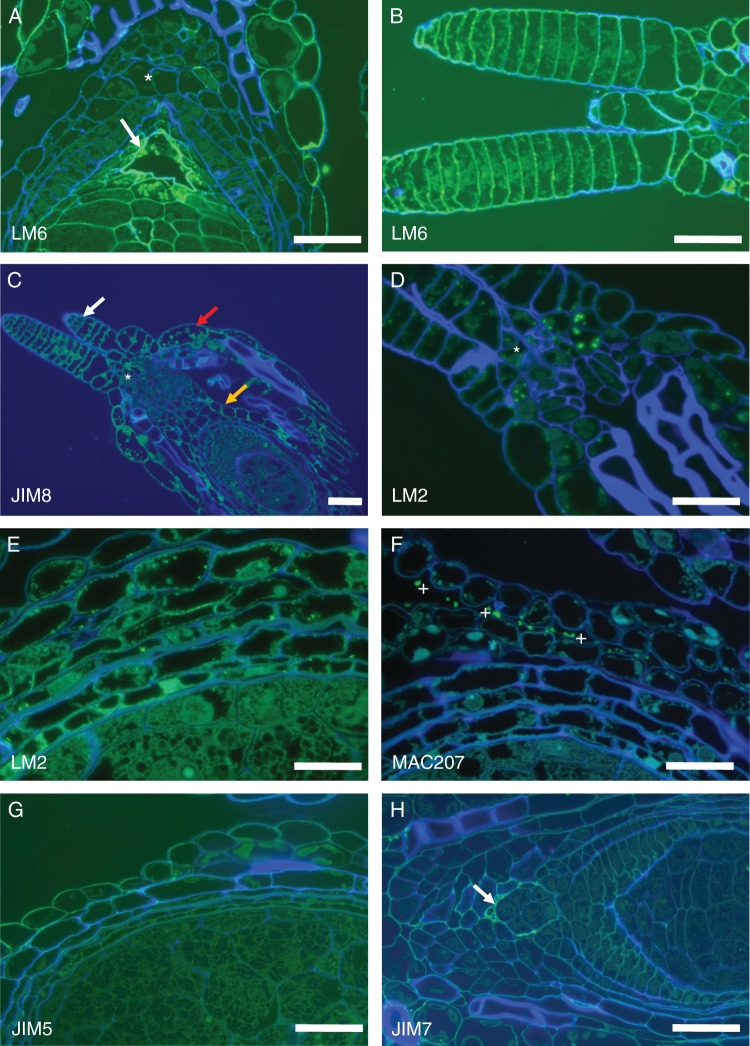
AGPs have been implicated in the sexual reproduction of several plant species and in Arabidopsis some AGPs were identified as molecular markers for gametophytic cell development (Coimbra et al., 2007). The monoclonal antibodies used that recognize AGP epitopes were JIM8, JIM13 and MAC207. For pectin epitopes, we used LM6, which recognizes a pentasaccharide of (1–5)-α-l-arabinans present in type I rhamnogalacturonan (Willats et al., 1998), although this is also present in some AGPs. Homogalacturonan epitopes were localized using JIM7 (Knox et al., 1990), which ligates to partially methyl-esterified homogalacturonans, and JIM5 (Knox et al., 1990), which has affinity for both partially methyl-esterified homogalacturonan and also non-esterified homogalacturonans. In pistils of T. submersa, the labelling obtained using LM6 was not uniform in all cell types (Fig. 2A). Cell walls are composed of cellulose microfibrils embedded in a polysaccharide matrix of hemicelluloses, pectins and other proteins, namely AGPs; the fact that the two integuments are not labelled with LM6 is significant (Fig. 2A). LM6 is present with high intensity in the embryo sac wall (Figs 2A and 3A) and in the outer ovary wall (Fig. 3A). In contrast, labelling with JIM8 is relatively intense in the ovary wall and the outer integument (Fig. 2B), in the stigmatic hairs and on the nucellus tissue at the entrance to the embryo sac (Fig. 3C). The stigmatic hairs are strongly labelled with the anti-pectin LM6 and with the anti-AGPs JIM8 (Fig. 3B). LM2 antibodies also label intensely the stigmatic hairs and the ovary entrance (Fig. 3D) as well as the integuments and ovary wall (Fig. 3E). Curiously, when the antibody used was MAC207, the ovule and ovary cells were not labelled, but some secreted material present in the extracellular spaces was consistently labelled (Fig. 3F). Homogalacturonan epitopes were identified by JIM5 (Fig. 3G) and by JIM7 (Fig. 3H), although the labeling was not intense and mainly in the outer ovary cells wall and embryo sac wall.
Fig. 2.
Labelling with LM6 and JIM8. (A) LM6 labelling, showing a high intensity of labelling in the embryo sac wall (arrows) and the outer ovary wall (arrowhead). The perisperm cell walls are also labelled. (B) JIM8 labelling is relatively intense in the ovary wall (red arrow), inner integument (blue arrow) and outer integument (green arrow). JIM8 labelling is also evident in some of the starch grains and in thickened cell walls. Scale bars = 200 µm.
Fig. 3.
(A) Details of LM6 labelling on the embryo sac wall (arrow) and nucellar beak (*). (B) Stigmatic hairs strongly labelled with LM6 which recognizes epitopes in pectins (i.e. the arabinan side-chains) and in AGPs. (C) JIM8 (as well as JIM13) labels the ovary wall (orange arrow), the outer integument (red arrow), the stigmatic hairs (white arrow) and the region at the base of the stigmatic hairs (*). (D) LM2 antibodies intensely label the stigmatic hairs and the ovary entrance (*). (E) LM2-recognized epitopes are strongly present in the integuments and ovary wall. (F) Monoclonal antibody MAC207 consistently labels some secreted material present in the extracellular spaces (*), but not the ovule or the ovary cell walls. (G) JIM5 only labels the outer ovary wall while (H) JIM7 labels the embryo sac wall (arrow) the outer ovary wall and, very faintly, the perisperm. Scale bars: (A, B, D, G, H) = 50 µm; (C) = 100 µm; (E, F) = 20 µm.
Starch grains of T. submersa
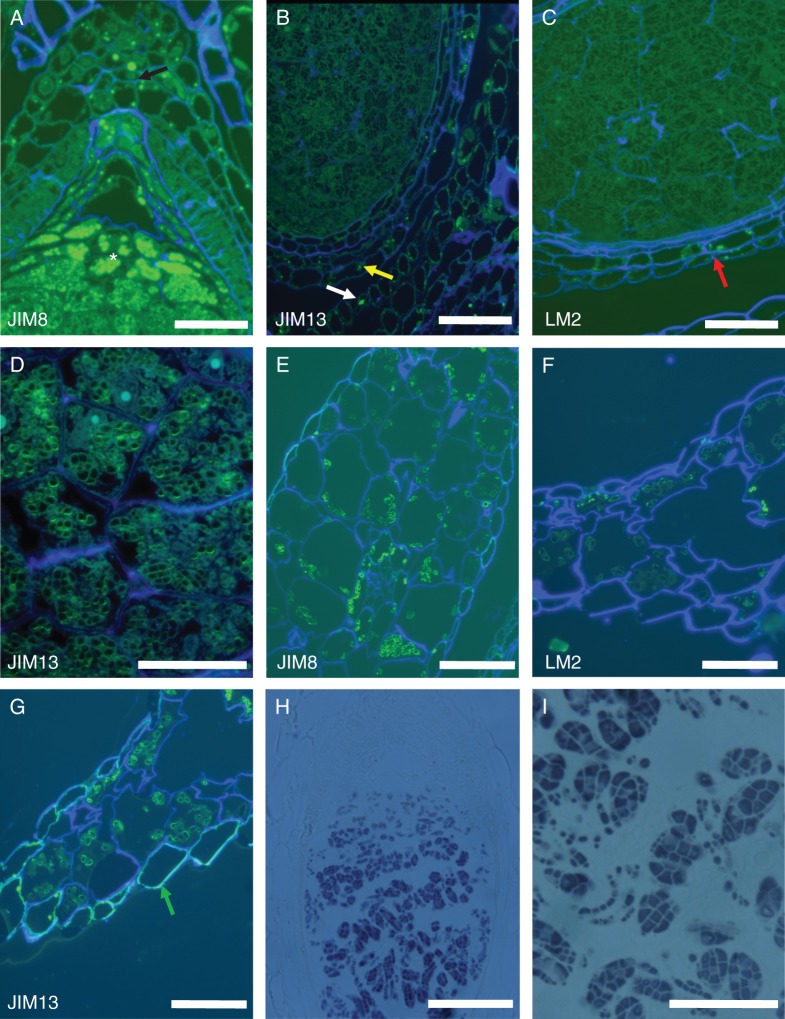
Remarkably, we found that the two different types of starch grain present in different tissues of the same RU of T. submersa were differentially labelled by very similar anti-AGP antibodies such as JIM8 and JIM13. Specifically, JIM8 and LM2 both labelled equally; they are both absent from the perisperm starch grains (Fig. 4B, C), but are present in the outer layer of the starch grains of the outer integument, ovary wall, stamen filaments and bract-like phyllomes (Fig. 4B, E, F). Conversely, JIM13-recognized epitopes were present in the outer layer of the perisperm starch grains (Fig. 4A, D) and also present in the starch grains of the ovary wall, stamen filaments and bract-like phyllomes (Fig. 4A, G). In these plants, the amyloplasts may contain one or more starch grains; several grains of joint origin form a compound amyloplast, as evidenced by staining with lugol (Fig. 4H, I).
Fig. 4.
Starch grains of different tissues in the same reproductive unit. (A) JIM13 labels the starch grains of the perisperm (*) and the outer integument (arrow). (B) JIM 8 and (C) LM2 both label only the starch grains present in the outer integument and ovary wall (arrows) and not the perisperm starch grains. (D) The labelling is associated with the outer layer of the perisperm starch grains. (E) JIM 8 and (F) LM2 both label equally the outer layer of the starch grains present in the bract-like phyllomes. (G) JIM13 labels intensely the outer layer of the starch grains present in the bract-like phyllomes and also the outer wall of the phyllome epidermal cells (arrow). (H) The amyloplasts are of the composed type, as evidenced by staining with lugol. (I) Higher magnification of the composed perisperm amyloplasts, stained with lugol. Scale bars: (A, B, E, F, G) = 50 µm; (C) = 20 µm; (D) = 10 µm; (H) = 100 µm.
Immunolocalization of AGPs and pectins in T. submersa anthers
Pollen of Trithuria is oblong or rounded and monosulcate (Remizowa et al., 2008). The aperture has a distinct margin and extends the full length of the pollen grain. It has already been shown that sometimes the aperture membrane bulges outwards (Prychid et al., 2011). Labelling with the antibodies JIM 8 and JIM 13 indicates some preferential presence for the AGPs identified by these antibodies in pollen grains, specifically at the intine wall and preferentially at the location where the pollen tube will emerge (Fig. 5A, B). At this stage of development, pollen is mature, the tapetum is already absent and the endothecium shows its typical wall thickenings. The entire intine wall is labelled with LM6 (Fig. 5C), rather than preferential labelling, as for the anti-AGP antibodies.
Fig. 5.
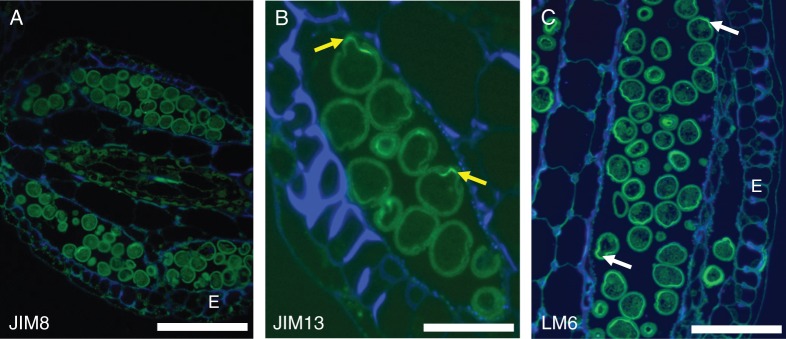
Anther sections. (A) JIM 8 and (B) JIM 13 preferential label the intine wall and especially at the aperture, where the pollen tube will emerge (arrows). (C) With LM6, the entire intine wall is labelled as expected, but the pectin wall is thicker at the aperture (arrows). E = endothecium. Scale bars: (A) = 50 µm, (B) = 10 µm, (C) = 20 µm.
DISCUSSION
AGPs and pectins in pistils of T. submersa
Although the molecular mechanism of the action of AGPs remains unknown, mainly due to the difficulties posed by their complex glycoproteins, the selective labelling obtained here in mature reproductive units using the AGP monoclonal antibodies JIM8, JIM13, MAC207 and LM2 leads us to postulate that some AGPs are important for cell differentiation in Trithuria submersa.
AGPs are ubiquitous throughout the entire plant kingdom and have been shown to be involved in important developmental processes. They are probably related not only to the transition from a sporophytic type of development to a gametophytic one (Coimbra et al., 2007), but also in pollen development and pollen tube growth (Wu et al., 1995; Levitin et al., 2008; Coimbra et al., 2010). AGPs are important as cell-wall structural glycoproteins, but the fact that they are anchored to the cytoplasmic membrane by a GPI anchor makes them credible candidates for signalling molecules, as they are exposed to the extracellular matrix and can be cleaved by specific phospholipases.
A study of the stigmatic hairs and pollen tubes of Hydatellaceae has already shown that the uniseriate, multicellular stigmatic hairs of Trithuria label intensely with LM2, indicating that the AGP epitopes identified by this monoclonal antibody could be involved in pollen-tube attraction (Prychid et al., 2011). Here, we show that in addition to intense labelling in the stigmatic hairs, LM2 also labels AGP epitopes present at the bases of the hairs, in the micropylar nucellus and in the ovule integuments. The same result is true for the epitopes recognized by JIM8 and JIM13. These results agree with labellings obtained for Arabidopsis (Coimbra et al., 2007), and confirm the importance of AGPs in the reproductive structures as essential structural components and probably important signalling molecules.
Pectin is the most structurally complex family of polysaccharides in nature, making up to 35 % of primary walls in eudicots and non-graminaceous monocots, 2–10 % of primary walls of grasses and other commelinid monocots, and up to 5 % of walls in woody tissue (Mohnen, 2008). Surprisingly, we obtained differential labelling in the pistil tissues using LM6, JIM5 and JIM7, the monoclonal antibodies that label pectin epitopes. The embryo sac wall, the wall of the nutritive tissue and the ovary cell walls are strongly labelled with LM6 while the integument cell walls are not labelled at all. This does not mean that these cells lack pectin in their walls, but that the pectins have different structures in different tissues of the pistil. The rhamnogalacturonan I (RG-I) type of pectin (the epitope recognized by LM6) is apparently absent from the integuments. Homogalacturonan epitopes labelled by JIM5 and JIM7 were evident only in the cell walls of the ovary and in the embryo sac wall. Labelling in the other cell types was faint or not present. Pectins are known to perform multiple functions in plant cells, starting with growth, development, morphogenesis, defence, cell–cell adhesion, wall structure, signalling, cell expansion, pollen tube growth, seed hydration, leaf abscission and fruit development (Mohnen, 2008). This is probably the reason that there are different wall constitutions in different tissues. The stigmatic hairs play an important role in adhesion for pollen tube growth, a function performed by AGPs and pectins. It is important to note that LM6 can also bind to AGPs (Lee et al., 2005).
As in Arabidopsis, no MAC207 labelling was present in the embryo sac wall of Trithuria (Coimbra et al., 2007). However, instead of being scattered throughout most cell types (as in Arabidopsis), this monoclonal antibody only labelled the extracellular secretions around the pistil in Trithuria. This difference could be consistent with the evolutionary distance between these two plant species, showing different constituents for equivalent organ tissues.
Starch grains of T. submersa
In leaves and stems, starch grains are stored transiently and provide for the immediate energy needs of the plant. In contrast, starch grains located in the ovules are storage starches used as long-term energy reserves. Starch grains contain two types of molecules composed of glucose residues, amylopectin and amylose. Amylopectin consists of ramified molecules consisting of glucose residues, while amylose is composed of linear glucose chains.
Unexpectedly, we found that AGP epitopes are associated with Trithuria starch grains. An even more unpredictable result was that the labelling of starch with anti-AGP antibodies was different according to the starch grain type. These results demonstrate that besides having different roles, these two types of starch grains are also different in composition.
AGPs and pectins in T. submersa anthers
The labelling obtained in the anthers with JIM8 and JIM13 was also significant. It is known that these two monoclonal antibodies specifically label the gametophytic cells in Arabidopsis (Coimbra et al., 2007). The labelling obtained for Trithuria was intense, but located in the intine wall at the aperture, associated with future pollen tube emergence. In many plants, AGPs are present in the pollen-tube wall (Mollet et al., 2002; Pereira et al., 2006; Qin et al., 2007; Dardelle et al., 2010) and they are probably involved in signalling during pollen tube growth, but it is interesting to note that the AGP epitopes present are different in these diverse plant species. With these results we can propose that AGPs are important surface molecules, involved in pollen tube growth and most probably also in pollen–pistil interactions.
Conclusions
In conclusion, the specific and strong labelling obtained with monoclonal anti-AGP antibodies demonstrate the importance of these proteins in Trithuria, a plant that is phylogenetically placed close to the root of extant angiosperms. Although important for plant reproduction due to the specific presence in the pollen tube pathway, AGPs also show the evolutionary divergence related to the cell-wall composition of specific cell types, such as pollen tubes, ovule tissues and style, probably related to the evolution of reproductive strategies.
ACKNOWLEDGEMENTS
This work was financed by FEDER through the COMPETE programme, and by Portuguese National funds through FCT – Fundação para a Ciência e Tecnologia (Project PTDC/AGR-GPL/115358/2009) and from an FCT PhD grant SFRH/BD/60995/2009 awarded to A.M.P.
LITERATURE CITED
- Borner GHH, Lilley KS, Stevens TJ, Dupree P. Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiology. 2003;132:568–577. doi: 10.1104/pp.103.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, McCann MC. The cell wall. In: Buchanan BB, Gruissem W, Jones R, editors. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 52–109. [Google Scholar]
- Coimbra S, Pereira LG. Arabinogalactan proteins in Arabidopsis pollen development. In: Çiftçi YO, editor. Transgenic plants-advances and limitations. Inteck: 2012. pp. 329–352. http://www.intechopen.com/ [Google Scholar]
- Coimbra S, Almeida J, Junqueira V, Costa M, Pereira LG. Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. Journal of Experimental Botany. 2007;58:4027–4035. doi: 10.1093/jxb/erm259. [DOI] [PubMed] [Google Scholar]
- Coimbra S, Costa ML, Mendes MA, Pereira A, Pinto J, Pereira LG. Early germination of Arabidopsis pollen in a double null mutant for the arabinogalactan protein genes AGP6 and AGP11. Sexual Plant Reproduction. 2010;23:199–205. doi: 10.1007/s00497-010-0136-x. [DOI] [PubMed] [Google Scholar]
- Dardelle F, Lehner A, Ramdani Y, et al. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiology. 2010;153:1563–1576. doi: 10.1104/pp.110.158881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Sørensen I, Willats WGT. The distribution of cell wall polymers during antheridium development and spermatogenesis in the charophycean green alga, Chara corallina. Annals of Botany. 2009;104:1045–1056. doi: 10.1093/aob/mcp193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder M, Tenhaken R, Driouich A, Lutz-Meindl U. Occurrence and characterization of arabinogalactan-like proteins and hemicelluloses in Micrasterias (Streptophyta) Journal of Phycology. 2008;44:1221–1234. doi: 10.1111/j.1529-8817.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- Friedman WE. Hydatellaceae are water lilies with gymnospermous tendencies. Nature. 2008;453:94–97. doi: 10.1038/nature06733. [DOI] [PubMed] [Google Scholar]
- Friedman WE, Bachelier JB, Harmaza JI. Embryology in Trithuria submersa (Hydatellaceae) and relationships between embryo, endosperm, and perisperm in early-diverging flowering plants. American Journal of Botany. 2012;99:1083–1095. doi: 10.3732/ajb.1200066. [DOI] [PubMed] [Google Scholar]
- Hamann U. Hydatellaceae. In: Kubitzki K, editor. Families and genera of vascular plants, vol. IV, Flowering plants—Monocotyledons—Alismatanae and Commelinanae. Berlin: Springer; 1998. pp. 231–234. [Google Scholar]
- Iles W, Rudall PJ, Sokoloff DD, et al. Molecular phylogenetics of Hydatellaceae (Nymphaeales): sexual-system homoplasy and a new sectional classification. American Journal of Botany. 2012;99:663–676. doi: 10.3732/ajb.1100524. [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta. 1990;181:512–521. doi: 10.1007/BF00193004. [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, Peart J, et al. Developmentally-regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. The Plant Journal. 1991;1:317–326. doi: 10.1046/j.1365-313X.1991.t01-9-00999.x. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Sakata Y, Mau S-L, et al. Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens. Plant Cell. 2005;17:3051–3065. doi: 10.1105/tpc.105.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitin B, Richter D, Markovich I, Zik M. Arabinogalactan proteins 6 and 11 are required for stamen and pollen function in Arabidopsis. The Plant Journal. 2008;56:351–363. doi: 10.1111/j.1365-313X.2008.03607.x. [DOI] [PubMed] [Google Scholar]
- Mohnen D. Pectin structure and biosynthesis. Current Opinion in Plant Biology. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Mollet JC, Kim S, Jauh GY, Lord EM. Arabinogalactan proteins, pollen tube growth, and the reversible effects of Yariv phenylglycoside. Protoplasma. 2002;219:89–98. doi: 10.1007/s007090200009. [DOI] [PubMed] [Google Scholar]
- Pennell RI, Knox JP, Scofield GN, et al. A family of abundant plasma membrane associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. Journal of Cell Biology. 1989;108:1967–1977. doi: 10.1083/jcb.108.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Janniche L, Kjellbom PP, et al. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. The Plant Cell. 1991;3:1317–1326. doi: 10.1105/tpc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LG, Coimbra S, Oliveira H, Monteiro L, Sottomayor M. Expression of arabinogalactan protein genes in pollen tubes of Arabidopsis thaliana. Planta. 2006;223:374–380. doi: 10.1007/s00425-005-0137-4. [DOI] [PubMed] [Google Scholar]
- Prychid CJ, Sokoloff DD, Remizowa MV, Tuckett RE, Yadav SR, Rudall PJ. Unique stigmatic hairs and pollen-tube growth within the stigmatic cell wall in the early-divergent angiosperm family Hydatellaceae. Annals of Botany. 2011;108:599–608. doi: 10.1093/aob/mcr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Chen D, Zhao J. Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tabacum L. Protoplasma. 2007;231:43–53. doi: 10.1007/s00709-007-0245-z. [DOI] [PubMed] [Google Scholar]
- Remizowa MV, Sokoloff DD, Macfarlane TD, Yadav SR, Prychid CJ, Rudall PJ. Comparative pollen morphology in the early-divergent angiosperm family Hydatellaceae reveals variation at the infraspecific level. Grana. 2008;47:81–100. [Google Scholar]
- Rudall PJ, Sokoloff DD, Remizowa MV, et al. Morphology of Hydatellaceae, an anomalous aquatic family recently recognized as an early-divergent angiosperm lineage. American Journal of Botany. 2007;94:1073–1092. doi: 10.3732/ajb.94.7.1073. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Remizowa MV, Beer A, et al. Comparative ovule and megagametophyte development in Hydatellaceae and water lilies reveal a mosaic of features among the earliest angiosperms. Annals of Botany. 2008;101:941–956. doi: 10.1093/aob/mcn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudall PJ, Remizowa MV, Prenner G, Prychid CJ, Tuckett RE, Sokoloff DD. Non-flowers near the base of extant angiosperms? Spatiotemporal arrangement of organs in reproductive units of Hydatellaceae, and its bearing on the origin of the flower. American Journal of Botany. 2009a;96:67–82. doi: 10.3732/ajb.0800027. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Eldridge T, Tratt J, et al. Seed fertilization, development and germination in Hydatellaceae (Nymphaeales): implications for endosperm evolution in early angiosperms. American Journal of Botany. 2009b;96:1581–1593. doi: 10.3732/ajb.0900033. [DOI] [PubMed] [Google Scholar]
- Saarela JM, Rai HS, Doyle JA, et al. Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature. 2007;446:312–315. doi: 10.1038/nature05612. [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. The biology of arabinogalactan proteins. Annual Review of Plant Biology. 2007;58:137–161. doi: 10.1146/annurev.arplant.58.032806.103801. [DOI] [PubMed] [Google Scholar]
- Showalter AM. Arabinogalactan-proteins: structure, expression and function. Cellular and Molecular Life Sciences. 2001;58:1399–1417. doi: 10.1007/PL00000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff DD, Remizowa MV, Macfarlane TD, Rudall PJ. Classification of the early-divergent angiosperm family Hydatellaceae: one genus instead of two, four new species, and sexual dimorphism in dioecious taxa. Taxon. 2008a;57:179–200. [Google Scholar]
- Sokoloff DD, Remizowa MV, Macfarlane TD, et al. Seedling diversity in Hydatellaceae: implications for the evolution of angiosperm cotyledons. Annals of Botany. 2008b;101:153–164. doi: 10.1093/aob/mcm274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff DD, Remizowa MV, Macfarlane TD, Yadav SR, Rudall PJ. Hydatellaceae: a historical review of systematics and ecology. Rheedea. 2011;21:115–138. [Google Scholar]
- Taylor ML, Williams JH. Pollen tube development in two species of Trithuria (Hydatellaceae) with contrasting breeding systems. Sexual Plant Reproduction. 2012;25:83–96. doi: 10.1007/s00497-012-0183-6. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Terry D, Macfarlane TD, Williams JH. Reproductive ecology of the basal angiosperm Trithuria submersa (Hydatellaceae) Annals of Botany. 2010;106:909–920. doi: 10.1093/aob/mcq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratt J, Prychid CJ, Behnke HD, Rudall PJ. Starch-accumulating (S-type) sieve-element plastids in Hydatellaceae: implications for evolution of sieve-element plastids in flowering plants. Protoplasma. 2009;237:19–26. doi: 10.1007/s00709-009-0067-2. [DOI] [PubMed] [Google Scholar]
- Tuckett RE, Merritt DJ, Rudall PJ, et al. A new type of specialised morphophysiological dormancy and seed storage behaviour in Hydatellaceae, an early-divergent angiosperm family. Annals of Botany. 2010a;105:1053–1061. doi: 10.1093/aob/mcq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckett RE, Merritt DJ, Hay F, Hopper SD, Dixon KW. Comparative longevity and low-temperature storage of seeds of Hydatellaceae and temporary pool species of south-west Australia. Australian Journal of Botany. 2010b;58:327–334. [Google Scholar]
- Willats WG, Marcus SE, Knox JP. Generation of monoclonal antibody specific to (1→5)-alpha-L-arabinan. Carbohydrate Research. 1998;308:149–152. doi: 10.1016/s0008-6215(98)00070-6. [DOI] [PubMed] [Google Scholar]
- Wu H-M, Wang H, Cheung A. A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell. 1995;82:395–403. doi: 10.1016/0092-8674(95)90428-x. [DOI] [PubMed] [Google Scholar]