Abstract
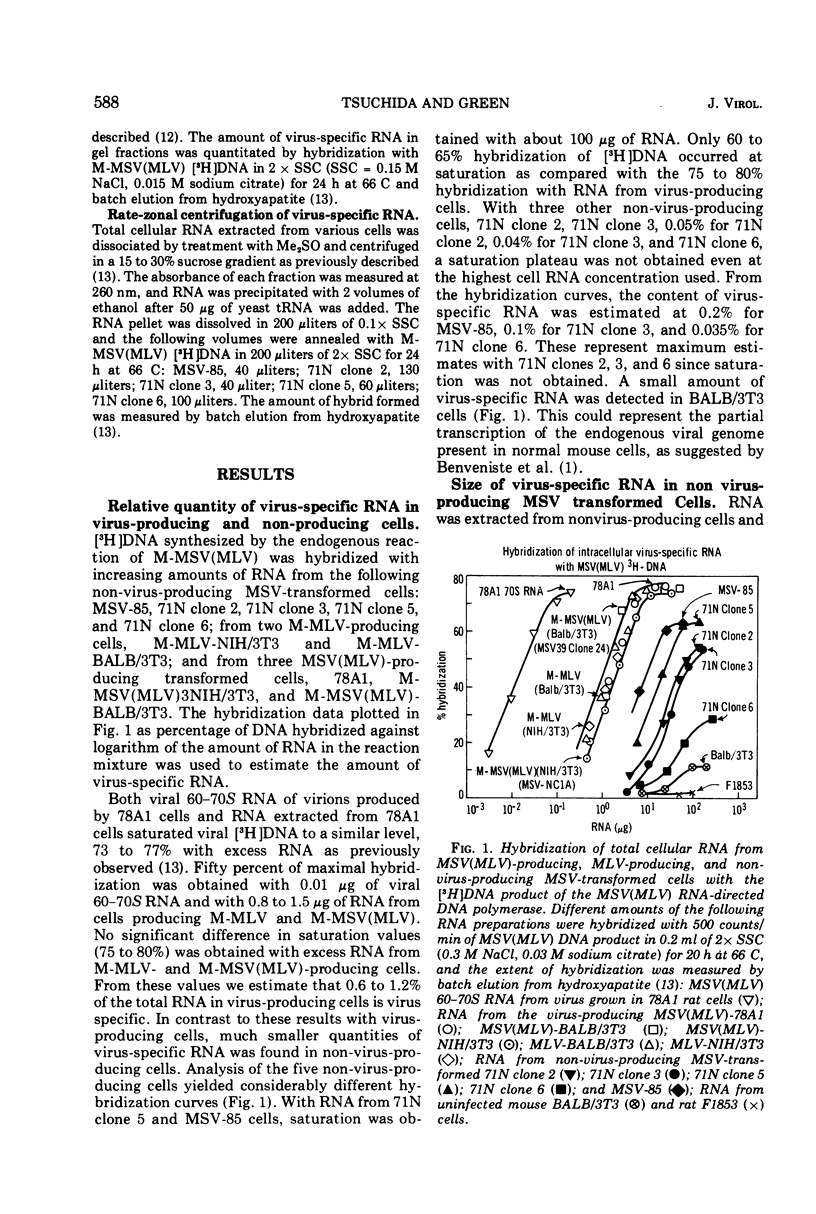
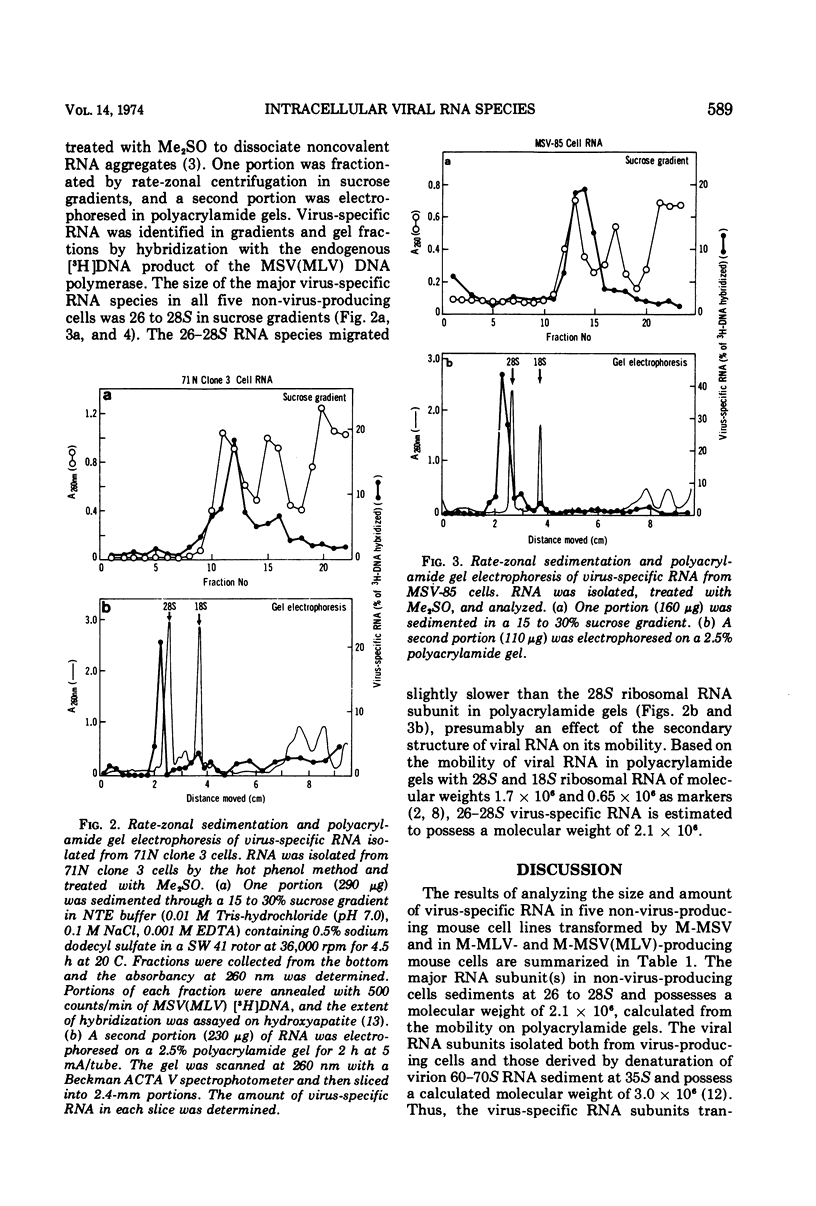
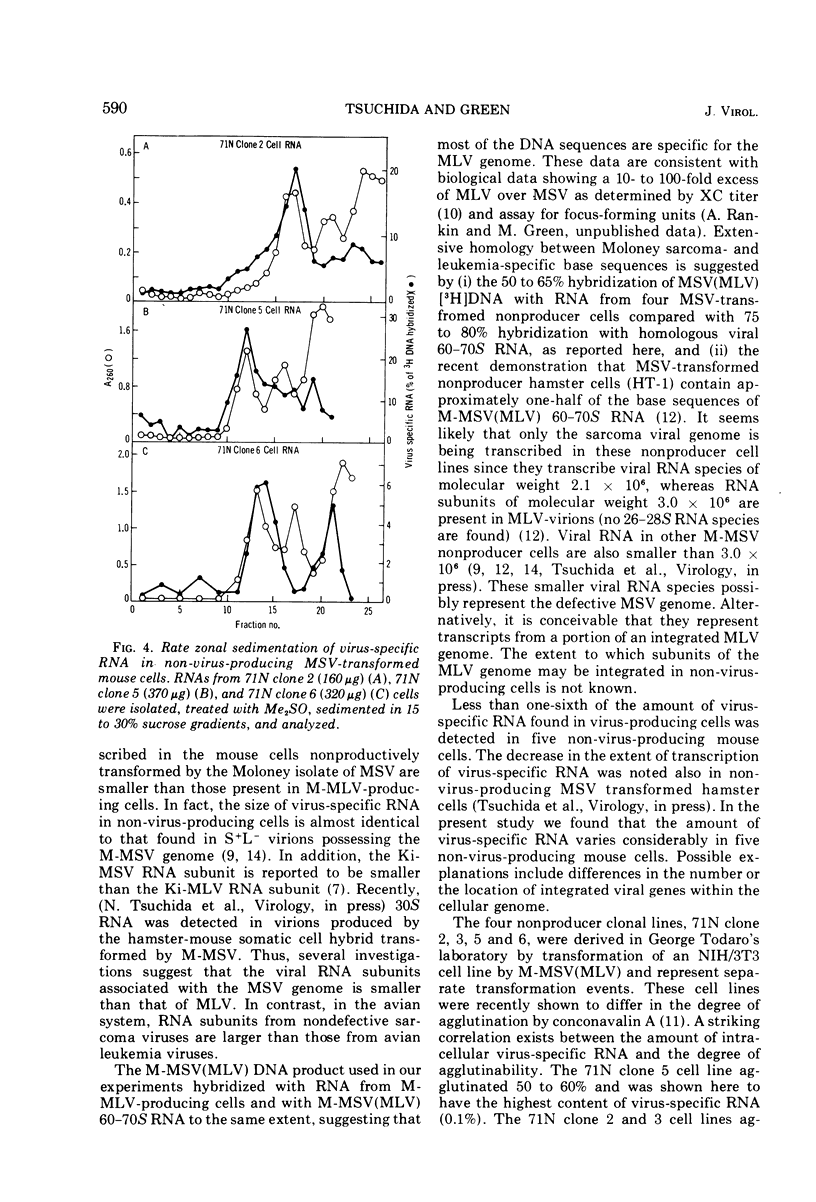
The size and quantity of virus-specific RNA in five non-virus-producing mouse cells transformed by the Moloney isolate of murine sarcoma virus (MSV) was determined. Hybridization of RNA from transformed cells with the [3H]DNA product of the RNA-directed DNA polymerase of the murine sarcoma-leukemia virus was used to detect and quantitate virus-specific RNA. The amount of virus-specific RNA in non-virus-producing cells was less than one-sixth of that found in virus-producing cells. A striking correlation was found between the amount of intracellular virus-specific RNA and the degree of agglutination by conconavalin A previously reported for the four non-virus-producing NIH/3T3 cell lines (Salzberg and Green, 1974). A major RNA subunit sedimenting at 26 to 28S was detected in all five MSV-transformed non-virus-producing cells. This could represent the RNA genome of defective MSV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Todaro G. J., Scolnick E. M., Parks W. P. Partial transcription of murine type C viral genomes in BALB c cell lines. J Virol. 1973 Oct;12(4):711–720. doi: 10.1128/jvi.12.4.711-720.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Rokutanda H., Rokutanda M. Virus specific RNA in cells transformed by RNA tumour viruses. Nat New Biol. 1971 Apr 21;230(16):229–232. doi: 10.1038/newbio230229a0. [DOI] [PubMed] [Google Scholar]
- Green M., Rokutanda M., Fujinaga K., Ray R. K., Rokutanda H., Gurgo C. Mechanism of carcinogenesis by RNA tumor viruses. I. An RNA-dependent DNA polymerase in murine sarcoma viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):385–393. doi: 10.1073/pnas.67.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J., Klement V., Lai M. M., Ostertag W., Duesberg P. Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3536–3540. doi: 10.1073/pnas.70.12.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Phillips L. A., Hollis V. W., Jr, Bassin R. H., Fischinger P. J. Characterization of RNA from noninfectious virions produced by sarcoma positive-leukemia negative transformed 3T3 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):3002–3006. doi: 10.1073/pnas.70.10.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Green M. Intracellular and virion 35 S RNA species of murine sarcoma and leukemia viruses. Virology. 1974 May;59(1):258–265. doi: 10.1016/0042-6822(74)90221-9. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Vidrine J. G., Harewood K. R., Bulfone L. M., Higdon C. R., Mayyasi S. A. High molecular weight RNA in a murine leukaemia virus helper-independent strain of Moloney sarcoma virus. J Gen Virol. 1973 Aug;20(2):239–242. doi: 10.1099/0022-1317-20-2-239. [DOI] [PubMed] [Google Scholar]



