Abstract
Background and Aims
Heterostyly and related style polymorphisms are suitable model systems to evaluate the importance of functional pollinators in the maintenance of population variability. In Narcissus papyraceus different functional pollinators, incompatibility system and flower morphology have been proposed to influence the maintenance of polymorphism through their effect on disassortative mating. Here a test is done to find out if the visitation rate of long- versus short-tongued pollinators correlates with the morph ratio and if the latter is related to other flower traits of the species across its main geographic range.
Methods
Floral traits from 34 populations in the south-west of the Iberian Peninsula and in north-west Africa were measured, perianth variation was described and a comparison was made of allometric relationships between sex organs and floral tube. Correlations between pollinator guilds, stigma–anther separation of reciprocal morphs (our proxy for disassortative mating) and morph-ratio variation were analysed. Finally, the incompatibility system of the species in the northern and southern borders of its distribution are described.
Key Results
Flowers from southern populations were significantly larger than flowers from centre and northern populations. The abundance of short-styled plants decreased gradually with increasing distance from the core region (the Strait of Gibraltar), with these disappearing only in the northern range. Although there was a significant difference in stigma–anther separation among populations, morph ratio was not associated with reciprocity or floral tube length. Long-style morph frequency increased with short-tongued pollinator visitation rate. Populations from both edges of the distribution range were self-incompatible and within- and between-morph compatible.
Conclusions
The style morph ratio changed gradually, whereas perianth trait variation showed abrupt changes with two morphotypes across the range. The positive relationship between the visitation rate of short-tongued pollinators and the decrease of the short-style morph supports our initial hypothesis. The results highlight the importance of different pollinators in determining the presence of style polymorphism.
Keywords: Disassortative mating, floral morphology, geographic variation, morph ratio, Narcissus papyraceus, pollinators, stylar dimorphism
INTRODUCTION
There is increasing evidence that pollinators are important drivers of floral variation at micro- and macroevolutionary levels (Stebbins, 1970; Fenster et al., 2004). Some studies have considered variation among populations as an incipient process of evolutionary divergence, leading to pollination ecotypes (Robertson and Wyatt, 1990; Johnson, 1997; Valiente-Banuet et al., 2004). The ensemble of floral traits that are thought to be the target of pollinator-mediated selection is wide (perianth and sex-organ form and size, colour, scent, pollinator rewards, etc.), and may vary both in a continuous and discrete mode among and within populations. Probably one of the most-studied cases of intrapopulation variation in animal-pollinated hermaphroditic plants is heterostyly (Barrett, 2002), which consists of two [long (L) and short (S)] or three [L, S and medium (M)]-styled morphs, that differ reciprocally at the height at which stigmas and anthers are positioned within the flower. Darwin's cross-pollination hypothesis highlights the relevance of pollinators in the maintenance of heterostyly, and the underlying mechanism is the promotion of pollen transfer between morphs by precise location of pollen grains on insects' bodies, which reduces the interference between the male (pollen delivery) and female (pollen receipt) functions (Darwin, 1877; Lloyd and Webb, 1992). Notwithstanding the intrinsic within-population variation, the expression of this polymorphism may also vary across populations (Ganders, 1979).
Typical heterostylous species usually present a heteromorphic incompatibility system that ensures fertilizations only between morphs (dissasortative mating; Lewis and Jones, 1992). Disassortative mating allows equal frequency of morphs (isoplethy) within a population. However, deviations from 1 : 1 morph ratio (anisoplethy) are often reported (Meeus et al., 2012) and several hypotheses have been put forward to explain these deviations. Stochastic processes (i.e. drift or founder effect), differences in fitness between morphs or modifications in the heteromorphic incompatibility systems may be the ultimate cause for that variation (Charlesworth and Charlesworth, 1979b; Heuch, 1979; Morgan and Barrett, 1988; Weller et al., 2007; Sosenski et al., 2010). In contrast, some heterostylous species present a self-incompatibility system that allows intra-morph (assortative) mating (Pérez-Barrales et al., 2006; Brys et al., 2008; Ferrero et al., 2012), thus variation in floral morphology which affects the position of sexual organs and pollen dispersal (Barrett et al., 2004; Hodgins and Barrett, 2008) could determine different rates of assortative vs. disassortative mating (Hodgins and Barrett, 2006). As a result, populations can deviate from isoplethy to anisoplethy and even to monomorphism. Biases towards the L-morph are common because of the simple Mendelian inheritance of heterostyly: the L-morph is usually a recessive homozygous condition and the S-morph is heterozygous (Lewis and Jones, 1992).
Evolutionary models of heterostyly suggest that stylar dimorphism (the putative ancestral stage of distyly) is a suboptimal and unstable condition because L-morph anther position does not match that of the stigma of the S-morph. This mismatch determines low reciprocity between morphs and low disassortative mating, if a heteromorphic incompatibility system is lacking (Charlesworth and Charlesworth, 1979a; Lloyd and Webb, 1992). Nonetheless, stylar dimorphism commonly occurs in some genera (Lithodora, Glandora, Nivenia, Anchusa and particularly Narcissus and probably in Linum; reviewed in Darwin, 1877; Barrett and Harder, 2005; Thompson, 2005; Ferrero et al., 2009; Sánchez et al., 2010) and its stability is apparently associated with sufficient levels of disassortative mating (Cesaro and Thompson, 2004; Pérez-Barrales and Arroyo, 2010). Indeed, previous studies suggest anisoplethy or the loss of one morph in populations of dimorphic species is associated with changes in pollinator guilds and floral traits (Pérez-Barrales et al., 2007; Pérez-Barrales and Arroyo, 2010).
Polymorphic Narcissus species very often display variation among populations in morph-ratio and perianth traits (Arroyo and Dafni, 1995; Baker et al., 2000; Barrett et al., 2004; Pérez-Barrales et al., 2007; Hodgins and Barrett, 2008; Pérez-Barrales et al., 2009), which frequently occur as geographical gradients. Morph-ratio variation in Narcissus may result from morph-specific differences in the rates of selfing, assortative and dissasortative mating in populations. All of these crosses are possible because polymorphic species are characterized by the absence of an intra-morph incompatibility system and some are also self-compatible (Arroyo and Dafni, 1995; Barrett et al., 1997; Baker et al., 2000; Arroyo et al., 2002; Pérez-Barrales et al., 2006). Pollinator environment has also been useful to understand variation in morph ratio across populations. For example, populations of N. tazetta in Israel show a shift from S-morph-biased populations in marshes with predominance of long-tongued pollinators to L-morph-biased populations in hills with short-tongued pollinators as main visitors (Arroyo and Dafni, 1995). In tristylous N. tirandrus, the shift to a pollinator with larger and more variable size was coupled with a change in flower size and the loss of the M-morph (Hodgins and Barrett, 2008). Finally, in N. papyraceus a shift in pollinator guilds was suggested to be related to the loss of the S-morph and small but gradual and consistent changes in perianth traits (Pérez-Barrales et al., 2007; Pérez-Barrales et al., 2009). Despite the available experimental evidence (Pérez-Barrales and Arroyo, 2010), it is still unknown whether the fixation of the L-morph in natural conditions is linked to a breakdown in the incompatibility system (Eckert and Barrett, 1992) or to differences in reproductive success between morphs (Brys et al., 2008). Furthermore the studies in N. papyraceus (Pérez-Barrales et al., 2007; Pérez-Barrales and Arroyo, 2010) did not include observations of insects in the species' southern range (only populations in the central and northern geographic range were sampled) and the study of flower morphology did not include the analysis of reciprocity and the influence of the perianth traits, which is crucial for mating patterns. Detailed understanding of the maintenance of style dimorphism requires thorough investigation across the species' range, which is rarely achieved in widespread species (Barrett et al., 2004; Hodgins and Barrett, 2008).
The present study aimed to analyse the relationship between geographic variation in pollinator guilds and variation in population morph ratios and perianth traits in N. papyraceus across most of the species' range, to provide a comprehensive account of the geographic differences in flower biology. In particular, if an increased role of short-tongued pollinators determines a lower success of S-morph plants, because these insects cannot transfer pollen to their stigmas, then we predict that short-tongued insect visit rate will correlate with a decreased frequency of S-morph plants. We also predict that clinal variation in floral morphology will be correlated with variation in morph ratio because of its effect on reciprocity between morphs. To test this, we sampled 34 populations along the main geographic range of the species and measured the morph ratio, perianth traits, stigma–anther separation between morphs and the pollinator visitation rate and determined the incompatibility system.
MATERIALS AND METHODS
Study species and population sampling
Narcissus papyraceus Ker Gawl. (Amaryllidaceae) is a style dimorphic species [see Fig. 1 in Pérez-Barrales and Arroyo (2010) for details of this polymorphism] distributed along the Mediterranean Basin, with most populations located in the south-west of the Iberian Peninsula and north-west Morocco (Santos-Gally et al., 2012). Flowers present basal nectaries and narrow floral tubes in which stamens (in two whorls) are adnated. The flowering season matches the rainy and cold season of the Mediterranean climate, peaking in November–December in coastal lowlands and February–March in mountain and inland areas.
We selected 34 populations spanning approx. 700 km along a latitudinal gradient (Fig. 1) to study the pattern of variation in morph ratio, flower morphology and pollinators. Based on previous published work (Arroyo et al., 2002) and preliminary observations we grouped populations into three regions (see Fig. 1): the Guadalquivir river basin (south-west Iberian Peninsula; mostly L-monomorphic populations); Strait of Gibraltar (both shores; mostly dimorphic isoplethic populations); and the Sebou river basin (central Morocco, larger flowered and anisoplethic populations). This grouping guided selection of populations for some exhaustive analysis, but most analyses involved continuous geographic variation including the whole population sampling. We collected 4519 flowers of N. papyraceus, of which 2905 were from 17 populations in the Sebou river basin, the southern region that we had not previously explored. For morphological measurements, we sampled the first flower of the umbel inflorescence in at least 100 different plants per population (depending of the population size; see Table 1 for details of morph numbers) separated by at least 1 m to avoid repetition of the same genet. We preserved flowers in 70 % ethanol until their measurement in the laboratory.
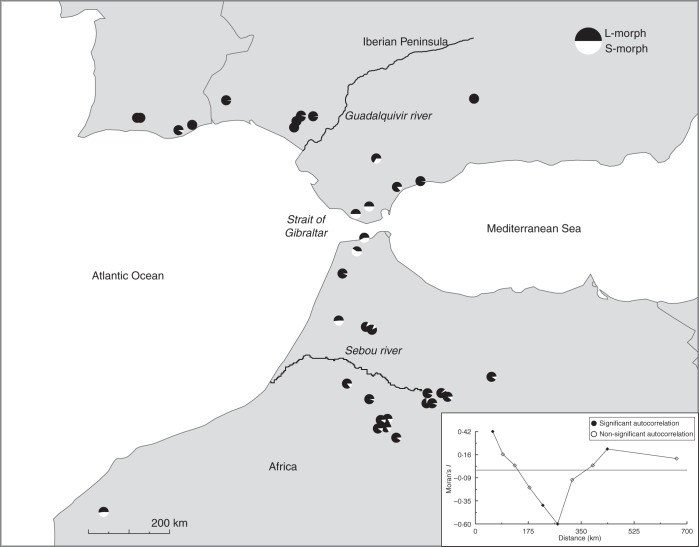
Fig. 1.
Distribution and morph-ratio variation of 34 populations of Narcissus papyraceus in the Guadalquivir river basin, Strait of Gibraltar and Sebou river basin. Each pie chart represents the morph ratio in a population (black = L-morph; white = S-morph). Moran's I correlogram of morph-ratio variation is shown in the inset graph (significant and non-significant autocorrelation coefficients as indicated, calculated from their moments; see PASSaGE v. 1·1 manual; Rosenberg, 2004).
Table 1.
Morph ratio and G-test for significant departure of isoplethy (1 : 1) in 34 populations of Narcissus papyraceus
| Population | Pop. no. | Co-ordinates | Elevation | Approx. population size | No. of flowers for morphometric measurments (L- : S-morph) | L-morph (%) | G-test |
|---|---|---|---|---|---|---|---|
| Morocco: Tánger-Tetuán, Oued Lediane | 1 | 35°50′N, 5°38′W | 20 | 5000 | 99 (57 : 42) | 57·00 | 2·28n.s. |
| Morocco: Tetuán-Larache, Souk el Arba Ayacha | 2 | 35°25′N, 5°53′W | 150 | 200 | 100 (95 : 5) | 96·30 | 98·93* |
| Morocco: Tánger-Tetuán, Ragaia | 3 | 35°41′ N, 5°43′ W | 100 | 100 | 100 (41 : 59) | 50·00 | 3·26n.s. |
| Spain: Málaga, Casares-Manilva | 4 | 36°26′N, 5°15′W | 300 | 1000 | 100 (87 : 13) | 87·40 | 61·35* |
| Spain: Málaga, San Pedro de Alcántara | 5 | 36°30′N, 4°59′W | 100 | 1000 | 67 (66 : 1) | 98·53 | 83·84* |
| Spain: Cádiz, Tarifa-Bolonia | 6 | 36°7′N 5°44′W | 100 | 1 000,000 | 100 (52 : 48) | 50·00 | 0n.s. |
| Spain: Cádiz, Los Barrios | 7 | 36°13′N 5°35′W | 100 | 250 000 | 100 (50 : 50) | 50·00 | 0n.s. |
| Spain: Cádiz, El Bosque | 8 | 36°46′N 5°30′W | 400 | 100 | 48 (32 : 16) | 66·00 | 10·42* |
| Spain: Huelva, Villanueva de los Castillejos | 9 | 37°27′N 7°15′W | 150 | 1000 | 100 (99 : 1) | 100·00 | 127·43* |
| Spain: Huelva, Hinojos, El Caoso | 10 | 37°15′N, 6°23′W | 50 | 100 | 24 | 100·00 | 138·63* |
| Spain: Huelva, Hinojos, Coto del Rey | 11 | 37°12′N, 6°26′W | 50 | 1000 | 100 | 100·00 | 138·63* |
| Spain: Huelva, Almonte, El Rocío | 12 | 37°9′N 6°27′W | 50 | 1000 | 99 (96 : 3) | 98·50 | 110·35* |
| Spain: Sevilla, Aznalcázar | 13 | 37°16′N, 6°14′W | 50 | 1000 | 98 | 100·00 | 135·86* |
| Spain: Córdoba, Carcabuey, Valdecañas | 14 | 37°28′N, 4°21′W | 700 | 1000 | 100 | 100·00 | 138·63* |
| Portugal: Algarve, Barranco São Miguel | 15 | 37°6′N, 7°49′W | 300 | 100 | 61 (55 : 6) | 90·60 | 45·34* |
| Portugal: Algarve, Mesines-Alte | 16 | 37°15′N, 8°15′W | 300 | 100 | 100 | 100·00 | 138·63* |
| Portugal: Algarve, Tavira | 17 | 37°10′N, 7°39′W | 100 | 500 | 100 | 100·00 | 138·63* |
| Morocco: Meknes, Boufakrane | 18 | 33°38′N, 5°26′W | 829 | 500 | 108 (91 : 17) | 91·95 | 123·09* |
| Morocco: Meknes, Boufakrane 2 | 19 | 33° 39′N, 5°26′W | 861 | 1000 | 95 (83 : 12) | 86·49 | 109·93* |
| Morocco: Meknes, Boufakrane 3 | 20 | 33°37′N, 5°26′W | 923 | 1000 | 97 (84 : 13) | 88·89 | 123·95* |
| Morocco: El Hajeb | 21 | 33°41′N, 5°23′W | 879 | 500 | 70 (50 : 20) | 83·54 | 200·16* |
| Morocco: Azrou | 22 | 33°29′N, 5°15′W | 1344 | 200 | 100 (99 : 1) | 90·16 | 181·38* |
| Morocco: Sefrou | 23 | 33°53′N 4°52′W | 697 | 1000 | 98 (92 : 6) | 94·83 | 397·54* |
| Morocco: Bhalil | 24 | 33°51′N, 4°51′W | 925 | 1000 | 97 (86 : 11) | 90·20 | 372·48* |
| Morocco: Jabariyine | 25 | 34°47′N, 5°36′20·7′ W | 211 | 70 | 22 (21 : 1) | 90·00 | 73·61* |
| Morocco: Ouezzane | 26 | 34°47′N, 5°36′13·97′W | 220 | 100 | 22 (10 : 12) | 90·00 | 73·61* |
| Morocco: Khemis Zemamra | 27 | 32°37′N, 8°38′W | 162 | 1000 | 98 (50 : 48) | 50·91 | 0·04n.s. |
| Morocco: Bounaz-Meknes | 28 | 34°7′N, 5°50′W | 890 | 100 | 100 (84 : 16) | 86·00 | 57·64* |
| Morocco: Meknes | 29 | 33°56′N, 5°34′W | 860 | 500 | 100 (95 : 5) | 95·00 | 98·93* |
| Morocco: Larache-Souk el Arba du Rhab | 30 | 34°52′N, 5°55′W | 100 | 500 | 63 (33 : 30) | 52·38 | 0·06n.s. |
| Morocco: Sidi Hazem-Taza | 31 | 34°0′N, 4°54′W | 500 | 100 | 60 (57 : 3) | 95·00 | 59·36* |
| Morocco: Sidi Hazem-Taza P236 | 32 | 33°58′N, 4°39′W | 670 | 100 | 62 (60 : 2) | 96·77 | 68·28* |
| Morocco: Bab-Merzouka, Fez | 33 | 34°12′N 4°08′W | 700 | 100 | 40 (37 : 3) | 92·50 | 34·14* |
| Morocco: Bab-Merzouka, Taourirt | 34 | 34°1′N, 4°44′W | 850 | 1000 | 48 (28 : 20) | 90·00 | 73·61* |
Note: morph ratio is expressed in terms of the percentage ratio of L-morph/L- + S-morph.
n.s., Non-significant; *, P < 0·05.
Morph ratios and spatial structure at a geographical scale
We classified flowers as L-morph when the style was above the lower anther whorl and S-morph when the style was below the lower anther whorl (Arroyo et al., 2002). Arroyo et al. (2002) showed that frequency distribution of style length is bimodal with the separation being coincident with height of lower anthers, as in other style-dimorphic Narcissus (Barrett et al., 1996). Morph ratios were calculated as number of sampled L-morph plants/sampled L- + S-morph plants. We assessed the significance of the departure of style morph frequencies from isoplethy (1 : 1) in each population of N. papyraceus with a G-test (Sokal and Rohlf, 1995).
The spatial structure of morph-ratio variation at the geographical scale was estimated by means of a spatial autocorrelation analysis (Moran's I) performed with PASSaGE v.1·1 (Rosenberg, 2004). From a distance matrix, we created ten distance classes with an equal number of population pairs in each division. To compute Moran's I autocorrelation coefficient, the distance class matrix was weighted as binary. For each data point we chose random distribution so the variances of the correlation coefficients were calculated given all possible random permutations of the data (Sokal and Oden, 1978).
Morphometric measurements and analyses
We followed Pérez-Barrales et al. (2009; see their fig. 2 for details) to measure five perianth traits – flower width, corona height and width and flower-tube length and width – and the sex organs – height of the upper- and lower-stamen whorls and style length. We analysed the variation in perianth traits within populations (L- vs. S-morph) and between regions (Guadalquivir river basin–Strait of Gibraltar and Sebou river basin) using a Student's t-test or Wilcoxon rank sum test when the data presented non-normal errors.
We applied a principal component analysis (PCA) on the correlation matrix from five perianth traits for all sampled individuals to examine patterns of variation among traits across populations. We extracted the mean and standard error of the scores of the first and second principal components for each population. Style length and lower- and upper-anther height were excluded in the PCA because the first presents a bimodal distribution and the second and third are highly correlated with tube length. Instead, we explored the allometric relationships between the sex organs and flower-tube length.
Because N. papyraceus has epipetalous stamens, the relationship between anther height and flower-tube length could differ among populations with different-sized flowers. In contrast, the style is placed in a different whorl, and it is likely to be less affected by tube length. The differential effect of flower-tube length on the anthers and style could influence sex-organ reciprocity within populations (Faivre and McDade, 2001; Barrett et al., 2004), and affect the population morph ratio (Barrett et al., 2004). We were interested in the slope of the regression between each sex organ and flower-tube length. Hence, we performed analyses of covariance (ANCOVA), where the response variables were the upper-anther height, lower-anther height and style length, and the covariate was flower-tube length. We included the factors population type (fixed) (Sebou river basin vs. Guadalquivir river basin–Strait of Gibraltar, clearly separated by PCA first component; see Results), population (random and nested within-population type) and morph (fixed) in the model. The interaction terms of interest for testing differences in slope between the two morphological types of populations (Guadalquivir river basin–Strait of Gibraltar and Sebou river basin, small and large-flowered populations, respectively; see Results,) were flower-tube length × population type for each of the three response variables related with sex organ height. We used a reduced dataset, including only those populations where L- and S-morphs were equally represented within each population sample to ensure accurate measures of floral morphology, keeping the two population types relatively balanced (five populations from Guadalquivir–Strait of Gibraltar and six populations from the Sebou river basin).
We measured stigma–anther separation between morphs as an estimate of reciprocity within populations of N. papyraceus (Barrett and Shore, 1987). We only analysed anisoplethic populations with more than three S-morph individuals. Because N. papyraceus presents two levels of anthers in each morph, we calculated the stigma–anther separation taking the upper anther level of the S-morph as reciprocal to the stigma of the L-morph, and lower anther level of the L-morph as reciprocal to the stigma of the S-morph. We explored the possible association between reciprocity and morph-ratio variation among populations with regression analyses, to evaluate the prediction that low reciprocity may decrease disassortative mating, and therefore the morph ratio could be biased. Because flower-tube length could, potentially, influence reciprocity, we included tube length in multiple regression analyses. Morph-ratio data were arcsin transformed (Sokal and Rohlf, 1995).
The PCA and multiple regression analyses were conducted in R using the lattice, MASS and scaterplot3D code (Everitt and Hothorn, 2006). The ANCOVAs were run in JMP (SAS, 1999).
Pollinators
Pollinator censuses were carried out in nine populations: two L-monomorphic and one L-anisoplethic population in the Guadalquivir river basin (population numbers 13, 11 and 17; see Table 1); three isoplethic populations in the Strait of Gibraltar (population numbers 3, 6 and 7; see Table 1); and one isoplethic and two L-anisoplethic populations in Sebou river basin (population numbers 27, 19 and 21; see Table 1). Data for diurnal observations of L-monomorphic were collected from the literature (Pérez-Barrales et al., 2007). We observed diurnal and nocturnal insects in 10-m2 plots for several days in 2008–2011. We selected plots with roughly similar numbers of flowers to avoid influence of flower number on visitation rate. Diurnal censuses started at 1000 h and continued until 1800 h, when diurnal pollinator visitation noticeably declined. Nocturnal observations started at 1800 h and continued until 2000 or 2100 h, depending on the weather conditions, which is crucial for pollinator activity. After that time, pollinator activity stopped. We used a head-lamp with red light to observe nocturnal visitors and also recorded videos with a digital video camera with near infrared to record in the dark (Sony DCR-SR70). We rotated observers randomly among plots, changing every 15 min. For each flower visited, we recorded the insect morphotype and if visits were legitimate (i.e. visitor's body came in contact with anthers and/or stigma). One specimen from each morphotype was captured and examined in the laboratory to check whether it bore pollen on its body (Beattie, 1971). Pollen was compared with reference samples made directly from flowers of N. papyraceus.
We checked if different insect morphotypes could reach the nectar column (which in N. papyraceus flowers is 4·2–5·8 mm in height; R. Santos-Gally and J. Arroyo, pers. obs.) and touch the S-stigmas. To do so, we sliced an S-styled flower across the sagittal plane, placed the head of the pollinator at the entrance of the flower tube with its proboscis extended, and measured the distance between the end of the proboscis and the floral nectary. If the tip of the proboscis was >5 mm away from the floral nectary the pollinator was considered as a short-tongued pollinator (ST), otherwise it was classified as a long-tongued pollinator (LT) (see Fig. S1 in Supplementary Data available online).
We calculated the visitation rate (number of visits per hour) by ST and LT pollinators and used correlation analyses to examine the relationship between population morph ratio and visitation rate from ST and LT pollinators.
Incompatibility system
We designed hand-pollination experiments to test whether the incompatibility system of N. papyraceus changes in the southern and northern margins of the species distribution. The experiments were performed for two anisoplethic populations in the Sebou river basin (populations number 18 and 21 in Table 1) and in one L-monomorphic population in the Guadalquivir river basin (population number 13 in Table 1). Thirty-three plants (population 18, 25 L-morph and 8 S-morph; population 21, 6 L-morph and 9 S-morph) were collected over a large area (approx. 3 ha) from the populations within the Sebou river basin and 16 L-morph plants from population 13 in the Guadalquivir river basin. These plants were grown in the greenhouse at the University of Seville.
For individuals of populations 18 and 21 we applied four hand-pollination treatments: self-pollination, within- and between-morph cross-pollination, and cross-pollination after prior self-pollination the day before. The later treatment is a test for ovule discounting due to late acting self-incompatibility, or early inbreeding depression. In these conditions, ovules do not produce seeds but are not available for further outcross fertilization (Barrett et al., 1997). For individuals of population number 13 we applied two treatments: self-pollination and within L-morph cross-pollination. Since resources for fruit development may vary according to the flower opening sequence and position in the inflorescence, we randomly assigned each of the four treatments to flowers at different positions in the inflorescence. The number of replicates per treatment was balanced across flower positions. Flowers of the within-morph and between-morph pollination treatments were emasculated before anthers dehisced.
We performed hand-pollinations daily for 6 weeks on inflorescences that were marked and bagged with exclusion nets (0·1-mm pore size) to avoid any potential pollen contamination. All treatments required two hand-pollinations (the second 24 h after the first, to exclude possible effects of unreceptive stigmas) and cross-pollinations involved a single, randomly chosen pollen donor. We harvested fruits 6–8 weeks after pollination, counted the number of plump seeds, aborted seeds and undeveloped ovules. The effect of different treatments on the seed set (seed to ovule number) were tested using a generalized estimating equation (GEE) with binomial distribution and logit link functions. We included treatment and population as categorical explanatory variables (function GEE in PASW version 18; SPSS, 2009), and flowers as repeated measures of plants, because four flowers per treatment in each plant were used. We analysed interactions as well as main effects.
RESULTS
Morph-ratio variation and its geographical structure
Of the 34 sampled populations, seven were L-monomorphic and were only distributed in the northern range margin (Fig. 1). All the rest (27) were dimorphic, and 21 of these deviated significantly from a 1 : 1 morph ratio (G-test for each population P < 0·001; see Table 1). Six populations were isoplethic, four of these were found in the Strait of Gibraltar, one on the western coast of Morocco and one in the Sebou river basin. Most of the southern populations in the Sebou river basin were anisoplethic (Fig. 1).
The spatial autocorrelogram of the morph ratio showed two positive peaks of similarity, corresponding to population pairs at distances between 0–56 km (populations in closer proximity have similar morph ratios) and 387–437 km (Fig. 1); the latter probably due to the anisoplethic populations located in the Sebou river basin and their association with the few anisoplethic populations from the Guadalquivir river basin. In contrast, two consecutive distances of negative similarity were found between 178 and 250 km, probably because of the marked change in morph ratio in mid-distance populations from the Strait of Gibraltar (Fig. 1).
Perianth morphology and sex-organ position
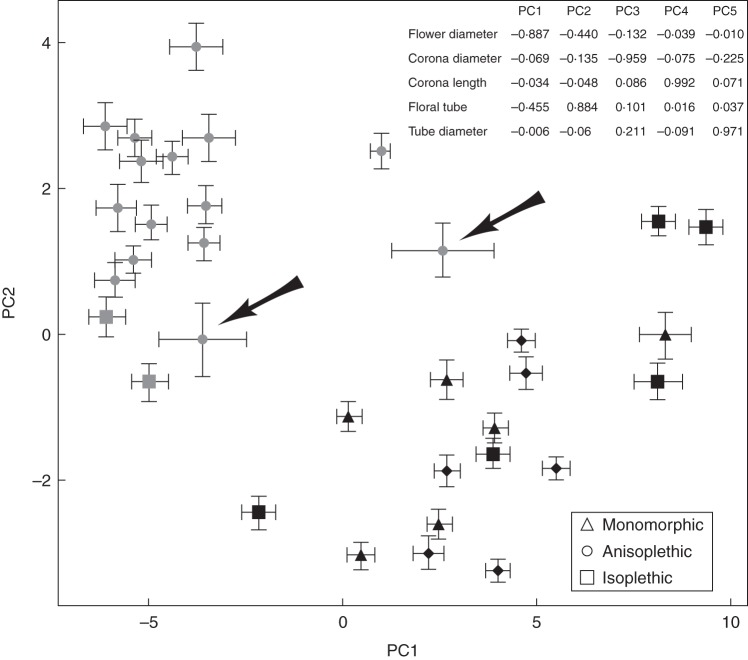
The PCA analyses showed that the first two components (PC1 and PC2) explained 95 % of the variance in the perianth measurements (PC1, 78 % of the variance explained; PC2, 17 %). Flower diameter loaded negatively on PC1, which separated populations of the Sebou river basin from populations of the Guadalquivir river basin and Strait of Gibraltar (Fig. 2). There was more variation in PC1 among populations from the Guadalquivir river basin and Strait of Gibraltar (bottom right in Fig. 2) than populations from the Sebou river basin (top left in Fig. 2). PC2 accounted for variation in flower-tube length (Fig. 2). It is remarkable that PC1 and PC2 accounted for most of the variation in flower diameter and flower-tube length, whereas the remaining flower traits were accounted for by the remaining low-variance components. Two populations between the Strait of Gibraltar and Sebou river basin (number 25 in the western Rif in Morocco and number 26 in northern central Morocco) presented the highest variation in the two PC axes (highlighted by arrows in Fig. 2). Differences between these regions in perianth traits are shown in Supplementary Data Table S1, and the most remarkable difference was associated with flower tube (length and diameter) and corolla diameter. Therefore, there were two clear population types differing in flower size (small-flowered populations from the Guadalquivir river basin and Strait of Gibraltar and large-flowered populations from the Sebou river basin). The corona showed some differences among populations in diameter but not in length. Intermorph differences were absent except in corona diameter in the northern region (Supplementary Data Table S1).
Fig. 2.
Principal component analysis of five morphological traits from the Guadalquivir river basin, Strait of Gibraltar and Sebou river basin populations of Narcissus papyraceus. The first two axes account for 78 % and 18 % of the total variation, respectively. Monomorphic, anisoplethic and isoplethic populations are as indicated in the key. The Guadalquivir river basin and Strait of Gibraltar populations are depicted by black symbols, the Sebou river basin populations by grey symbols. Each population is represented with standard error of PCA1 and PCA2 factor loadings. Loadings for all the variables of the PCA are given in the top right corner. Arrows indicate populations from an intermediate geographic location between the Strait of Gibraltar and the Sebou river basin.
The ANCOVAs showed that there was a significant effect of flower-tube length on the position of all sex organs (see Supplementary Data Table S2). Allometric relationships between floral tube and sex-organ height or length differed across population types (Guadalquivir river basin–Strait of Gibraltar and Sebou river basin). The interaction tube length × population type was not significant for style length (F = 0·0102, d.f. = 1,846, P = 0·9198; i.e. both population types had similar regression slopes between tube and style length). In contrast, this interaction was significant for upper-anther height (F = 5·6735, d.f. = 1,846, P = 0·017) and particularly for lower-anther height (F = 9·9863, d.f. = 1,846, P = 0·0016), indicating that population types had different slopes when regressing flower-tube length and anther heights. The three-way interaction term (tube length × morph × population type) was non-significant in all analyses (F = 0·0855–0·5178, d.f. = 1,846, P = 0·47–0·77).
Separation between L-stigma and upper anthers of S plants was shorter than separation between S-stigma and lower anthers of L plants (see Supplementary Data Fig. S2). There was a significant difference between Guadalquivir river basin–Strait of Gibraltar populations (small-flowered) and Sebou river basin populations (large-flowered) in the separation between S-stigma and lower anthers of L plants (W = 10, P = 0·0003), but not in the separation between L-stigma and upper anthers of S plants (W = 49, P = 0·40). The multiple regression analyses showed that neither stigma–anther separation (reciprocity), nor flower-tube length had an effect on the morph ratio (for L-morph, β = 0·10, P = 0·4 and β = 0·032, P = 0·07, reciprocity and tube length, respectively; and for S-morph, β = −0·0004, P = 0·99 and β = 0·035, P = 0·19, reciprocity and tube length, respectively).
Pollinators
We found similar guilds of pollinators in all population types (Guadalquivir river basin, Strait of Gibraltar and Sebou river basin: L-monomorphic, isoplethic and anisoplethic, respectively) of N. papyraceus (see Table 2 for details). Syrphidae (Eristalis sp., Eupeodes luniger, Merodon sp.) and Apidae (Amegilla sp., Anthophora sp. and Andrena sp.) were the most frequent families of diurnal short-tongued (ST) visitors, which only fed on pollen. The diurnal hummingbird hawk-moth (Macroglossum stellatarum, Sphingidae) and two nocturnal moths, Autographa gamma and other Sphingidae species, were the most frequent long-tongued (LT) pollinators, those able to access the nectar in the tube base (see Supplementary Data Fig. S1 for details). All pollen preparations from insects bodies observed under the microscope presented pollen of N. papyraceus.
Table 2.
Total number of pollinators observed in three types of Narcissus papyraceus populations (ST, short-tongued; LT, long-tongued; see text for details on this classification). The total hours of time effort of the observations is indicated
| Flower visitors | Isoplethic populations | Anisoplethic populations | Monomorphic populations |
|---|---|---|---|
| Diurnal | Total time effort: 99·5 | Total time effort: 92 | Total time effort: 25·5* |
| Diptera | |||
| Eupeodes luniger (ST) | 101 | ||
| Eupeodes sp. (ST) | 17 | ||
| Merodon (ST) | 5 | 9 | |
| Eristalis sp. (ST) | 26 | ||
| Eristalis tenax (ST) | 83 | 925 | |
| Bombylidae (LT) | 9 | 21 | |
| Other Syrphidae (ST) | 271 | 70 | 68 |
| Other Diptera (ST) | 28 | 45 | 2 |
| Hymenoptera | |||
| Wasps (ST) | 6 | 2 | |
| Amegilla sp. (ST) | 80 | 4 | |
| Anthophora sp (ST) | 1 | 282 | |
| Apis mellifera (ST) | 24 | 5 | 4 |
| Andrena sp (ST) | 1 | ||
| Bombus sp (LT) | 14 | 56 | |
| Other bees (LT) | 12 | 5 | |
| Other bees (ST) | 137 | 7 | 1 |
| Coleoptera (ST) | |||
| Tropinota squalida | 4 | ||
| Meligethes sp. | 246 | ||
| Lepidoptera | |||
| Gonepteryx cleopatra (LT ) | 10 | 4 | |
| Cynthia sp (LT) | 10 | 40 | |
| Macroglossum stellatarum (LT) | 301 | 158 | 2 |
| Pieris brassicae (LT) | 45 | ||
| Other moths (LT) | 1 | ||
| Other moths (ST) | 56 | 1 | |
| Nocturnal | Total time effort: 50·91 | Total time effort: 43·35 | Total time effort: 10·8 |
| Lepidoptera | |||
| Autographa gamma (LT) | 50 | 145 | 23 |
| Sphingidae (LT) | 4 | 23 | 7 |
| Shrankia sp. (ST) | 3 | ||
| Other moths (LT) | 15 |
* Data from Pérez-Barrales et al. (2007).
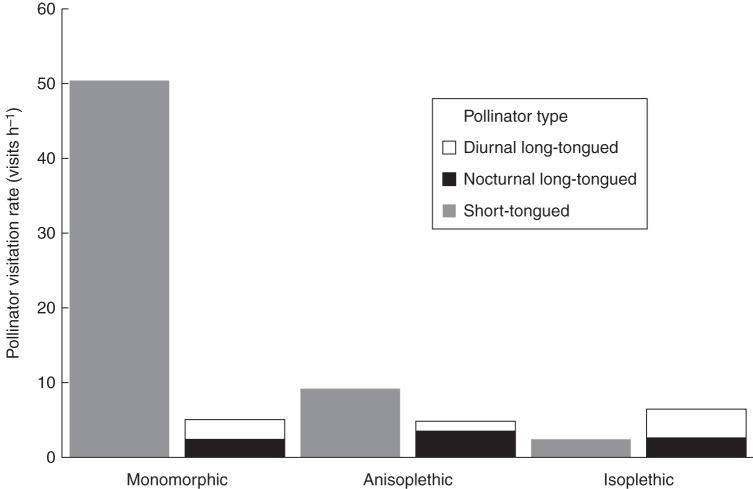
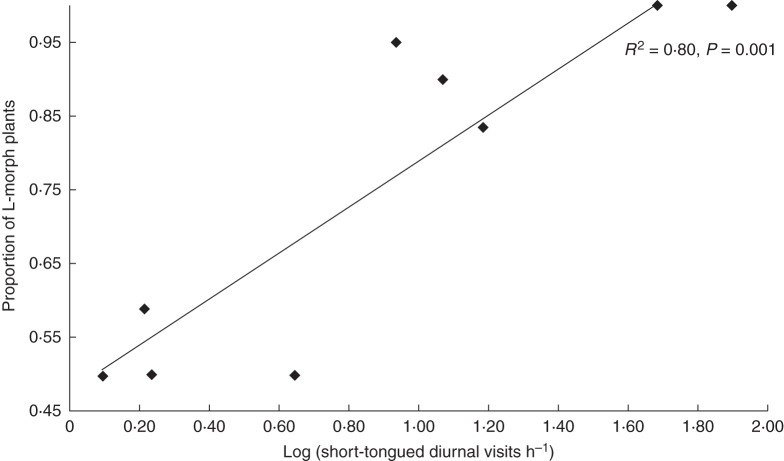
Populations differed in the pollinator visitation rate. The mean relative proportion of visits by ST insects was higher in L-monomorphic populations (89·2 %, s.d. = 0·6, n = 2) than in L-biased (62·7 %, s.d. = 12·4, n = 3) and isoplethic populations (17·9 %, s.d. = 4·7, n = 4). Diurnal and nocturnal LT pollinators presented the highest proportion of visits in isoplethic populations (73·3 %, s.d. = 15·8), and the frequency of their visits decreased in L-biased (32·6 %, s.d. = 10) and notably more in L-monomorphic (10·7 %, s.d. = 0·6) populations (Fig. 3). Correlation analysis revealed a significant positive relationship between morph ratio and visitation rate of ST pollinators; the higher the visit rate of ST pollinators the higher the proportion of the L-morph (R2 = 0·80, P = 0·0012; n = 9) (Fig. 4); whereas there was no effect of visitation rates of LT pollinators on morph ratio (R2 = 0·0002, P = 0·968; n = 9).
Fig. 3.
Diurnal and nocturnal, and short- and long-tongued pollinator visitation rates (total visits h−1) in nine populations of Narcissus papyraceus. Data from two monomorphic, three anisoplethic and four isoplethic populations (population numbers 3, 6, 7, 11, 13, 17, 19 and 21; see Table 1 for details). Diurnal short-tongued pollinators of the three types of population are shown separately (grey); long-tongued pollinators are divided into diurnal and nocturnal pollinators, as indicated.
Fig. 4.
Relationship between short-tongued pollinator visitation rates (total visits h−1) and the proportion of L-morph plants in nine populations of Narcissus papyraceus.
Incompatibility system
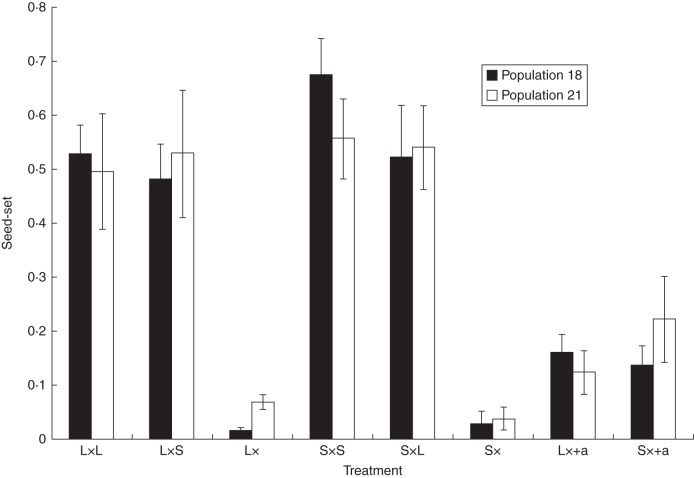
Hand-pollination experiments showed self-incompatibility and inter- and intra-morph compatibility in both dimorphic populations examined (Fig. 5). Average seed set was very similar between inter and intra-morph treatments but contrasted markedly with outcrossed pollination one day after self-pollination (Fig. 5). Generalized estimating equation analyses showed that the interaction population × treatment was significant (χ2 Wald = 14·21, P = 0·048, d.f. = 7), but the main effect of population was not significant (χ2 Wald = 0·683, d.f. = 1, P = 0·41), whereas the main effect of treatment was highly significant (χ2 Wald = 285·11, d.f. = 7, P < 0·0001). Ovule discounting by self-pollination reduced the seed set by 65–73 % depending on the morph (Fig. 5). In the monomorphic population, the self-pollination treatment produced nearly no seeds, whereas cross-pollination produced 50·2 % of seeds.
Fig. 5.
Seed-set (seed to ovule number) of L- and S-morphs in two anisoplethic population of Narcissus papyraceus from the Sebou river basin after hand pollination experiments (see Table 1 for location of populations). Values indicated are the means ± s.e. of seed production following intramorph (L × L, S × S), intermorph (L× S, S× L), self- pollination (L× , S×) and prior self-pollination plus cross-pollination (L× +a, S× +a).
DISCUSSION
Narcissus papyraceus is a species with a remarkable among-population variation in morph ratio, which is mirrored by variation in flower traits, sex-organ reciprocity and visitation rates of morpho-functionally different pollinators. The results obtained in this study, particularly in the southern range of the species, showed distinctly different patterns, albeit complementary, from those previously reported for the northern range (Arroyo et al., 2002; Pérez-Barrales et al., 2009). Taken together these results provide strong evidence supporting the hypothesis that long- and short-tongued pollinators are involved in the maintenance, and loss, of style polymorphism in N. papyraceus, supporting the Darwinian view.
Morph-ratio variation and its geographical structure
Morph ratio exhibited (a) non-random variation among populations throughout the geographic range (Fig. 1), (b) predominance of L-biased populations in the southern range, and (c) absence of S-biased and S-monomorphic populations. Some of these patterns are consistent with the results of previous studies in stylar polymorphic N. assoanus and N. dubius (Baker et al., 2000), N. tazetta (Arroyo and Dafni, 1995) and N. triandrus (Barrett et al., 2004). Only the latter study showed a clear geographically explicit structure of morph ratio variation. Some of these studies showed an association between small population size and increased frequency of the L-morph and eventually L-monomorphism. It is remarkable that fixation of the L-morph was never found in the southern range of N. papyraceus where most populations were small (<1000 individuals; see Table 1). On the other hand, in the northern range polymorphism is lost in small populations (Arroyo et al., 2002). Thus large population size may not be a necessary condition to maintain the S-morph.
Perianth morphology, sex-organ separation and morph-ratio variation
Narcissus papyraceus populations from Morocco, the southern range of the species, had larger flowers than those from the northern range. It is noteworthy that there is larger flower-size variation across populations from the northern range (Strait of Gibraltar and Guadalquivir river basin) than those from the southern inland range (Sebou river basin) (as depicted by PC1 in Fig. 2). The two populations from the latter group that showed the highest within-population variation for both PC1 and PC2 (Fig. 2, highlighted by the arrows) are in an intermediate geographic location between the two groups of populations. It is possible that they contain plants derived from both regions, which would enhance the within-population variation. Ongoing research on population genetic and morph ratio will reveal if this is the case (V. I. Simón, unpubl. res.).
The regression slope between flower-tube length and style length was identical for populations in the northern and southern range, but the slopes of the relationship between tube length and both upper- and lower-anther heights differed between populations. The ultimate consequence of the dissimilar relationship between male and female organs in different populations is that reciprocity is affected (Faivre and McDade, 2001). Our results confirm this view, as there was a significant difference in reciprocity between Strait of Gibraltar–Guadalquivir river basin (large-flowered) and Sebou river basin (short-flowered) populations (see Supplementary Data Fig. S2). However, although flower allometry affects reciprocity, there is no relationship between the latter and morph ratio, contrary to our prediction and in contrast to the scarce available evidence in other style-polymorphic species (e.g. Barrett et al., 2004). Experimental evaluation of pollen transfer rates and fertility in style-dimorphic Narcissus species have shown that species with higher reciprocity present higher rates of disassortative than assortative mating (i.e. N. assoanus; Cesaro and Thompson, 2004) compared with species with lower reciprocity (i.e. N. papyraceus; Pérez-Barrales and Arroyo, 2010).
As shown in other stylar dimorphic Narcissus, stigma–anther separation of the S-morph was higher than in the L-morph (see Supplementary Data Fig. S2). Moreover, the latter have reciprocal positions of stigmata with upper anthers of both L- and S-morphs (data not shown for the L-morph), which favours assortative mating. This advantage of the L-morph could lead to L-biased populations. It has been argued that the frequency of the S-morph is dependent on its reciprocity, as a surrogate for disassortative mating, and an experimental and a correlative study support this prediction in long-tongued-pollinated N. assoanus (Cesaro and Thompson, 2004; Thompson et al., 2012). However, our results do not support this hypothesis, as reciprocity does not vary with the morph ratio in N. papyraceus populations. It is possible that, in N. papyraceus, short-tongued pollinators reduce selection for S-morph reciprocity.
Pollinator influence on morph-ratio variation
Although reciprocity in N. papyraceus is lower compared with truly heterostylous species, the position of the sexual organs may nonetheless facilitate reciprocal mating. The hidden stigma (6·4 mm deep into the tube) of the S-morph requires the action of LT insects to receive pollen. Although LT insects seem to be adequate for pollen transfer to the S-stigma (Cesaro et al., 2004; Pérez-Barrales and Arroyo, 2010), we did not find a significant relationship between morph ratio and the visitation rate of LT pollinators across the studied populations. Instead, visitation rate of ST pollinators was positively related to morph ratio (Fig. 4). Because ST pollinator visitation rate is clearly correlated with overall visitation rate (r = 0·9, d.f. = 7, P < 0·0001) it is possible that the morph ratio is also a consequence of overall visitation rate. We suggest a role of ST pollinators on morph-ratio variation, rather than overall rate, simply because they are by far the most abundant in monomorphic and anisoplethic populations, whereas LT pollinators are equally uncommon in all populations. Furthermore, there is no correlation between LT pollinators and overall visitation rate (r = 31, d.f. = 7, P = 0·42). Tackling this question will require a detailed study of ST- and LT-pollinator efficiency on morphs and populations, which is currently ongoing. Despite our results being correlative, these findings may suggest that an increase in short-tongued visitors can deviate the morph ratio towards L dominance, probably much through increased assortative mating among L-plants (Pérez-Barrales and Arroyo, 2010). Note that the L-morph has been suggested to be recessive homozygous (Dulberger, 1964); if true crosses between L-morph plants would give only this morph. The lack of a relationship between S-morph reciprocity (stigma–anther separation) and morph ratio is thus probably due to the fact that extremely frequent ST pollinators do not play a role in pollinating S-flowers.
We propose that pollen availability in the anthers could be reduced by the numerous visits of pollen-collecting syrphids and solitary bees. The activity of these insects may hence reduce pollen availability for other pollinators, such as hawkmoths, one of the most frequent LT visitors, and the only ones able to reach the S-stigmas to deliver the pollen. As a result, S-morph flowers may suffer reduced fertility (Pérez-Barrales and Arroyo, 2010). Indeed, we observed that (a) pollen from the upper anthers was almost entirely collected by Andrena sp. after a single visit (see Supplementary Data Fig. S1) and (b) the hoverfly Eristalis tenax may deposit about 23 pollen grains on an L-stigma after a visit (V. I. Simón, R. Santos-Gally and J. Arroyo, unpubl. res.) and ovaries contain an average of 44·8 ± 0·8 ovules. Moreover, females of Antophora sp. and Amegilla spp. actively gather pollen to transport back to the nest for larval nutrition (Alcock, 1999). Interestingly, the S-morph is never lost in the southern range of the species, where ST pollinators were noticeably less abundant than in the L-monomorphic northern range.
Incompatibility system
Hand-pollination experiments from marginal northern L-monomorphic and southern anisoplethic populations showed that the incompatibility system is maintained and similar to that reported in central populations of N. papyraceus (Arroyo et al., 2002): self-incompatibility and within- and between-morph compatibility (Fig. 5). In sex polymorphic species, the breakdown of the incompatibility system can influence morph ratio variation (Husband and Barrett, 1991) or cause the fixation of one of the morphs (Alves dos Santos, 2002); however in N. papyraceus we can discard this possibility.
Conclusions
In this study, we found that allometric effects of discrete variation in flower size were associated with variation in reciprocity, but neither tube length or stigma–anther separation account for morph-ratio variation. We also showed that the loss of stylar polymorphism in populations of N. papyraceus is correlated with high visitation rates of ST pollinators, which suggests that their abundance may overwhelm any possible effects of LT pollinators. These ecological data, combined with the maintenance of the incompatibility system across the distribution range of the species reinforces the argument that pollinators could be the main ecological and evolutionary drivers for the maintenance versus loss of stylar dimorphism in N. papyraceus.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
Alejandra de Castro, A. Peña del Valle, M. Serra, J. Ruiz and I. Cancio assisted with field and laboratory work. A. Gonzalez-Voyer improved early drafts of the manuscript, including English correction and P. L. Valverde helped with regression models for sexual organs. M. A. Marcos identified some of the insect. The greenhouse service at the University of Seville assisted with plant care. This work was supported by funding from the Spanish Ministerio de Ciencia e Innovación (grants CGL-2006-13847-C02-01 and CGL-2009 12565) and PAIDI (Andalousian regional government grant P09-RNM-5280). R.S.G. was supported by a fellowship from Consejo Nacional de Ciencia y Tecnología (CONACyT) and a postdoctoral research contract from the Andalousian regional government (P09-RNM- 5280).
LITERATURE CITED
- Alcock J. The nesting behavior of Dawson's Burrowing Bee, Amegilla dawsoni (Hymenoptera: Anthophorini), and the production of offspring of different sizes. Journal of Insect Bahavior. 1999;12:363–384. [Google Scholar]
- Alves dos Santos I. Flower-visiting bees and the breakdown of the tristylous breeding system of Eichhornia azurea (Swartz) Kunth (Pontederiaceae) Biological Journal of the Linnean Society. 2002;77:449–507. [Google Scholar]
- Arroyo J, Dafni A. Variations in habitat, season, flower traits and pollinators in dimorphic Narcissus tazetta (Amaryllidaceae) in Israel. New Phytologist. 1995;129:135–145. doi: 10.1111/j.1469-8137.1995.tb03017.x. [DOI] [PubMed] [Google Scholar]
- Arroyo J, Barrett SCH, Hidalgo R, Cole WW. Evolutionary maintenance of stigma-height dimorphism in Narcissus papyraceus (Amaryllidaceae) American Journal of Botany. 2002;89:1242–1249. doi: 10.3732/ajb.89.8.1242. [DOI] [PubMed] [Google Scholar]
- Baker AM, Thompson JD, Barrett SCH. Evolution and maintenance of stigma-height dimorphism in Narcissus. II. Fitness comparisons between style morphs. Heredity. 2000;84:514–524. doi: 10.1046/j.1365-2540.2000.00686.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD. The evolution of polymorphic sexual systems in daffodils (Narcissus) New Phytologist. 2005;165:45–53. doi: 10.1111/j.1469-8137.2004.01183.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. Variation and evolution of breeding systems in the Turnera ulmifolia complex (Turneraceae) Evolution. 1987;41:340–354. doi: 10.1111/j.1558-5646.1987.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Lloyd DG, Arroyo J. Stylar polymorphisms and the evolution of heterostyly in Narcissus (Amaryllidaceae) In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman & Hall; 1996. [Google Scholar]
- Barrett SCH, Cole WW, Arroyo J, Cruzan MB, Lloyd DG. Sexual polymorphisms in Narcissus triandrus (Amaryllidaceae): is this species tristylous? Heredity. 1997;78:135–145. [Google Scholar]
- Barrett SCH, Harder LD, Cole WW. Correlated evolution of floral morphology and mating-type frequencies in a sexually polymorphic plant. Evolution. 2004;58:964–975. doi: 10.1111/j.0014-3820.2004.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Beattie A. A technique for the study of insect-borne pollen. Pan Pacific Entomologist. 1971;47:82. [Google Scholar]
- Brys R, Jacquemyn H, Hermy M, Beeckman T. Pollen deposition rates and the functioning of distyly in the perennial Pulmonaria officinalis (Boraginaceae) Plant Systematics and Evolution. 2008;273:1–12. [Google Scholar]
- Cesaro AC, Thompson JD. Darwin's cross-promotion hypothesis and the evolution of stylar polymorphism. Ecology Letters. 2004;7:1209–1215. [Google Scholar]
- Cesaro AC, Barrett SCH, Maurice S, Vaissiere BE, Thompson JD. An experimental evaluation of self-interference in Narcissus assoanus: functional and evolutionary implications. Journal of Evolutionary Biology. 2004;17:1367–1376. doi: 10.1111/j.1420-9101.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. The maintenance of distyly. American Naturalist. 1979a;114:499–513. [Google Scholar]
- Charlesworth D, Charlesworth B. A model for the evolution of distyly. American Naturalist. 1979b;114:467–498. [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- Dulberger R. Flower dimorphism and self-incompatibility in Narcissus tazetta L. Evolution. 1964;18:361–363. [Google Scholar]
- Eckert CG, Barrett SCH. Stochastic loss of style morphs from populations of tristylous Lythrum salicaria and Decodon verticillatus (Lythraceae) Evolution. 1992;46:1014–1029. doi: 10.1111/j.1558-5646.1992.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Everitt BS, Hothorn T. A handbook of statistical analyses using R. London: Chapman & Hall/CRC; 2006. [Google Scholar]
- Faivre AE, McDade LA. Population-level variation in the expression of heterostyly in three species of Rubiaceae: does reciprocal placement of anthers and stigmas caracterize heterostyly? American Journal of Botany. 2001;88:841–853. [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Ferrero V, Arroyo J, Vargas P, Thompson JD, Navarro L. Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae) Perspectives in Plant Ecology Evolution and Systematics. 2009;11:111–125. [Google Scholar]
- Ferrero V, Arroyo J, Castro S, Navarro L. Unusual heterostyly: style dimorphism and self-incompatibility are not tightly associated in Lithodora and Glandora (Boraginaceae) Annals of Botany. 2012;109:655–665. doi: 10.1093/aob/mcr222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganders FR. The biology of heterostyly. New Zealand Journal of Botany. 1979;17:607–635. [Google Scholar]
- Heuch I. Equilibrium populations of heterostylous plants. Theoretical Population Biology. 1979;15:43–57. [Google Scholar]
- Hodgins KA, Barrett SCH. Female reproductive success and the evolution of mating-type frequencies in tristylous populations. New Phytologist. 2006;171:569–580. doi: 10.1111/j.1469-8137.2006.01800.x. [DOI] [PubMed] [Google Scholar]
- Hodgins K, Barrett SCH. Geographic variation in floral morphology and style-morph ratios in a sexually polymorphic daffodil. American Journal of Botany. 2008;95:185–192. doi: 10.3732/ajb.95.2.185. [DOI] [PubMed] [Google Scholar]
- Husband BC, Barrett SCH. Colonization history and population genetic structure of Eichhornia paniculata in Jamaica. Heredity. 1991;66:287–296. [Google Scholar]
- Johnson SD. Pollination ecotypes of Satyrium hallackii (Orchidaceae) in South Africa. Botanical Journal of the Linnean Society. 1997;123:225–235. [Google Scholar]
- Lewis D, Jones DA. The genetics of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. [Google Scholar]
- Lloyd DG, Webb CJ. The evolution of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. [Google Scholar]
- Meeus S, Honnay O, Brys R, Jacquemyn H. Biased morph ratios and skewed mating success contribute to diversity in the distylous Pulmonaria officinalis. Annals of Botany. 2012;109:227–235. doi: 10.1093/aob/mcr272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MT, Barrett SCH. Historical factors and anisoplethic population structure in tristylous Pontederia cordata L., a re-assessment. Evolution. 1988;42:496–504. doi: 10.1111/j.1558-5646.1988.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Barrales R, Arroyo J. Pollinator shifts and the loss of style polymorphism in Narcissus papyraceus (Amaryllidaceae) Journal of Evolutionary Biology. 2010;23:1117–1128. doi: 10.1111/j.1420-9101.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Barrales R, Vargas P, Arroyo J. New evidence for the Darwinian hypothesis of heterostyly: breeding systems and pollinators in Narcissus sect. Apodanthi. New Phytologist. 2006;171:553–567. doi: 10.1111/j.1469-8137.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Barrales R, Arroyo J, Ambruster WS. Differences in pollinator faunas may generate geographic differences in floral morphology and integration in Narcissus papyraceus (Amaryllidaceae) Oikos. 2007;116:1904–1918. [Google Scholar]
- Pérez-Barrales R, Pino R, Albaladejo RG, Arroyo J. Geographic variation of flower traits in Narcissus papyraceus (Amaryllidaceae): do pollinators matter? Journal of Biogeography. 2009;36:1411–1422. [Google Scholar]
- Robertson JL, Wyatt R. Evidence for pollination ecotypes in the yellow-fringed orchid, Plantathera ciliaris. Evolution. 1990;44:121–133. doi: 10.1111/j.1558-5646.1990.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg MS. PASSaGE. pattern analyses, spatial statistics, and geographic exegesis. Tempe, AZ: Arizona State University; 2004. Version 1·1. [Google Scholar]
- Sánchez JM, Ferrero V, Arroyo J, Navarro L. Patterns of style polymorphism in five species of the South African genus Nivenia (Iridaceae) Annals of Botany. 2010;106:321–331. doi: 10.1093/aob/mcq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Gally R, Vargas P, Arroyo J. Insights into Neogene Mediterranean biogeography based on phylogenetic relationships of mountain and lowland lineages of Narcissus (Amaryllidaceae) Journal of Biogeography. 2012;39:782–789. [Google Scholar]
- SAS I. JMP statistics and graphics guide. Cary, NC: SAS Institute; 1999. Version 3·2·5. [Google Scholar]
- Sokal RK, Oden NL. Spatial autocorrelation in biology. 1. Methodology. Biological Journal of the Linnean Society. 1978;10:199–128. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York, NY: W. H. Freeman and Co. 1995 [Google Scholar]
- Sosenski P, Fornoni J, Molina-Freaner FE, Weller SG, Domínguez CA. Changes in sexual organ reciprocity and phenotypic integration during the tristyly-distyly transition in Oxalis alpina. New Phytologist. 2010;185:829–840. doi: 10.1111/j.1469-8137.2009.03105.x. [DOI] [PubMed] [Google Scholar]
- SPSS I. PASW Statistics 18. Chicago, IL: SPSS Inc; 2009. Version 18. [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in Angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Thompson JD. Plant evolution in the Mediterranean. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Thompson JD, Cesaro AC, Arroyo J. Morph ratio variation and sex organ reciprocity in style dimorphic Narcissus assoanus. International Journal of Plant Sciences. 2012;173:885–893. [Google Scholar]
- Valiente-Banuet A, Molina-Freaner F, Torres A, Arizmendi MdelC, Casas A. Geographic differentiation in the pollination system of the columnar cactus Pachycereus pecten-aboriginum. American Journal of Botany. 2004;91:850–855. doi: 10.3732/ajb.91.6.850. [DOI] [PubMed] [Google Scholar]
- Weller SG, Domínguez CA, Molina-Freaner F, Fornoni J, Gretchen L. The evolution of distyly from tristyly in populations of Oxalis alpina (Oxalidaceae) in the Sky Islands of the Sonoran Desert. American Journal of Botany. 2007;94:972–985. doi: 10.3732/ajb.94.6.972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.