Abstract
Background and Aims
Under stress-promoting conditions unicellular algae can undergo programmed cell death (PCD) but the mechanisms of algal cellular suicide are still poorly understood. In this work, the involvement of caspase-like proteases, DNA cleavage and the morphological occurrence of cell death in wasp venom mastoparan (MP)-treated Chlamydomonas reinhardtii were studied.
Methods
Algal cells were exposed to MP and cell death was analysed over time. Specific caspase inhibitors were employed to elucidate the possible role of caspase-like proteases. YVADase activity (presumably a vacuolar processing enzyme) was assayed by using a fluorogenic caspase-1 substrate. DNA breakdown was evaluated by DNA laddering and Comet analysis. Cellular morphology was examined by confocal laser scanning microscopy.
Key Results
MP-treated C. reinhardtii cells expressed several features of necrosis (protoplast shrinkage) and vacuolar cell death (lytic vesicles, vacuolization, empty cell-walled corpse-containing remains of digested protoplast) sometimes within one single cell and in different individual cells. Nucleus compaction and DNA fragmentation were detected. YVADase activity was rapidly stimulated in response to MP but the early cell death was not inhibited by caspase inhibitors. At later time points, however, the caspase inhibitors were effective in cell-death suppression. Conditioned medium from MP-treated cells offered protection against MP-induced cell death.
Conclusions
In C. reinhardtii MP triggered PCD of atypical phenotype comprising features of vacuolar and necrotic cell deaths, reminiscent of the modality of hypersensitive response. It was assumed that depending on the physiological state and sensitivity of the cells to MP, the early cell-death phase might be not mediated by caspase-like enzymes, whereas later cell death may involve caspase-like-dependent proteolysis. The findings substantiate the hypothesis that, depending on the mode of induction and sensitivity of the cells, algal PCD may take different forms and proceed through different pathways.
Keywords: Caspase-like proteases, cell death phenotype, Chlamydomonas reinhardtii, DNA disintegration, mastoparan, programmed cell death, signalling, YVADase
INTRODUCTION
Programmed cell death (PCD) is an active process of cellular suicide present throughout animal and plant kingdoms. In plants it is involved in embryogenesis, development and senescence and in the response to a variety of biotic and abiotic challenges (Pennell and Lam, 1997; Fukuda, 2000; Heath 2000; Lam, 2004; Bozhkov et al., 2005; Rogers, 2006; van Doorn and Woltering, 2005). In mammalian cells, a type of PCD commonly referred to as apoptosis, is often mediated by a caspase (cysteine-aspartic protease) enzymatic cascade (Sanmartin et al., 2005) resulting in a typical phenotype comprised of cytoplasm shrinkage, chromatin condensation and ordered nuclear DNA cleavage into oligonucleosomal fragments of 180 bp and multiples thereof (Kerr et al., 1972). Plant PCD might also be accompanied by the occurrence of apoptotic-like DNA fragmentation and nuclear condensation but this is not always observed (Mur et al., 2008; van Doorn and Woltering, 2010). Although no structural homologues of animal caspases are present in plants, there is evidence that plant proteases with substrate specificity and functional similarity to animal caspases do exist, and inhibitors designed to target the caspase-active sites in animal cells have been proven as potent PCD inhibitors in plant systems (del Pozo and Lam, 1998; Sun et al., 1999; de Jong et al., 2000; Elbaz et al., 2002; Woltering et al., 2002; Chichkova et al., 2004; Danon et al., 2004; Rotari et al., 2005; Bonneau et al., 2008; Twumasi et al., 2010). Vacuolar processing enzyme (VPE), a plant asparaginyl-specific cysteine protease was identified as one of the plant protease targets of human caspase-1 inhibitors (Hiraiwa et al., 1999; Lam and del Pozo 2000; Rotari et al., 2001; Yamada et al., 2005; Hara-Nishimura et al., 2005) and shown to be implicated in cell death in tobacco induced by tobacco mosaic virus (Hatsugai et al., 2004) and in bacteria, fungus and virus-induced cell death in Arabidopsis thaliana (Rojo et al., 2004). The proteasome β1 subunit was demonstrated as a target of a caspase-3 inhibitor (Hatsugai et al., 2009) and caspase-like activity has been established for subtilisin-like serine proteases (saspases) involved in victorin-induced cell death in oat (Coffeen and Wolpert, 2004). Mediators of plant PCD are also metacaspases that different to caspases hydrolyse proteins after arginine or lysine and not after aspartate residue (Sundström et al., 2009; Carmona-Gutierrez et al., 2010). Additionally, PCD signal transduction may proceed through a caspase-independent proteolysis that may operate in concert with a caspase-like cascade (Woltering et al., 2002; Woltering, 2010; Rotari et al., 2005; Bonneau et al., 2008; van Doorn et al., 2011). Other key components of plant cell death mechanism are, among others, mitochondrial factors, reactive oxygen species (ROS), nitric oxide, ethylene and other hormones (Wang et al., 1996; Hoeberichts and Woltering, 2003; Lam, 2004; van Doorn and Woltering, 2005; Mur et al., 2008; Reape and McCabe, 2008). Similar to animal systems plant anti-apoptotic factors such as defender against apoptotic death (dad1) expressed in response to UV-C stress in Chlamydomonas and Bax inihibitor-1 (BI-1) in arabidopsis challenged with heat shock or with the mycotoxin fumonisin B1 have been identified (Kawai-Yamada et al., 2001; Watanabe and Lam, 2006; Moharikar et al., 2007).
According to recently recommended morphological classification, two major classes of plant cell death are recognized (van Doorn et al., 2011). The cell death, which is associated with the formation of autophagosome-like and small lytic structures, growing larger vacuoles, activation of VPE, tonoplast rupture and vacuole-mediated engulfment of the protoplast to form a virtually empty cell corpse, has been defined as ‘vacuolar cell death’. Cell death showing swelling of mitochondria, protoplast shrinkage, early rupture of the plasma membrane resulting in a largely unprocessed cell corpse, and biochemical features of mitochondrial membrane permeabilization, respiratory decline, ATP depletion and accumulation of ROS and reactive nitrogen species has been termed ‘necrosis’. The laddering pattern of DNA degradation may occur during both PCD categories. The hypersensitive response (HR) at plant–microbe interactions, self-incompatibility (SI) response – a PCD mechanism aimed at preventing self-fertilization in angiosperm plants, fungal toxin victorin-induced cell death in oat and cell death in cereal starchy endosperm have been categorized as cell-death modalities expressing mixed or atypical phenotype of vacuolar and necrotic cell deaths (van Doorn et al., 2011).
Under certain stress-promoting conditions, unicellular algae are able to undergo PCD which determines them as a useful model system for examining the physiological factors and molecular components of cell death-signalling pathways. It is proposed that PCD may control the fate of individuals and, by selecting the fittest cells to orchestrate a co-ordinated response to stresses, and thus provide a selective advantage for preservation of the algal population (Vardi et al., 1999; Segovia et al., 2003; Bidle and Bender, 2008; Deponte, 2008; Segovia and Berges, 2009). It has been suggested that, depending on intensity and type of stress stimulus, the single-cell algae may express morphological and biochemical features of different cell death types. For example, cell death showing necrotic morphology and an increase in DEVDase (a plant protease expressing caspase-3-like activity) has been detected in Micrasterias denticulata exposed to low concentrations of hydrogen peroxide (H2O2) (Darehshouri et al., 2008), whereas in response to another cell death inducer (salt stress), in the same alga, symptoms of vacuolar cell death and DNA fragmentation have been simultaneously observed (Affenzeller et al., 2009). Very recently, Andosch et al. (2012) showed that under Cd stress Micrasterias can also undergo autophagic cell death as shown by the occurrence of autophagosome-like structures. In heat- and salt-stressed Chlorella saccharophila symptoms of necrotic PCD have been described (Zuppini et al., 2010). UV radiation, nitrogen starvation, heat shock and senescence have been demonstrated to produce cell death in Dunaliella viridis conforming to a mix of morphological PCD features associated with activation of DEVDase (Jiménez et al., 2009). Furthermore, metacaspases have also been shown to have a role in algal cell death (Bidle and Bender, 2008). Participation of ROS in stress-induced cell-death signalling in microalgae has been clearly documented (Vardi et al., 1999; Darehshouri et al., 2008; Segovia and Berges, 2009; Zuppini et al., 2010). Comparative analysis of PCD-related genome sequences including unicellular green algae revealed existence of anti-apoptotic regulators homologous to those in animals. Among them are apoptosis antagonizing transcription factor (AATF), tumour suppressor transcription factor p53, baculovirus inhibitor of apoptosis protein repeat (BIR) domain, proteinase inhibitor I32 and apoptosis inhibitory protein 5 (AP15) (Nedelcu, 2006, 2009 and references therein).
The unicellular photoautotrophic alga Clamydomonas reinhardtii (Chlorophyta) is an established model for research on important physiological processes such as photosynthesis, respiration, cell division and nutrient uptake, genome stability and adaptive response in presence of radiation, UV and chemical mutagens, heavy-metal tolerance in relation to phytoremediation and for stress validation of, for example, salinity, high and low temperature and water, osmotic and oxidative stresses (Harris, 2001; Hanikenne, 2003; Hema et al., 2007; Dimova et al., 2008). Recently, Chlamydomonas has been employed in investigations into PCD (Moharikar et al., 2006, 2007; Pérez-Pérez et al., 2010; Yordanova et al., 2009, 2010; Durand et al., 2011; Zuo et al., 2012). Exposure of Chlamydomonas reinhardtii to UV-C was shown to induce markers of animal apoptosis such as DNA laddering, occurrence of terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL)-positive nuclei and externalization of phosphatidylserine (Moharikar et al., 2006). Similar features of cell death have been found in conditions of acetic acid stress (Zuo et al., 2012). C. reinhardtii was also demonstrated to undergo autophagic PCD in response to treatment with rapamycin (an inhibitor of the protein kinase target of rapamycin – a negative regulator of autophagy in mammalian cells) (Pérez-Pérez et al., 2010).
In plant cell biology, C. reinhardtii has also been used as a classical model for studies on phospholipid signalling downstream of trimeric G-proteins stimulated by the wasp venom mastoparan (MP) – a cationic amphipathic 14-residue peptide toxin which is recognized as a modulator of G-proteins. It has been found that MP stress triggers pathways involving Ca2+, inositol triphosphate (IP3) turnover, phospholipases C and D (PLC and PLD, respectively), phosphatidic acid and oxidative stress, and causes cell mortality (Legendre et al., 1992; Quarmby et al., 1992; Ross and Higashijima, 1994; Munnik et al., 1998; van Himbergen et al., 1999). In the earlier works, the morphological occurrence of cell death in C. reinhardtii was estimated mainly by excising the flagella (Quarmby et al., 1992). More up-to-date details regarding MP-induced PCD features have not yet been described. In human cells MP has been shown to induce apoptosis executed through a caspase cascade (e.g. Ellerby et al., 1997). MP has also been demonstrated as a potent PCD inducer in plant models. Low concentrations of 2·5 and 5 µm MP were established to trigger vacuolar collapse and DNA fragmentation during differentiation of the tracheary elements in zinnia cell culture (a process of xylogenesis in vitro, representing a type of developmental PCD) (Groover and Jones, 1999). The application of 25-μm MP induced nuclear DNA cleavage and death of the cells in pollen tubes resembling the SI PCD response (Franklin-Tong et al., 1996; Jordan et al., 2000). Our previous studies indicated that in C. reinhardtii MP induces cell death associated with morphological features of PCD (Yordanova et al., 2010). In the presence of specific PLC and PLD inhibitors MP-induced cell death was strongly suppressed suggesting the involvement of lipid-derived signals downstream of G-proteins (Yordanova et al., 2009). By employing a laser-based technology to detect ethylene and NO, simultaneous increases in the production of both gases in MP-treated C. reinhardtii was established, indicating that NO and ethylene may act synchronously in the signalling of MP-induced PCD in this alga (Yordanova et al., 2010). However, the mechanisms through which MP triggers PCD are still far from well known. No information is available on whether MP-triggered PCD in plants might be similar to that in human cells which engage caspase-like proteolytic pathways. The characterizsation of MP-induced cell death in model organism such as C. reinhardtii may help us to understand better the plant response to stresses and, more specifically, to toxins of biotic origin (e.g. at plant–insect or plant–microbe interactions). In a wider aspect, the elucidation of the mode of action of MP on PCD in algae will expand the existing information on general questions regarding the altruistic biological role of PCD as a survival mechanism in algal populations and will provide more insight into the evolutionary conserved/diversified mechanisms of PCD in the eukaryotic cells.
The present study was a follow-up to our previous work and was aimed to characterize morphological, biochemical and molecular determinants of PCD in MP-treated C. reinhardtii, with a focus on the possible dependency of cell death on caspase-like proteases, including YVADase (caspase-1-like protease, presumably representing VPE), DNA disintegration and the phenotypic appearance of cell death.
MATERIALS AND METHODS
Chemicals
Acetyl-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-CMK), benzyoxycarbonyl-Asp-2,6-dichlorobenzoyloxymethylketone (Z-Asp-CH2-DCB), N-aAcetyl-Asp-Glu-Val-l-aspartic acid aldehyde (Ac-DEVD-CHO), N-acetyl-Tyr-Val-Ala-Asp-AMC (7-amino-4-methylcoumarin) (Ac-YVAD-AMC) and N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethylketone (MeOSuc-AAPV-CMK) were purchased from Bachem AG. SYBR Green and LysoTracker® Red DND-99 (LT) were obtained from Molecular Probes and all other chemicals from Sigma-Aldrich.
Algae growth and culture conditions
Chlamydomonas reinhardtii wild-type strain 137 C (+) was maintained on solid Sager-Granick (SG) medium (Harris, 1989) in glass tubes, at 25 °C, under continuous illumination at a light intensity of 90 µmol m−2 s−1 (Philips cool fluorescent tubes, 40-W bulbs). Vegetative cells of C. reinhardtii were grown in liquid Tris acetate phosphate medium (Harris, 1989), in an orbital shaker at 18·5 g under the same light and temperature conditions. Cultures with a cell density of 3 × 106 cells mL−1 at the late logarithmic phase of growth were used for all experiments.
Chemical treatments
To induce cell death, a range of 1 to 30 µm of the wasp venom peptide MP isolated from Vespula lewisii was used. MP was applied to 10 mL of cell culture in 30-mL bottles.
To study the involvement of caspase-like proteases in MP-induced cell death, pharmacological experiments including co-treatments with MP and synthetic peptide caspase inhibitors were undertaken. Irreversible caspase-1 inhibitor Ac-YVAD-CMK, reversible caspase-3 inhibitor Ac-DEVD-CHO and irreversible broad-range caspase inhibitor Z-Asp-CH2-DCB were tested in concentrations of 1 nm, 10 nm, 100 nm, 1 µm, 10 µM, 50 µm and 100 µm and the cell death was scored at time points 0·2, 0·5, 2, 12 and 24 h. As a negative control the caspase-unrelated peptide MeOSuc-AAPV-CMK was applied. To obtain information on whether MP-induced cell death might also involve other more-general proteolytic events, the cells were treated with broad-range serine protease inhibitors 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) and Nα-p-tosyl-l-lysine chloromethylketone (TLCK) and, the broad-range cysteine protease inhibitors iodoacetamide (IA) and N-ethylmaleimide (NEM), tested in concentrations ranging from nm to mm. MP-treated cells were also exposed to treatments with a range of concentrations of antioxidants: the NADPH oxidase inhibitors diphenyleneiodonium (DPI) and imidazole and the immediate precursor of ascorbic acid l-galactone-γ-lactone (L-gal). The effect of serine and cysteine protease inhibitors and antioxidants on MP-induced cell death was determined 24 h after the treatments.
All inhibitors and all concentrations of the chemicals were tested alone and in combination with MP. To allow uptake into the cells, the chemicals were introduced 20 min before the treatment with MP. The lowest concentrations of applied inhibitors giving significant suppression of MP-induced cell death are presented. The chemicals were taken from concentrated stocks allowing μL volumes to be added to the cell cultures. MP, IA and AEBSF were dissolved in water, Ac-YVAD-CMK, Z-Asp-CH2-DCB, Ac-DEVD-CHO, MeOSuc-AAPV-CMK, TLCK and NEM were dissolved in DMSO and diluted 1000 times to the final applied concentration. DMSO was tested alone and at a final concentration of 0·1 % (v/v); no effect on cell viability was detected.
To examine the release of putative cell death-protecting molecules that might be produced by the dying cells, fresh algal cells were grown in conditioned medium collected from culture treated for 24 h with 1-μm MP and exposed to additional treatment with 1 µm MP. Without further treatment with MP, fresh cells were also grown in conditioned medium from MP-treated and non-MP-treated cells. In these experiments cell death was scored after 2, 12 and 24 h.
Cell death determination
Cell death was determined through fluorescence microscopy. The cells were stained with 0·002 % fluorescein diacetate (FDA). FDA allows easy quantification of cell death. Esterases in the living cells metabolize this dye to a green fluorescence-producing compound fluorescein which remains trapped in the living cells, whereas dead cells do not cleave the dye and are distinguished by red fluorescence emitted from chlorophyll. Cell death was calculated as a percentage of dead cells to the total number of cells. Cell counting of each sample was executed in three different randomly chosen non-overlapping microscopic fields, each containing at least 80–100 cells.
Assessment of YVADase proteolytic activity
Activity of a plant caspase-1 like protease (VPE) was determined according to the combined method described in del Pozo and Lam (1998) and Danon et al. (2004). The cells were collected after centrifugation at 370 g for 2 min and kept at –80 °C before further processing. Frozen samples were ground to a fine powder with a mortar and pestle and homogenized after addition of 400 µL caspase extraction buffer (50 mm Hepes, pH 7·5, 20 % glycerol, 1 mm EDTA, 1 mm DTT, 1 % BSA, 1 mm PMSF). Samples were transferred to a 2-mL microfuge tube and incubated with shaking on ice for 15 min, centrifuged for 10 min (13 200 g, 4 °C) and the supernatants collected. Fifty microlitres from the samples were mixed with 50 µL caspase assay buffer (caspase extraction buffer containing 150 µm of the caspase-1 fluorogenic substrate Ac-YVAD-AMC). Half of the mixture (50 µL) was immediately transferred to a tube containing 25 µL stop solution (1 % sodium acetate in 175 µm acetic acid) and this sample was designated as t0. The remaining amount of the sample was incubated at 30 °C for 2 h before the stop solution was added (sample t1). After the reactions, samples were diluted in 2 mL water and the fluorescence (F) was measured with a Perkin Elmer 3000 (USA) fluorometer using 380/460 nm excitation/emission filters. Relative enzyme activity at the different time points was calculated as F(t1) – F(t0), normalized to a cell number of 3 × 106 cells mL−1. Samples containing 10 µm of the caspase-1 inhibitor Ac-YVAD-CMK were used as a negative control.
DNA laddering
Isolation, purification and separation of DNA from C. reinhardtii were performed according to combined protocol of Moharikar et al. (2006) and de Jong et al. (2000) with modifications. DNA was collected from 10 mL of the suspension with cell density of 3 × 106 cells mL−1, yielding 50 mg f. wt of pelleted cells after 2 min centrifugation at 370 g. The cells were ground to a fine powder in liquid nitrogen. Hexadecyltrimethyl ammonium bromide (CTAB) isolation buffer (2 % w/v CTAB, 1·4 m NaCl, 20 mm EDTA, 100 mm Tris–HCl, pH 8·0), freshly supplemented with 10 mm mercaptoethanol, was added to broken cells and mixed thoroughly. The mixture was incubated at 65 °C for 20 min. DNA extraction procedure was optimized by additional rounds with equal volume phenol and chloroform–isoamyl alcohol (24 : 1 v/v). DNA was precipitated with ice-cold isopropanol and centrifuged at 15 490 g for 15 min. The nucleic acid pellet was washed with a mixture of ice-cold 70 % v/v ethanol and 3-mm sodium acetate (pH 5·2), at a ratio of 1 : 9, and centrifuged for 10 min. The washing procedure was repeated with cold 70 % ethanol and, after centrifugation, the pellet was air dried and dissolved in 25 µL TE buffer (10 mm Tris–HCl, pH 8·0, 1 mm EDTA). DNase-free RNase A (0·1 µg μL−1) was added and the mixture incubated at 37 °C for 30 min. DNA was separated on 1·8 % agarose gel stained with ethidium bromide with equal amounts of 6 µg DNA per lane.
Comet assay
The alkaline Comet (single-cell gel electrophoresis) assay was applied as described by Miloshev et al. (2002) and Collins et al. (2008) with modifications. One millilitre of Chlamydomonas suspension cells was collected and centrifuged for 5 min at 130 g. The pellet was washed with 500 µL S-buffer (50 mm Tris–HCl pH 7·5, 10 mm MgCl2, 1 m sorbitol), centrifuged again and re-suspended in S-buffer. Aliquots from the cell suspension containing 103 cells mL−1 were mixed with low-melting agarose to a final concentration of 0·7 % and were spread as micro-gels on microscopic slides.To set the gels the slides were incubated for 5 min at 4 °C and thereafter submerged in lysis solution (1 m NaCl, 50 mm EDTA, pH 8, 30 mm NaOH, 0·1 % N-lauroylsarcozine) for 1 h at 4 °C; washed three times for 20 min at 4 °C with denaturing solution (30 mm NaOH, 10 mm EDTA, pH 8) and subjected to electrophoresis (0·45 V cm−1) for 10 min at 4 °C in the same denaturing solution. Following electrophoresis, the gels were neutralized for 5 min with dH2O and dehydrated at room temperature by successive washes in 75 % and 95 % ethanol for 5 min each. After dehydration the gels were stained with 1× SYBR green I and the DNA comets were observed under a Leitz epifluorescence microscope (VARIO Orthomat 2; Bunton Instrument Company Inc., Wetzlar, Germany) using an 450–490-nm band-pass filter. One hundred objects per slide were scored and statistically analysed. The percentage of comet nuclei was calculated from the total number of observed objects. By visual scoring, the comets were classified into four classes from 0 (no tail) to 4 (almost all DNA in the tails) as described by Collins (2004). Images were taken with a digital camera (Olympus μ8) at a resolution of 3 Mpx.
Imaging
Fluorescent field images of C. reinhardtii suspension cells were collected using fluorescent microscope (Nikon Eclipse, TS 100, filter B-2A, exciter 450–490, DM 505, BA 520; Vienna, Austria) equipped with a Nikon DXM 1200 digital camera. Cell morphology was detected using a TCS SP2 AOBS CLSM system (Leica-Microsystems GmbH, Mannheim, Germany) mounted on an inverted Leica DM IRE2 microscope (CLSM). Three different lasers (405, 488 and 561 nm) were employed for excitation, three emission channels for fluorescence imaging and one separate channel for non-confocal transmission imaging. Overlays and orthogonal projections were made using the Leica Confocal software. The living cells were visualized with FDA, and propidium iodide (PI) (penetrates the damaged plasma membrane) was used to distinguish the nuclei in the dead cells. LysoTracker® Red DND-99 (LT) – a specific lysosome staining dye – was used to stain acidic cellular compartments.
Data analysis
The presented data for cell death experiments, Comet assay and YVADase activity are average values from at least four independent experiments and are compared by standard error of the means (s.e.m.). Additionally, in the cell death experiments with caspase inhibitors, the means of values at the individual time points of analysis were pairwise compared using Fisher's LSD (P ≤ 0·05). The statistical processing and graphical artworks were performed using computer software MS Office Excel and GraphPad Prism.
RESULTS
Effect of MP on cell death
Chlamydomonas reinhardtii suspension cells (3 × 106 cells mL−1) were collected at the late logarithmic growth phase and exposed to treatment with a range of concentrations (0·5, 1, 5, 10 and 30 µm) of MP. Cell death was scored at intervals of 24 h until 120 h. In control, non-treated cultures, cell death averaged between 2 and 6 %. MP induced cell lethality in a dose-dependent manner with cell death almost completed within 30 min of treatment. Approximately 50–60 % cell death was observed in 1-μm MP-treated cultures, whereas higher MP concentrations increased the lethality up to 80 or 100 % (Fig. 1A). In further experiments the cells were routinely treated with 1-μm MP. MP was also administrated in mid-log phase of culture growth, but no effect on cell death was detected, indicating that during the phase of active growth the cells are unresponsive to MP treatment. The cell death as measured by FDA-negative cells 24 h after MP treatment was confirmed by increased ion leakage (data not shown).
Fig. 1.
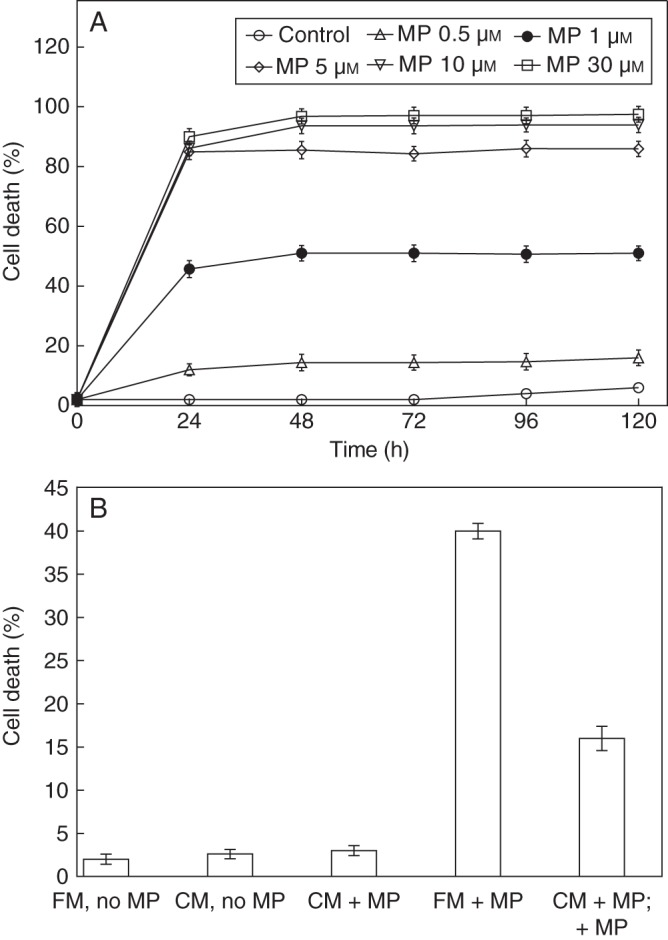
Effect of MP on cell death in C. reinhardtii. (A) Cell-death kinetics in 1 to 30 μm MP-treated cell suspension (approx. 3 × 106 cells mL−1). Cell death was scored at 24-h intervals (0–120 h) after MP application following FDA staining of the living cells, and was calculated as a percentage of dead cells to total number of cells. (B) MP-induced cell death in algal suspension grown in fresh (FM) or in conditioned (CM) medium. Fresh algal cells were grown in conditioned medium collected from cultures treated for 24 h with 1 μm MP and cell death was scored after an additional 24-h treatment with 1 μm MP. Fresh cells without further MP treatment were also grown in conditioned medium from MP-treated and non-MP-treated cells. FM-no MP, Control non-treated cells grown in fresh medium; CM-no MP, cells grown in conditioned medium collected from non-MP-treated cells; CM + MP, cells grown in conditioned medium collected from MP-treated cells; FM + MP, MP-treated cells grown in fresh medium; CM + MP; +MP, cells grown in conditioned medium collected from MP-treated cells and exposed to additional treatment with MP. Error bars indicate ± s.e.m.
Experiments with fresh cells, grown in conditioned medium collected from cells grown for 24 h in 1 μm MP showed reduced cell death in response to an additional 24-h application of 1 μm MP (Fig. 1B). The effect of the conditioned medium was also tested after 2 and 12 h, but at these time points the alleviation of the severity of MP stress was not detected (data not shown). This indicated that after longer exposure to MP the dying cells might release substances preventing the cell death in the remaining living cells in the culture. No increase of cell death over the control cells was observed when the cells were grown in conditioned medium from MP-treated cells and not exposed to additional treatment with the elicitor (Fig. 1B).
Contribution of caspase-like proteases to MP-induced cell death
To elucidate the possible contribution of caspase-like proteases to MP-induced cell death the temporal pattern of cell death was assayed from 0 to 24 h in cultures treated with MP and caspase inhibitors (10 µm of the irreversible pan-caspase inhibitor Z-Asp-CH2-DCB, 1 µm of the caspase-3 inhibitor Ac-DEVD-CHO (Fig. 2A) or 10 µm of the caspase-1 inhibitor Ac-YVAD-CMK) (Fig. 2B). In the same time span the dose effect of the caspase inhibitors applied alone or in combination with MP was also monitored. When the inhibitors were tested alone in the concentrations indicated above and in lower concentrations, no effect on cell death was observed in comparison with non-treated control cells. However, the single application of concentrations higher than 10 µm Z-Asp-CH2-DCB and Ac-YVAD-CMK and higher than 1 µm Ac-DEVD-CHO caused >15 % cell death in 2 h (data not shown). No additional inhibition of MP-induced cell death with the higher doses of the caspase inhibitors was established. For this reason, the higher concentrations were excluded from further experiments.
Fig. 2.
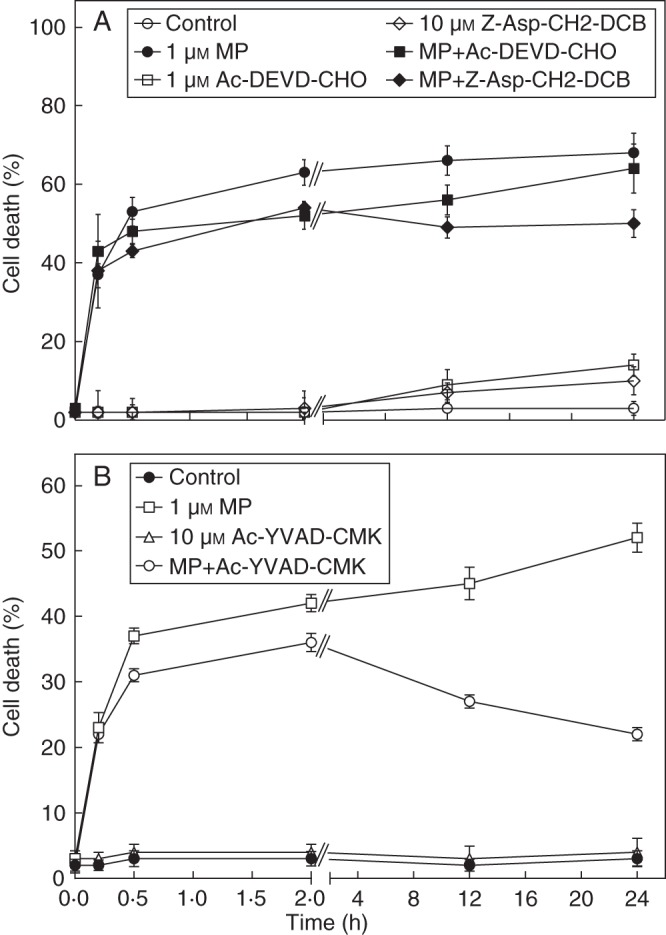
Temporal pattern of the effect of caspase inhibitors on MP-induced cell death in C. reinhardtii. The cells were treated with 1 μm MP and combinations of MP and inhibitors. (A) Cell death in response to 10 µm of the broad-range caspase inhibitor Z-Asp-CH2-DCB and 1 µm of caspase-3 inhibitor Ac-DEVD-CHO. (B) Cell death in response to 10 µm of caspase-1 inhibitor Ac-YVAD-CMK. Cell death was scored at the indicated time points after application of the chemicals following FDA staining of the living cells, and was calculated as a percentage of dead cells to total number of cells. Error bars indicate ± s.e.m. Cell-death effect of the chemicals at the individual time points of analyses is compared by LSD (P ≤ 0·05) as follows: (A) 0·2 h, 9·14; 0·5 h, 10·62; 2·0 h, 7·16; 12·0 h, 8·54; 24·0 h, 11·82; (B) 0·2 h, 6·17; 0·5 h, 7·65; 2·0 h, 4·73; 12·0 h, 8·82; 24·0 h, 11·41.
Until 2 h after administration of the chemicals, no significant suppression of MP-induced cell death was detected in response to Z-Asp-CH2-DCB and Ac-DEVD-CHO (Fig. 2A), and a slight, but statistically significant, decrease of MP-induced cell death was found in the presence of Ac-YVAD-CMK (Fig. 2B). An effect of Ac-YVAD-CMK, however, was observed from 2 h onwards resulting in an approx. 40 % inhibition of cell death at 24 h (Fig. 2B). At the later time points cell death was also suppressed by Z-Asp-CH2-DCB (30 % inhibition at 24 h) (Fig. 2A). Caspase-3 inhibitor Ac-DEVD-CHO suppressed cell death at 12 h but not at 24 h (Fig. 2A). The significance of the effect of caspase inhibitors on cell death at the later time points of analyses was confirmed by LSD (P ≤ 0·05) (Fig. 1A, B). As a negative control, the algal cells were treated with 1 µm and 10 µm of the caspase-unrelated peptide N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethylketone (MeOSuc-AAPV-CMK) carrying a similar ketone moiety as the caspase-1 inhibitor. The analyses were performed at 2, 12 and 24 h. This peptide did not affect MP-induced cell death at the time points tested (data not shown). Cumulatively, the inhibitory analyses indicated that caspase-like proteases most probably were not involved in the rapid cell death of a major part of the MP-responsive cells.
To verify if MP-induced cell death is indeed an active process and not accidental death, the effect of broad-range serine and cysteine protease inhibitors and antioxidants on MP-induced cell death was determined 24 h after the treatments. Serine protease inhibitors AEBSF (100 µm) and TLCK (10 µm), and cysteine protease inhibitors NEM (1 µm) and IA (10 µm) reduced MP-induced cell death by an average of 30–60 % (Supplementary Data Fig. S1A). These results indicated that, at least in the late stages, MP-induced cell death in C. reinhardtii involves processes of general proteolysis. In treatments with the ascorbic acid precursor L-gal and the NADPH oxidase inhibitors DPI and imidazole (thought to interfere with flavonoid-containing enzymes), after 24 h, over 50 % reduction of MP-induced cell death was observed (Supplementary Data Fig. S1B). This indicated that oxidative stress is implicated in MP-induced cell death in C. reinhardtii.
Effect of MP on YVADase activity
The kinetics of cell death inhibition on administration of 10 µm of the caspase-1 inhibitor Ac-YVAD-CMK showed a remarkable pattern. Whereas the rapid cell death during the first 2 h of MP treatment was not influenced by the inhibitor, at later time points in the presence of the inhibitor the culture showed an increased viability. This suggested that MP might stimulate activity of a caspase-1-like protease that might be involved in later time points of cell death. To verify this suggestion the activity of YVADase (presumably VPE) was assayed at consecutive time points after administration of MP and the inhibitor. To avoid eventual cleavage of the fluorogenic substrate Ac-YVAD-AMC by other plant proteases, the caspase assay buffer contained PMSF (serine protease inhibitor) and EDTA (metalloprotease inhibitor) that do not inhibit animal caspases. In MP-treated cells YVADase activity increased very rapidly within the first 30 min and continued to rise until 2 h, consecutively reaching a plateau after 12–24 h. In the samples treated with a combination of MP and Ac-YVAD-CMK or with the inhibitor alone the enzyme activity was inhibited to zero. In control non-treated cells, activity remained low throughout the experiment (Fig. 3). This showed that MP stimulates caspase-1-like activity. However, until 2 h the MP-induced cell death was not inhibited by the caspase-1 inhibitor, which suggested that that YVADase was not involved in the early cell-death process. The gradual decrease in the percentage of dead cells at later time points in caspase-1 inhibitor-treated cultures indicated that a caspase-1-like activity might be involved in the later stages of cell death.
Fig. 3.
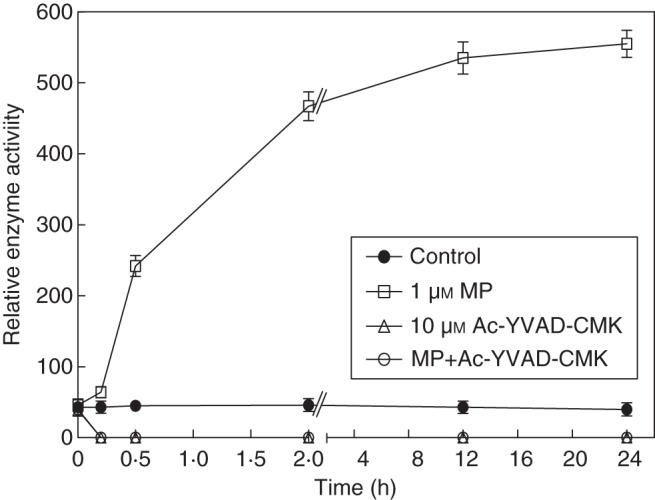
Kinetics of YVADase activity in MP-treated C. reinhardtii. Enzymatic activity was assayed in a time span of 0–24 h at consecutive time points after administration of the chemicals (1 µm MP, 10 µm Ac-YVAD-CMK and their combination). Control cells were left untreated. The fluorescence was measured and the relative caspase activity (normalized by cell count – 3 × 106 cells mL−1) was calculated as F(t1) – F(t0) (for details refer to Materials and methods). Error bars indicate ± s.e.m.
Effect of MP on cellular morphology
Field fluorescent images revealed living (green FDA fluorescence) and dead (red chlorophyll fluorescence) cells of different size in control non-treated culture (Fig. 4A) and in the cultures treated with MP and MP with inhibitors. This indicated that the cells in the cultures were in different stage of development. In the presence of MP and 10 µM of the caspase-1 inhibitor Ac-YVAD-CMK, 24 h after the treatments the number of dead cells was significantly reduced (Fig. 4C) in comparison with the MP-only-treated culture (Fig. 4B).
Fig. 4.
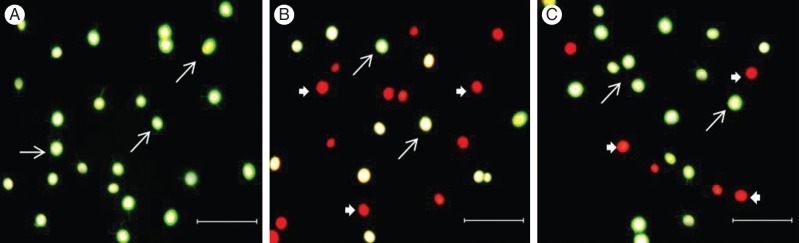
Fluorescent field images of C. reinhardtii. The living cells were stained with FDA and the dead cells were distinguished by chlorophyll fluorescence: (A) untreated control cells; (B) cells treated with 1-μm MP; (C) cells treated with 1-μm MP + 10 µm of the caspase-1 inhibitor Ac-YVAD-CMK. The images were collected 24 h after administration of the chemicals and cell counting was carried out at ×200 magnification using a fluorescent microscope (Nikon Eclipse; Vienna, Austria; TS 100, filter B-2A, exciter 450–490, DM 505, BA 520) equipped with Nikon DXM 1200 digital camera. Thick arrows indicate dead cells; thin arrows indicate living cells. Scale bars = 50 µm.
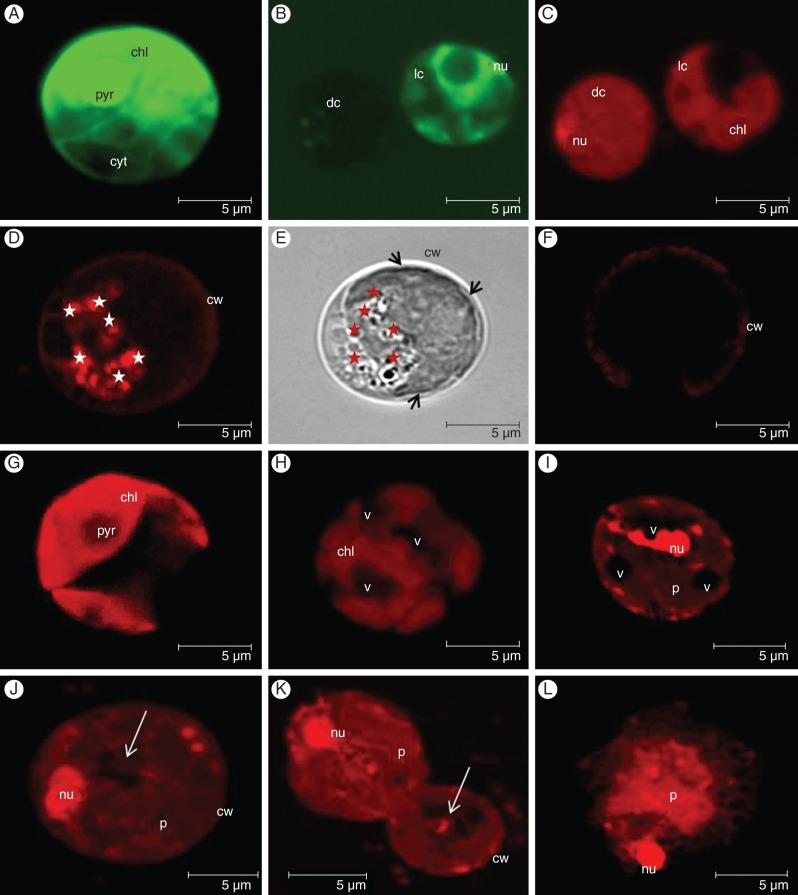
Morphology of vital and dead cells was examined by CLSM. Intact cup-shaped protoplasts with preserved integrity and diffuse nuclei were observed in non-treated FDA-positive cells (Fig. 5A, B). FDA-stained dead cells did not show green fluorescence (Fig. 5B). PI-positive nuclei were detected in MP-treated dead cells (Fig. 5C, I–K).
Fig. 5.
CLSM images representing the cellular morphology of MP-treated C. reinhardtii cells: (A) FDA-positive living cell showing intact protoplast; (B) FDA staining of a living (diffuse nuclei visible) and a dead cell (FDA negative); (C) PI staining of the cells in (B) – note the PI-positive compacted nucleus and the homogenous content of the dead cell; (D) fluorescent image showing a multitude of lysosome-like LT-positive vesicles (white stars); (E) transmission light image of (D) in which the lytic vesicles (red stars) appear as dark round organelles – note retraction of the protoplast (black arrows) from the cell wall; (F) LT-stained living cell – no LT-positive vesicles are detected; (G) chlorophyll fluorescence emitted from the intact cup-shaped chloroplast of a living cell; (H) chlorophyll fluorescence emitted from a disintegrated chloroplast in a dead cell with large vacuoles; (I) PI-stained vacuolated dead cell with shrunken protoplast and compacted nucleus; (J) PI-stained dead cell with compacted nucleus and nearly empty cell-walled corpse (white arrow) containing only the remains of the digested protoplast; (K) PI-stained dead cells expressing features of different cell-death types – the upper cells show necrotic morphology with shrunken protoplast and condensed nucleus while the lower cells show an empty cell-walled corpse which is a feature of vacuolar cell death (white arrow); (L) PI-stained dead cell showing a feature of necrotic cell death – the remains of a largely unprocessed protoplast and nucleus. Images (A–C) and (G–K) were collected 24 h after treatment with 1 μm MP; images (D–F) were collected 2 h after treatment with 1 μm MP; image (L) was taken 24 h after treatment with 5 μm MP. Cellular morphology was examined using a TCS SP2 AOBS CLSM (Leica-Microsystems GmbH, Mannheim, Germany) mounted on an inverted Leica DM IRE2 microscope. Three different lasers (405, 488 and 561 nm) were employed for excitation and three emission channels for fluorescence imaging and one separate channel for non-confocal transmission imaging. Overlays and orthogonal projections were made using the Leica Confocal software. Abbreviations: chl, chloroplast; dc, dead cell; lc, living cell; nu, nucleus; p, protoplast; pyr, pyrenoid; cw, cell wall; v, vacuole. Scale bars = 5 µm.
Until 2 h after exposure to 1 μm MP in a portion of the dead cells, the staining with LysoTracker Red revealed a multitude of LT positive organelles (Fig. 5D). In the same cells a slight retraction of the plasma membrane from the cell wall was observed, indicating the beginning of protoplast shrinkage (Fig. 5E). No LT-positive structures were found in non-treated cells or in the living and dead cells 12 and 24 h after MP treatment (Fig. 5F). This showed that the LT-positive vesicles occurred as a part of the early cell-death process and were not formed due to other processes (e.g. cell division or differentiation). Within the extent of the microscopy performed it was not possible to distinguish if the vesicles are double-membrane bound autophagosome-like organelles. The appearance of small lytic vacuoles resembled a component of vacuolar cell death and suggested that the cell-death process in MP-treated C. reinhardtii cells might pass through an early stage which is associated with the formation of lysosome-like compartments. Protoplast shrinkage detected in same cells resembled a component of necrotic cell death.
Large vacuoles and condensed nuclei were clearly visible in PI-positive cells (Fig. 5H, I). Bright red chlorophyll fluorescence emitted from the chloroplasts in living cells indicated an intact structure (Fig. 5G). With cell-death progression, the chloroplasts disintegrated in parallel with the occurrence of larger vacuoles (Fig. 5H). These growing vacuoles appeared LT negative. In vacuolated cells (vacuolization – a symptom of vacuolar cell death) protoplast shrinkage (a feature of necrosis) was also observed (Fig. 5I), indicating a combination of components of both cell-death types in the same cells. In the later cell-death stages, dead cells with a compact PI-positive nucleus and a nearly empty cell-walled corpse were distinguished (Fig. 5J). This resembled a final stage of vacuolar cell death which is typically characterized by vacuole-mediated engulfment of the protoplast followed by clearance of the cellular content resulting in an empty cell-walled corpse. Interestingly, in the same culture, cells expressing symptoms of either necrotic or vacuolar cell death were found next to each other (Fig. 5K): note the upper cell with a shrunken protoplast without distinguishable large vacuoles at the top of an empty wall-bound cell (the lower cell). This showed that different cells may undergo different types of PCD. When 5 μm MP was administered, in a portion of the dead cells (approx. 30 %) a PI-positive nucleus and largely unprocessed remains of the protoplast were observed (Fig. 5L). This resembled a feature of necrotic cell death resulting after early rupture of the plasma membrane and indicated that the type of cell death might be dependent on the severity of the applied stressor. The established mix of phenotypes showed that the low concentration of 1 μm MP triggers PCD that expresses an atypical morphological pattern comprising components of necrotic (protoplast shrinkage) and vacuolar (formation of small lytic vesicles, growing vacuoles and nearly empty cell-walled corpse) cell deaths. The morphology of the dead and living C. reinhardtii cells was also assessed in the presence of the inhibitors, alone and in combination with MP, and after double and triple staining with FDA, PI and LT. No effect of the inhibitors on cell-death phenotypes was observed, including the occurrence of LT-positive vesicles that were not eliminated in the MP + Ac-YVAD-CMK-treated culture (images not shown).
Effect of MP on DNA disintegration
DNA degradation into oligonucleosomal fragments of around 180 bp is a hallmark of animal apoptotic cell death and might occur during plant necrosis. A laddering pattern of DNA cleavage was detected within 10 min of MP treatment and, in the time interval between 30 min and 2 h (Fig. 6). At later time points (12 and 24 h) no DNA laddering in MP-treated cells was found. In control cultures DNA cleavage was not detected. This indicated that DNA fragmentation is initiated as an early event after administration of the elicitor. DNA laddering assay also occurred at the same time points with cells treated with 1 µm MP and 10 µm of the peptide caspase-1 inhibitor Ac-YVAD-CMK. In response to the inhibitor, no inhibition of DNA degradation was found (data not shown). This showed that MP-induced DNA cleavage occurs independently on caspase-1-like enzyme.
Fig. 6.
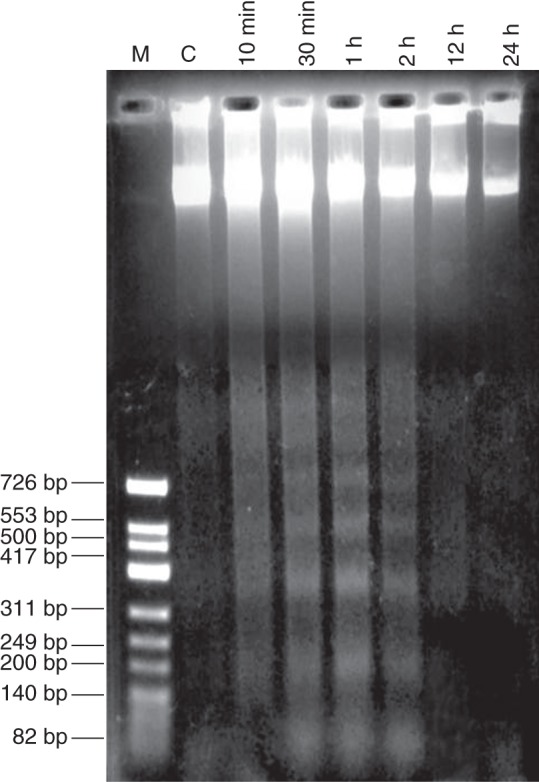
DNA laddering in MP-treated C. reinhardtii. DNA was isolated from a 1 μm MP-treated suspension of C. reinhardtii cells and the analysis were performed at consecutive time points of 10 min, 30 min, 1, 2, 12 and 24 h after treatment with MP. DNA was subjected to agarose gel electrophoresis using equal amounts of 6 µg DNA per lane as described in Materials and methods. Lane 1: M, marker; lane 2: C, non-treated control; lanes 3–8, time points after MP application.
To gain additional information about the effect of MP on DNA disintegration, the cells were analysed by the Comet assay which is a sensitive method for measuring double- and single-strand breaks in DNA The samples were collected from cell suspensions exposed to 1 µm MP for 10 min, 30 min, 2 h and 24 h. No comet pattern appeared in control non-MP-treated cells (Fig. 7A), which indicated intact DNA. Slight DNA degradation was observed 10 min after administration of MP. The comet tail was extended 30 min after the treatment, showing that DNA was undergoing a relaxation of the structural loop with an increasing number of breaks. Two hours after MP administration, DNA appeared granular both in the tail and in the comet head which is a symptom of progressive breakage. After 24 h of MP treatment, class 4 comets, lacking a compact comet head and with almost all DNA fragments in the tail, indicated irreversible DNA damage (Fig. 7A). Fluorescence images reflected the increase in the percentage of comet nuclei found at the consecutive time points of analysis (Fig. 7B). The results of the Comet assay provided supportive evidence that in MP-treated C. reinhardtii DNA cleavage proceeded concomitantly with the progression of cell death.
Fig. 7.
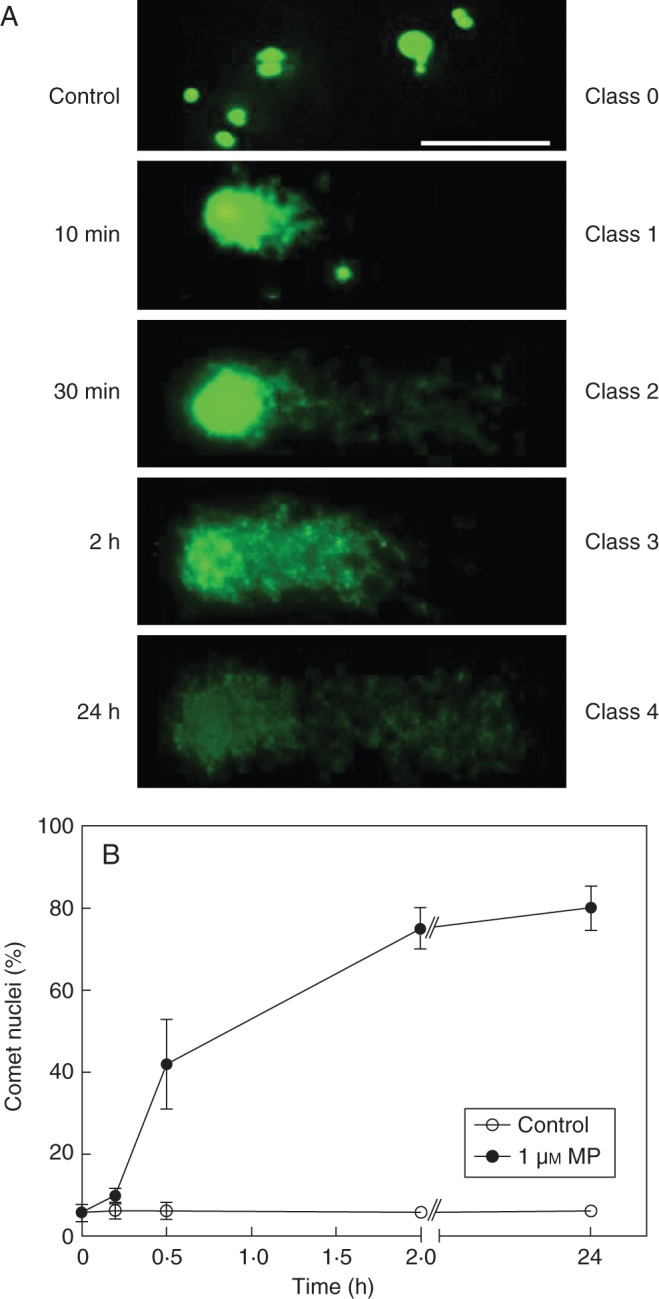
Comet assay of DNA integrity in MP-treated C. reinhardtii: (A) fluorescent images of comets (scale bar = 50 µm); (B) percentage of comet-positive nuclei. Chlamydomonas reinhardtii cells in suspension were treated with 1 μm MP. Samples were taken and analysed for comet DNA at consecutive time points at 10 min, 30 min, 2 h and 24 h. Comets were visually categorized in five classes as described in Materials and methods. Error bars indicate ± s.e.m.
DISCUSSION
Dying cells produce survival factors
The effect of MP on cell death occurred in a concentration-dependent manner (Fig. 1A). We have earlier shown that about 40 % of the cells were scored dead within 30 min after administration of 1 μm MP; increasing up to 50 % at 2 h (Yordanova et al., 2010). After 2 h the cell death percentage increased only slightly and stabilized after approx. 24 h (Fig. 1A). Although a dramatic increase in cell death (up to 80 %) was detected in presence of 5-μm MP, in this case also the portion of dead cells did not increase further after 24 h. This suggested that dying cells may produce factors that protect the still-living cells and/or reduce their susceptibility to MP. Indeed, when fresh cells were grown in conditioned medium from MP-treated cells, the cells were much less responsive to MP treatment (Fig. 1B). A protective effect of conditioned media on cell death in UV-treated Chlamydomonas has been earlier reported (Moharikar et al., 2006). Also, it has been found that the cellular content released from C. reinhardtii undergoing PCD in response to heat shock is beneficial, whereas the cellular content liberated during non-PCD (induced by sonic waves) is detrimental to the fitness of the population (Durand et al., 2011). It is suggested that volatile organic compounds released from C. reinhardtii cells undergoing acetic acid-induced PCD transmit the message of cell death in a population (Zuo et al., 2012). Mobile molecules, such as ethylene and nitric oxide, might also contribute to cell-to-cell communication (Yordanova et al., 2010). To assess whether MP-treated cells might emit low molecular-mass messengers we have performed PTR-MS analysis, and volatiles with antioxidant properties were identified. The substances were produced at the earliest 12 h after MP treatment (data not presented). Judging by the relatively late effect of the conditioned medium (after 24 h), we hypothesize that compounds preventing cell death might not be produced immediately or may have to accumulate to a certain level before exerting their cell-death protecting effect. These findings support the suggestion that under stress conditions the dying C. reinhardtii cells may send information to the living cells helping to keep the population vital.
Caspase-like proteases contribute to the later but not to the early stages of MP-induced cell death
The lack of suppression of MP-induced cell death by the general caspase inhibitor Z-Asp-CH2-DCB and caspase-3 inhibitor Ac-DEVD-CHO, and only the slight inhibition of cell death by caspase-1 inhibitor Ac-YVAD-CMK (Fig. 2A, B) during the first 2 h of treatment indicated that caspase-like proteases might not contribute to early cell death events. However, at later time points the caspase-1 inhibitor Ac-YVAD-CMK evoked a gradual recovery of culture vitality (Fig. 2B). A small but significant decrease in cell death was also recorded in the presence of Ac-DEVD-CHO and Z-Asp-CH2-DCB (Fig. 2A). This indicated that at later time points the cell-death process possibly becomes caspase-like dependent. The efficiency of the caspase inhibitors to affect late MP-induced cell death was confirmed by the lack of cell-death suppression in the presence of the caspase-unrelated peptide MeOSuc-AAPV-CMK. Elsewhere it was reported that this peptide was also not effective in preventing camptothecin- and Cd-induced PCD in tomato suspension cells where specific caspase inhibitors carrying the same ketone moiety were highly effective (de Jong et al., 2000; Yakimova et al., 2006). The reason for the eventual switch from caspase-like-independent to -dependent regulation might be a change in the physiological stage or of the sensitivity of algal cells to applied stressor which, in turn, might result in involvement of different signalling pathways. Developmental stage is an important parameter that determines the cell response to various stresses and this was also observed in MP-treated C. reinhardtii cells. At the stage of exponential growth, MP did not exert a cell-death effect. Moreover, in non-treated, but also in MP-treated cultures, the cells differed in size, indicating different developmental stages (Fig. 4).
A peculiar observation is that at later time points in caspase-1 inhibitor-treated cultures the percentage of dead cells seems to have decreased. A possible reason for this phenomenon is that the inhibitor might, through unknown mechanism, promote a gradual recovery over time of some of the cells that immediately after MP treatment were severely stressed but not really dead. It may also be that the caspase inhibitor offered protection to cell death in cells that perceived MP as a milder stress and died at later time points (presumably cells that had a lesser sensitivity to MP). These two possibilities can explain the lack of a further rise in cell death, but cannot explain a decrease in percentage cell death over time. The latter can only be explained if we assume that the presence of the caspase inhibitor may have ‘revitalized’ the remaining living cells, leading to extra cell divisions. This hypothesis needs further verification.
In MP-treated cells, YVADase activity was stimulated in a fast mode but inhibition of the activity did not correspond to a reduction in cell death at the early time points, thus providing an additional indication that the rapid cell-death response is most probably not mediated by caspase-like pathways. These results corroborate the findings of Moharikar et al. (2006) which also suggest that early stages of cell death in C. reinhardtii might be caspase independent. By using anti-caspase-3 antibody, the authors detected a 28-kDa procaspase-3-like protein. The antibody could recognize a sequence in the prodomain in the inactive proform which is activated by proteolytic removal of the prodomain and separation of the catalytic subunits. In UV-C-treated cells, the uncleaved proenzyme decreased starting as early as 1 h after the treatment and disappearing at 6 h, whereas cell death continued to increase until 24 h. This demonstrated that the early stage of cell death in this alga might be indeed caspase-independent.
The early stimulation of YVADase (presumably a VPE) coincided with the occurrence of lysosome-like structures. It is not clear whether the small lytic vacuoles might host a VPE, but lysosomal proteins might be involved in VPE activation as suggested for cathepsin B (a lysosome-localized papain-like non-caspase cysteine protease) during senescence and HR in arabidopsis, tomato and tobacco (Gilroy et al., 2007; McLellan et al., 2009). The fact that YVADase inhibition did not affect the early stage of cell death might be at least partially explained by the hypothesis of Danon et al. (2004) who assumed that in UV-C-treated arabidopsis protoplasts a constitutive YVADase activity might be necessary to prime the plant cell for death, but it is by itself insufficient to induce the process. Interestingly, different to our observation showing that Ac-YVAD-CMK did not prevent MP-induced DNA cleavage, the same authors showed that in their experimental model YVADase participated in the mediation of DNA fragmentation, which was inhibited in the presence of the reversible caspase-1 inhibitor Ac-YVAD-CHO. These point to a possibility of a different role for caspase-like enzymatic pathways depending on the cell-death inducer, the stage of cell-death process and the model under study.
Suggested signal transduction during MP-induced cell death
In C. reinhardtii, MP is shown to activate Ca2+ signalling, phospholipid turnover and oxidative stress downstream of G-proteins (Quarmby et al., 1992; Ross and Higashijima, 1994; Munnik et al., 1998; van Himbergen et al., 1999). In other model systems, MP has also been established to induce Ca2+-dependent processes (e.g. in tobacco suspension cells, arabidopsis seedlings and in staminal hairs of Setcreasea purpurea) (Tucker and Boss, 1996; Takahashi et al., 1998; Whalley et al., 2011). In cultured soybean cells, other G-protein modulators, such as cholera toxin, pertussis toxin and an elicitor extracted from the pathogenic fungus Verticillium dahlia, have been found to induce oxidative stress and synthesis of lipid intermediates (Legendre et al., 1992, 1993). MP-induced cell death and DNA fragmentation in differentiating tracheary elements in zinnia cell culture were inhibited by Ca2+ channel blockers and stimulated by calcium ionofore A23187, suggesting that MP might exert a PCD effect through a mechanism requiring Ca2+ influx (Groover and Jones, 1999). A similar mode of MP action through IP3-mediated elevation of Ca2+ levels has been demonstrated during MP-induced SI PCD in Papaver rhoeas (Franklin-Tong et al., 1996; Jordan et al., 2000). In MP-treated C. reinhardtii we have found that calmodulin inhibitor W-7 and Ca2+ channel blocker LaCl3 inhibited the cell death by about 80 % and 40 %, respectively (Z. P. Yordanova et al., our unpubl. res.), which also pointed to Ca2+-mediated cell-death signalling. Our earlier findings indicated the involvement of phospholipid factors, ethylene and NO (Yordanova et al., 2009, 2010). Here we provide novel information suggesting that caspase-like proteases might contribute to the later but not to the early stages of cell death and that the response of algal cells might be dependent on their physiological state and susceptibility to MP. Inhibition of later stages of PCD by serine and cysteine protease inhibitors and antioxidants indicates that the process also involves other proteolytic pathways and oxidative stress. Taken together, the information from the literature and our findings suggest that in C. reinhardtii the effect of MP on PCD might be exerted through pathways downstream of G-proteins engaging lipid-derived factors and Ca2+ that, in turn, might cross-talk with other messengers such as ethylene and NO. These pathways might converge at the level of ROS. ROS have been established as important mediators of caspase-like-associated cell death in algae (Vardi et al., 1999; Darehshouri et al., 2008; Segovia and Berges, 2009; Zuppini et al., 2010). Potentially, the levels of Ca2+ and ethylene, and the severity of oxidative stress might regulate the subsequent caspase-like-related cell-death cascade (de Jong et al., 2002). For example, higher amounts of ethylene, Ca2+ and ROS might trigger rapid caspase-like-independent cell death, whereas reliable levels of these factors might drive the process toward caspase-dependent cell death that occurs at a slower pace. However, this hypothetical mechanism through which MP might trigger PCD effects needs further verification.
MP-induced PCD is associated with DNA degradation
DNA damage during MP-induced cell death was analysed by two methods: DNA laddering and Comet assay. The DNA laddering assay revealed a PCD pattern of DNA cleavage into fragments smaller than 180 bp which occurred at the early time points of cell death – until 2 h after MP treatment (Fig. 6). This shows that DNA disruption is initiated as a quick response to MP treatment. In other experiments (Yordanova et al., 2010) and also here at later time points (12 and 24 h) DNA laddering was not detected. However, the Comet assay demonstrates that DNA disintegration proceeds during the whole 24-h period of MP-induced cell death (Fig. 7). It has to be noted that although DNA laddering has been shown in C. reinhardtii challenged with heat stress (Nedelcu, 2006) and UV radiation (Moharikar et al., 2006), the occurrence of this PCD event in unicellular algae is not a common feature. For example, in Micrasterias denticulata exposed to oxidative stress, biochemical and phenotypic features of PCD have been found but they have not been associated with DNA fragmentation (Darehshouri et al., 2008). DNA laddering analysis specifically identifies small internucleosomal fragments, whereas the Comet analysis detects high molecular-weight DNA resulting from double-strand breaks. In the Comet method the small cut-off fragments that leave the nucleus cannot always be observed, so it is disputable, to some extent, whether this analysis is an appropriate marker for PCD (Collins, 2004; Collins et al., 2008). However, as proven in other model systems, when used in conjunction with other PCD markers (e.g. DNA laddering, cellular morphology) the comet DNA can be informative for identification of cells in which PCD has actually taken place (Courtois-Moreau et al., 2009). The comet pattern of DNA damage confirmed that DNA breakage is characteristic of MP-induced PCD in C. reinhardtii.
MP induces cell death-expressing components of vacuolar and necrotic phenotype
To describe the cell-death phenotype in MP-treated C. reinhardtii, we have strictly followed the criteria recommended by van Doorn et al. (2011). CLSM characterization of 1-μm MP-treated Chlamydomonas cells revealed markers of necrotic and vacuolar cell death expressed in one single cell but also in different individual cells (Fig. 5E, I–K). In the cells with lytic vacuoles (presumably a feature of vacuolar cell death), a feature of necrosis appearing as a slight detachment of the protoplast from the cell wall was observed (Fig. 5E), whereas more-pronounced protoplast shrinkage occurred at later time points in cells containing larger LT-negative vacuoles (Fig. 5I). This suggests that symptoms of both cell-death types might occur in the same cells at different stages of cell death. The lytic organelles reminiscent of lysosomes are a suggested component of very early stages of plant PCD, prior to the formation of large lytic vacuoles; the latter followed by the final stage of cell death executed after tonoplast rupture, resulting in massive self-degradation (van Doorn and Woltering, 2010; van Doorn et al., 2011). In our samples, cells with nearly empty cell-walled corpses containing only a few remains of the protoplast were detected (Fig. 5J). This resembles HR PCD in which different to typical vacuolar cell death the vacuolar burst might not necessarily lead to complete protoplast clearance (Beers and McDowell, 2001; Hatsugai et al., 2004; Mur et al., 2008). Interestingly, features of necrotic (shrunken protoplast) or vacuolar (empty cell-walled corpses) cell deaths were expressed not only in one single cell but also in different, even neighbouring, cells (Fig. 5K). These observations also indicated that different cells in the culture might undergo different cell-death types. A higher concentration of 5 μm MP caused cell death expressing necrotic morphology (largely unprocessed protoplast due to possible early rupture of plasma membrane) (Fig. 5L) which complies with the suggestion that the severity of the stress stimulus might determine the type of cell death (Moharikar et al., 2006; Jiménez et al., 2009). The established morphological peculiarities of cell death in response to MP corroborate the assumption of other authors that the unicellular algae might undergo PCD-expressing features of different or intermediate categories (Segovia et al., 2003; Mohoarikar et al., 2006; Darehshouri et al., 2008; Affenzeller et al., 2009; Deponte, 2008; Jiménez et al., 2009; Pérez-Pérez et al., 2010; Zuppini et al., 2010).
Concluding remarks
Here we describe morphological, molecular and biochemical PCD events induced in C. reinhardtii in response to the wasp toxin MP, an activator of G-protein-mediated stress signalling. The work addresses questions related to the temporal pattern of involvement of caspase-like proteases, DNA integrity and phenotypic occurrence of cell death. Broad-range caspase inhibitor Z-Asp-CH2-DCB and specific caspase-3 (Ac-DEVD-CHO) and caspase-1 (Ac-DEVD-CMK) inhibitors did not inhibit the MP-induced cell death at the early time points but they were effective in suppressing the later stages of the process. Activity of YVADase (presumably a VPE) was strongly stimulated by the elicitor and inhibited in the presence of caspase-1 inhibitor but this did not correspond to inhibition of the rapid cell-death response. DNA analysis showed that cell death was accompanied with DNA disintegration, but the early-occurring ladder type of fragmentation was not prevented in the presence of Ac-YVAD-CMK. These results indicated that caspase-like proteases may not be crucial players in the early stages of MP-induced cell death but they may contribute to late cell death. This may explain the revitalisation of an MP-treated algal population in the presence of caspase inhibitors at later time points. We suggest that the response of algal cells might be determined by the developmental stage and sensitivity of the cells to MP. At earlier time points, a portion of the cells may recognize MP as severe stress and may die immediately in a non-caspase-mediated manner, whereas at later time points the cells that survived are possibly less responsive to MP and may perceive the elicitor as milder stress and die at a slower pace engaging caspase-like pathways. A release of survival-promoting factors from MP-treated cells that might contribute to mitigation of cell death was suggested to play a role in sustaining the fitness of a population.
MP-induced cell death revealed the components of the two major categories of vacuolar and necrotic cell deaths, as they are defined in van Doorn et al. (2011). The established symptoms resembled vacuolar (formation of small lytic vacuoles, growing vacuoles, activation of a presumed VPE and, finally, possibly vacuole-mediated clearance of cellular content, resulting in an empty cell-walled corpse) and necrotic (protoplast shrinkage) cell deaths. These features were accompanied by nuclear condensation and DNA fragmentation that may occur at any of the cell-death classes. Such an atypical combination of cell-death components gives us reason to assume that in C. reinhardtii MP induces PCD of an intermediate morphological type, reminiscent of the modality of HR. Our findings suggest that hallmarks specific to different types of cell death might be expressed within the same cells but also in different cells at different stages of the cell-death process which, depending on the physiological state of the cells, may proceed through caspase-like-dependent or -independent manner. More profound analyses including the use of mutant Chlamydomonas strains are necessary to characterize additionally the enzymes giving rise to caspase-like activities in this alga. We believe that the information reported here discloses novel elements in the performance of C. reinhardtii in stress conditions and, at the same time, substantiates the recent discoveries in unicellular algae, showing that even in one and the same organism cell death may occur in different forms and may follow different pathways depending either on the sensitivity of cells or on the mode of induction.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Dr Stephka Chankova, Institute for Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences (BAS), Sofia, Bulgaria, for kindly providing us with C. reinhardtii, the work team of Dr George Miloshev, Laboratory of Yeast Molecular Genetics, Institute of Molecular Biology, BAS, for help with Comet assay and Dr Diana Petkova, Institute for Biophysics and Biomedical Engineering, BAS, for technical support. The authors are in debt to Dr Elisabeth S. Pierson, Department of General Instrumentation, Faculty of Sciences, Radboud University, Nijmegen, The Netherlands, for her valuable assistance with the LSCM. The support and the intellectual contribution of Dr Simona M. Cristescu, Department of Molecular and Laser Physics, Institute for Molecules and Materials, Faculty of Science, Radboud University, Nijmegen are greatly acknowledged. We sincerely appreciate the stimulating comments of the two anonymous reviewers who helped us to improve an earlier version of the manuscript. This work was partially supported by University of Sofia and Ministry of Education, Youth and Science, Bulgaria (PhD project Biochemical and Molecular Programmed Cell Death Events in C. reinhardtii); EU FP6-2004-Infrastructures-5 Project (026183-TRACEGASFAC), Radboud University, Nijmegen, The Netherlands and Operational Programme Human Resources Development co-financed by the European Union through the European Social Fund (ISUN BG051P0001-3·3.04/42).
LITERATURE CITED
- Affenzeller MJ, Darehshouri A, Andosch A, Cornelius Lütz C, Lütz-Meindl U. Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata. Journal of Experimental Botany. 2009;60:939–954. doi: 10.1093/jxb/ern348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andosch A, Affenzeller MJ, Lütz C, Lütz-Meindl U. A freshwater green alga under cadmium stress: ameliorating calcium effects on ultrastructure and photosynthesis in the unicellular model Micrasterias. Journal of Plant Physiology. 2012;169:1489–1500. doi: 10.1016/j.jplph.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Beers EP, McDowell JM. Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Current Opinion in Plant Biology. 2001;4:561–567. doi: 10.1016/s1369-5266(00)00216-8. [DOI] [PubMed] [Google Scholar]
- Bidle KD, Bender SJ. Iron starvation and culture age activate metacaspases and programmed cell death in the marine diatom Thalassiosira pseudonana. Eukaryotic Cell. 2008;7:223–236. doi: 10.1128/EC.00296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau L, Ge Y, Drury GE, Gallois P. What happened to plant caspases? Journal of Experimental Botany. 2008;59:491–499. doi: 10.1093/jxb/erm352. [DOI] [PubMed] [Google Scholar]
- Bozhkov PV, Suarez MF, Filonova LH, et al. Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proceedings of the National Academy of Sciences of the USA. 2005;102:14463–14468. doi: 10.1073/pnas.0506948102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Gutierrez D, Fröhlich K-U, Kroemer G, Madeo F. Metacaspases are caspases. Doubt no more. Cell Death and Differentiation. 2010;17:377–378. doi: 10.1038/cdd.2009.198. [DOI] [PubMed] [Google Scholar]
- Chichkova NV, Kim SH, Titova ES, et al. A plant caspase-like protease activated during the hypersensitive response. The Plant Cell. 2004;16:157–171. doi: 10.1105/tpc.017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffeen WC, Wolpert TJ. Purification and characterization of serine proteases that exhibit caspase-like activity and are associated with programmed cell death in Avena sativa. The Plant Cell. 2004;16:857–873. doi: 10.1105/tpc.017947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR. The comet assay for DNA damage and repair – principles, applications, and limitations. Molecular Biotechnology. 2004;25:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- Collins AR, Oscoz AA, Brunborg G, et al. The comet assay: topical issues. Mutagenesis. 2008;23:143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- Courtois-Moreau CL, Pesquet E, Sjödin A, et al. A unique program for cell death in xylem fibers of Populus stem. The Plant Journal. 2009;58:260–274. doi: 10.1111/j.1365-313X.2008.03777.x. [DOI] [PubMed] [Google Scholar]
- Danon A, Rotari VI, Gordon A, Mailhac N, Gallois P. Ultraviolet-C overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, pp35 and Defender against apoptotic death. Journal of Biological Chemistry. 2004;279:779–787. doi: 10.1074/jbc.M304468200. [DOI] [PubMed] [Google Scholar]
- Darehshouri A, Affenzeller M, Lütz-Meindl U. Cell death upon H2O2 induction in the unicellular green alga Micrasterias. Plant Biology. 2008;10:732–745. doi: 10.1111/j.1438-8677.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo O, Lam E. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Current Biology. 1998;8:1129–1132. doi: 10.1016/s0960-9822(98)70469-5. [DOI] [PubMed] [Google Scholar]
- Deponte M. Programmed cell death in protists. Biochemica et Biophysica Acta. 2008;1783:1396–1405. doi: 10.1016/j.bbamcr.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Dimova EG, Bryant PE, Chankova SG. Adaptive response – some underlying mechanisms and open questions. Genetics and Molecular Biology. 2008;31:396–408. [Google Scholar]
- van Doorn WG, Woltering EJ. Many ways to exit? Cell death categories in plants. Trends in Plant Science. 2005;10:117–122. doi: 10.1016/j.tplants.2005.01.006. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. What about the role of autophagy in PCD? Trends in Plant Science. 2010;15:361–362. doi: 10.1016/j.tplants.2010.04.009. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Beers EP, Dangl JL, et al. Morphological classification of plant cell deaths. Cell Death and Differentiation. 2011;18:1241–1246. doi: 10.1038/cdd.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand PM, Rashidi A, Michod RE. How an organism dies affects the fitness of its neighbors. The American Naturalist. 2011;177:224–232. doi: 10.1086/657686. [DOI] [PubMed] [Google Scholar]
- Elbaz M, Avni A, Weil M. Constitutive caspase-like machinery executes programmed cell death in plant cells. Cell Death and Differentiation. 2002;9:726–733. doi: 10.1038/sj.cdd.4401030. [DOI] [PubMed] [Google Scholar]
- Ellerby HM, Martin SJ, Ellerby LM, et al. Establishment of a cell-free system of neuronal apoptosis: comparison of premitochondrial, mitochondrial, and postmitochondrial phases. The Journal of Neuroscience. 1997;17:6165–6178. [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong VE, Drøbak BK, Allan AC, Watkins PAC, Trewavas AJ. Growth of pollen tubes of Papaver rhoeas is regulated by a slow moving calcium wave propagated by 1,4,5-trisphosphate. The Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H. Programmed cell death of tracheary elements as a paradigm in plants. Plant Molecular Biology. 2000;44:245–253. doi: 10.1023/a:1026532223173. [DOI] [PubMed] [Google Scholar]
- Gilroy EM, Hein I, van der Hoorn R, et al. Involvement of cathepsin B in the plant disease resistance hypersensitive response. The Plant Journal. 2007;52:1–13. doi: 10.1111/j.1365-313X.2007.03226.x. [DOI] [PubMed] [Google Scholar]
- Groover A, Jones AM. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiology. 1999;119:375–384. doi: 10.1104/pp.119.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M. Chlamydomonas reinhardtii as a eukaryotic photosynthetic model for studies of heavy metal homeostasis and tolerance. New Phytologist. 2003;159:331–340. doi: 10.1046/j.1469-8137.2003.00788.x. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I, Hatsugai N, Nakaune S, Kuroyanagi M, Nishimura M. Vacuolar processing enzyme: an executor of plant cell death. Current Opinion in Plant Biology. 2005;8:404–408. doi: 10.1016/j.pbi.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas sourcebook: a comprehensive guide to biology and laboratory use. San Diego, CA: Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- Harris EH. Chlamydomonas as a model organism. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:363–406. doi: 10.1146/annurev.arplant.52.1.363. [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Kuroyanagi M, Yamada K, et al. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305:855–858. doi: 10.1126/science.1099859. [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Iwasaki S, Tamura K, et al. A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes & Development. 2009;23:2496–2506. doi: 10.1101/gad.1825209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath MC. Hypersensitive response-related death. Plant Molecular Biology. 2000;44:323–334. doi: 10.1023/a:1026592509060. [DOI] [PubMed] [Google Scholar]
- Hema R, Senthil-Kumar M, Shivakumar S, Chandrasekhara Reddy P, Udayakumar M. Chlamydomonas reinhardtii, a model system for functional validation of abiotic stress responsive genes. Planta. 2007;226:655–670. doi: 10.1007/s00425-007-0514-2. [DOI] [PubMed] [Google Scholar]
- van Himbergen JAJ, ter Riet B, Meijer HJG, van den Ende H, Musgrave A, Munnik T. Mastoparan analogues stimulate phospholipase C- and phospholipase D-activity in Chlamydomonas: a comparative study. Journal of Experimental Botany. 1999;50:1735–1742. [Google Scholar]
- Hiraiwa N, Nishimura M, Hara-Nishimura I. Vacuolar processing enzyme is selfcatalytically activated by sequential removal of the C-terminal and N-terminal propeptides. FEBS Letters. 1999;447:213–216. doi: 10.1016/s0014-5793(99)00286-0. [DOI] [PubMed] [Google Scholar]
- Hoeberichts FA, Woltering EJ. Multiple mediators of plant programmed cell death: interplay of conserved cell death mechanisms and plant-specific regulators. Bioessays. 2003;25:47–57. doi: 10.1002/bies.10175. [DOI] [PubMed] [Google Scholar]
- Jiménez C, Capasso JM, Edelstein CL, et al. Different ways to die: cell death modes of the unicellular chlorophyte Dunaliella viridis exposed to various environmental stresses are mediated by the caspase-like activity DEVDase. Journal of Experimental Botany. 2009;60:815–828. doi: 10.1093/jxb/ern330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong AJ, Hoeberichts FA, Yakimova ET, Maximova E, Woltering EJ. Chemical-induced apoptotic cell death in tomato cells: involvement of caspase-like proteases. Planta. 2000;211:656–662. doi: 10.1007/s004250000341. [DOI] [PubMed] [Google Scholar]
- de Jong AJ, Yakimova ET, Kapchina VM, Woltering EJ. A critical role of ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta. 2002;214:537–545. doi: 10.1007/s004250100654. [DOI] [PubMed] [Google Scholar]
- Jordan ND, Franklin FCH, Franklin-Tong VE. Evidence for DNA fragmentation triggered in the self-incompatibility response in pollen of Papaver rhoeas. The Plant Journal. 2000;23:471–479. doi: 10.1046/j.1365-313x.2000.00811.x. [DOI] [PubMed] [Google Scholar]
- Kawai-Yamada M, Jin L, Yoshinaga K, Hirata A, Uchimiya H. Mammalian Bax-induced plant cell death can be down-regulated by overexpression of Arabidopsis Bax Inhibitor-1 (AtBI-1) Proceedings of the National Academy of Sciences of the USA. 2001;98:12295–12300. doi: 10.1073/pnas.211423998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E. Controlled cell death, plant survival and development. Nature Reviews Molecular Cell Biology. 2004;5:305–315. doi: 10.1038/nrm1358. [DOI] [PubMed] [Google Scholar]
- Lam E, Del Pozo O. Caspase-like protease involvement in the control of plant cell death. Plant Molecular Biology. 2000;44:417–428. doi: 10.1023/a:1026509012695. [DOI] [PubMed] [Google Scholar]
- Legendre L, Heinstein P, Low P. Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. Journal of Biological Chemistry. 1992;267:20140–20147. [PubMed] [Google Scholar]
- Legendre L, Yueh Y, Crain R, Haddock N, Heinstein P, Low P. Phospholipase C activation during elicitation of the oxidative burst in cultured plant cells. Journal of Biological Chemistry. 1993;268:24559–24563. [PubMed] [Google Scholar]
- McLellan H, Gilroy EM, Yun B-W, Birch PRJ, Loake GJ. Functional redundancy in the Arabidopsis Cathepsin B gene family contributes to basal defence, the hypersensitive response and senescence. New Phytologist. 2009;183:408–418. doi: 10.1111/j.1469-8137.2009.02865.x. [DOI] [PubMed] [Google Scholar]
- Miloshev G, Mihaylov I, Anachkova B. Application of the single cell gel electrophoresis on yeast cells. Mutation Research. 2002;513:69–74. doi: 10.1016/s1383-5718(01)00286-8. [DOI] [PubMed] [Google Scholar]
- Moharikar S, D'Souza JS, Kulkarni AB, Rao BJ. Apoptotic-like cell death pathway is induced in unicellular chlorophyte Chlamydomonas reinhardtii (Chlorophyceae) cells following UV irradiation: detection and functional analysis. Journal of Phycology. 2006;42:423–433. [Google Scholar]
- Moharikar S, D'Souza JS, Rao BJ. A homologue of the defender against the apoptotic death gene (dad1) in UV-exposed Chlamydomonas cells is downregulated with the onset of programmed cell death. Journal of Biosciences. 2007;32:261–270. doi: 10.1007/s12038-007-0026-z. [DOI] [PubMed] [Google Scholar]
- Munnik T, van Himbergen JAJ, ter Riet B, et al. Detailed analysis of the turnover of polyphosphoinositides and phosphatidic acid upon activation of phospholipases C and D in Chlamydomonas cells treated with non-permeabilizing concentrations of mastoparan. Planta. 1998;207:133–145. [Google Scholar]
- Mur LAJ, Kenton P, Lloyd AJ, Ougham H, Prats E. The hypersensitive response; the centenary is upon us but how much do we know? Journal of Experimental Botany. 2008;59:501–520. doi: 10.1093/jxb/erm239. [DOI] [PubMed] [Google Scholar]
- Nedelcu AM. Evidence for pp53–like-mediated stress responses in green algae. FEBS Letters. 2006;580:3013–3017. doi: 10.1016/j.febslet.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Nedelcu AM. Comparative genomics of phylogenetically diverse unicellular eukaryotes provide new insights into the genetic basis for the evolution of the programmed cell death machinery. Journal of Molecular Evolution. 2009;68:256–268. doi: 10.1007/s00239-009-9201-1. [DOI] [PubMed] [Google Scholar]
- Pennell RI, Lamb C. Programmed cell death in plants. The Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez ME, Florencio FJ, Crespo JL. Inhibition of TOR signaling and stress activate autophagy in Chlamydomonas reinhardtii. Plant Physiology. 2010;152:1874–1888. doi: 10.1104/pp.109.152520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby LM, Yueh YG, Cheshire JL, Keller LR, Snell WJ, Crain CR. Inositol phospholipid metabolism may trigger flagellar excision in Chlamydomonas reinhardtii. The Journal of Cell Biology. 1992;116:737–744. doi: 10.1083/jcb.116.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reape TJ, McCabe PF. Apoptotic-like programmed cell death in plants. New Phytologist. 2008;180:13–26. doi: 10.1111/j.1469-8137.2008.02549.x. [DOI] [PubMed] [Google Scholar]
- Rogers HJ. Programmed cell death in floral organs: how and why do flowers die? Annals of Botany. 2006;97:309–315. doi: 10.1093/aob/mcj051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Martin R, Carter C, et al. VPEγ exhibits a caspase-like activity that contributes to defense against pathogens. Current Biology. 2004;14:1897–1906. doi: 10.1016/j.cub.2004.09.056. [DOI] [PubMed] [Google Scholar]
- Ross EM, Higashijima T. Regulation of G-protein activation by mastoparan and other cationic peptides. Methods in Enzymology. 1994;237:26–37. doi: 10.1016/s0076-6879(94)37050-8. [DOI] [PubMed] [Google Scholar]
- Rotari VI, Dando PM, Barrett AJ. Legumain forms from plants and animals differ in their specificity. Biological Chemistry. 2001;382:953–959. doi: 10.1515/BC.2001.119. [DOI] [PubMed] [Google Scholar]
- Rotari VI, He R, Gallois P. Death by proteases in plants: whodunit. Physiologia Plantarum. 2005;123:376–385. [Google Scholar]
- Sanmartin M, Jaroszewski L, Raikhel N, Rojo E. Caspases: regulating death since the origin of life. Plant Physiology. 2005;137:841–847. doi: 10.1104/pp.104.058552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia M, Berges JA. Inhibition of caspase-like activities prevents the appearance of reactive oxygen species and dark-induced apoptosis in the unicellular chlorophyte Dunaliella tertiolecta. Journal of Phycology. 2009;45:1116–1126. doi: 10.1111/j.1529-8817.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- Segovia M, Haramaty L, Berges JA, Falkowski PG. Cell death in the unicellular chlorophyte Dunaliella tertiolecta: a hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant Physiology. 2003;132:99–105. doi: 10.1104/pp.102.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y-L, Zhao Y, Hong X, Zhai Z-H. Cytocrome c release and caspase activation during menadion-induced apoptosis in plants. FEBS Letters. 1999;462:317–321. doi: 10.1016/s0014-5793(99)01539-2. [DOI] [PubMed] [Google Scholar]
- Sundström JF, Vaculova A, Smertenko AP, et al. Tudor staphylococcal nuclease is an evolutionarily conserved component of the programmed cell death degradome. Nature Cell Biology. 2009;11:1347–1354. doi: 10.1038/ncb1979. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Isobe M, Muto S. Mastoparan induces an increase in cytosolic calcium ion concentration and subsequent activation of protein kinases in tobacco suspension culture cells. Biochimica et Biophysica Acta. 1998;1401:339–346. doi: 10.1016/s0167-4889(97)00134-1. [DOI] [PubMed] [Google Scholar]
- Tucker EB, Boss WF. Mastoparan-induced intracellular Ca2+ fluxes may regulate cell-to-cell communication in pIants. Plant Physiology. 1996;111:459–467. doi: 10.1104/pp.111.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twumasi P, Iakimova ET, Qian T, et al. Caspase inhibitors affect the kinetics and dimensions of tracheary elements in xylogenic Zinnia (Zinnia elegans) cell cultures. BMC Plant Biology. 2010;10:162. doi: 10.1186/1471-2229-10-162. http://dx.doi.org/10.1186/1471-2229-10-162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi A, Berman-Frank I, Rozenberg T, Hadas O, Kaplan A, Levine A. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Current Biology. 1999;9:1061–1064. doi: 10.1016/s0960-9822(99)80459-x. [DOI] [PubMed] [Google Scholar]
- Wang H, Juan LI, Bostock RM, Gilchrist DG. Apoptosis: a functional paradigm of programmed cell death induced by a host-selective phytotoxin and invoked during development. The Plant Cell. 1996;8:375–391. doi: 10.1105/tpc.8.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Lam E. Arabidopsis Bax inhibitor-1 functions as an attenuator of biotic and abiotic types of cell death. The Plant Journal. 2006;45:884–894. doi: 10.1111/j.1365-313X.2006.02654.x. [DOI] [PubMed] [Google Scholar]
- Whalley HJ, Sargeant AW, Steele JFC, et al. Transcriptomic analysis reveals calcium regulation of specific promoter motifs in Arabidopsis. The Plant Cell. 2011;23:4079–4095. doi: 10.1105/tpc.111.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering EJ. Death proteases: alive and kicking. Trends in Plant Science. 2010;15:185–188. doi: 10.1016/j.tplants.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Woltering EJ, van der Bent A, Hoeberichts FA. Do plant caspases exist? Plant Physiology. 2002;130:1764–1769. doi: 10.1104/pp.006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimova ET, Kapchina-Toteva VM, Laarhoven L-J, Harren FM, Woltering EJ. Involvement of ethylene and lipid signalling in cadmium-induced programmed cell death in tomato suspension cells. Plant Physiology and Biochemistry. 2006;44:581–589. doi: 10.1016/j.plaphy.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Yamada K, Shimada T, Nishimura M, Hara-Nishimura I. A VPE family supporting various vacuolar functions in plants. Physiologia Plantarum. 2005;123:369–375. [Google Scholar]
- Yordanova ZP, Kapchina-Toteva VM, Woltering EJ, Cristescu SM, Harren FJM, Iakimova ET. Mastoparan-induced cell death signalling in Chlamydomonas reinhardtii. Biotechnology & Biotechnological Equipment. 2009;23:730–734. [Google Scholar]
- Yordanova ZP, Iakimova ET, Cristescu SM, Harren FJM, Kapchina-Toteva VM, Woltering EJ. Involvement of ethylene and nitric oxide in cell death in mastoparan-treated unicellular alga Chlamydomonas reinhardtii. Cell Biology International. 2010;34:301–308. doi: 10.1042/CBI20090138. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Zhu Y, Bai Y, Wang Y. Acetic acid-induced programmed cell death and release of volatile organic compounds in Chlamydomonas reinhardtii. Plant Physiology and Biochemistry. 2012;51:175–184. doi: 10.1016/j.plaphy.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Zuppini A, Gerotto C, Baldan B. Programmed cell death and adaptation: two different ways of abiotic stress response in a unicellular chlorophyte. Plant and Cell Physiology. 2010;51:884–895. doi: 10.1093/pcp/pcq069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.