Abstract
Background
Type 2 diabetes mellitus is a progressive metabolic disease necessitating therapies with sustained efficacy and safety over time. Exenatide once weekly (ExQW), an extended-release formulation of the glucagon-like peptide-1 receptor agonist exenatide, has demonstrated improvements in glycemic and cardiometabolic measures from 30 weeks to 2 years of treatment. Here, the efficacy and safety of treatment with ExQW for 3 years are described.
Methods
Patients were initially randomized to receive either ExQW (2 mg) or exenatide twice daily for 30 weeks. Following the initial 30 weeks, all patients were treated with ExQW in an open-label extension. Analyses of primary glycemic endpoints, beta-cell function, and cardiometabolic measures were assessed for patients who completed 3 years of ExQW treatment and for the intention-to-treat population. Safety and tolerability analyses were provided for the intention-to-treat population.
Results
Sixty-six percent of the intention-to-treat population (n = 295) completed 3 years of treatment (n = 194). At 3 years, a significant reduction in hemoglobin A1c (least squares mean ± standard error) of −1.6% ± 0.08% was observed, with 55% and 33% of patients achieving hemoglobin A1c targets of <7% and ≤6.5%, respectively. Consistent with a sustained reduction in hemoglobin A1c, improvements in beta-cell function were also observed. Body weight was significantly reduced by −2.3 ± 0.6 kg. Reductions in blood pressure, total cholesterol, low-density lipoprotein cholesterol, and triglycerides were also observed. Adverse events reported most frequently during both controlled and uncontrolled periods included diarrhea, nausea, and vomiting of mostly mild intensity. The incidence of these adverse events decreased over time. Incidence of minor hypoglycemia was low and no major hypoglycemia was observed.
Conclusion
ExQW produced clinically meaningful improvements in glycemic control that were durable through 3 years of treatment. Significant improvements in cardiometabolic measurements were also observed. ExQW was well-tolerated during long-term treatment and no new adverse events were noted.
Trial registration
ClinicalTrials.gov NCT00308139.
Keywords: diabetes, exenatide, GLP-1 receptor agonist, hyperglycemia, DURATION-1
Background
Type 2 diabetes mellitus (T2DM) is a progressive metabolic disease characterized by the presence of persistent hyperglycemia.1 While diet and exercise alone may be initially sufficient to improve glycemia and some cardiometabolic measures, pharmacological treatment is eventually required. Once initiated, the need for further pharmacological therapy escalates over time as the severity of the disease progresses. Due to the chronicity of the disease and the necessity of long-term pharmacological intervention, long-term studies are important to establish continued efficacy and safety. A Diabetes Outcome Progression Trial (ADOPT) evaluated the durability of several oral antidiabetes monotherapies over 5 years. Patients in all treatment groups experienced eventual failure of glycemic control; however, the observed superior durability of rosiglitazone was suggested to be due to a slowed rate of loss of beta-cell function.2 In the long-term UK Prospective Diabetes Study (UKPDS), a 10-year follow-up suggested that although the durability of the glycemic control was not sustained, a reduction of adverse diabetes-related endpoints was observed in the group of patients treated with early, intensive pharmacotherapy.3 Such long-term studies as ADOPT and UKPDS examine therapies for maintenance of glycemic control and other clinical benefits despite the progression of the disease over time. Studies of extended duration also permit observation of important safety and tolerability profiles over a longer period.
Exenatide twice daily (ExBID) is a subcutaneously administered, glucagon-like peptide-1 (GLP-1) receptor agonist used to treat T2DM as monotherapy or in combination with other antidiabetes drugs.4 Exenatide has been shown to promote glucose-dependent insulin secretion, inhibit elevated glucagon secretion, reduce food intake, and slow gastric emptying.5–10 Together, these mechanisms integrate to improve glycemic control in a potent manner. In addition, exenatide has also been shown to promote beta-cell proliferation and islet neogenesis from precursor cells in both in vitro and in vivo models of diabetes.11 These findings suggest that exenatide may benefit beta-cell health and are consistent with clinical observations of sustained improvement in markers of beta-cell health such as insulinogenic index, proinsulin/insulin ratio, and homeostasis model assessment of beta-cell function (HOMA-B). Improvement in the disposition index after a 4-week off-drug period in patients treated with ExBID for 3 years was also reported.12
In randomized, controlled trials, ExBID treatment was associated with improvements in hemoglobin A1c (HbA1c) and cardiometabolic measures such as body weight, lipid profile, and blood pressure.8,9,13–15 Longer-term studies of ExBID in patients continuing on therapy have shown that the reductions in HbA1c achieved during the initial 12 weeks of the study (−1.1%) were sustained for up to 3 years (−1.0%).15 Furthermore, improvements in body weight, triglycerides, high- and low-density lipoprotein cholesterol, and systolic and diastolic blood pressure were also sustained or newly observed with ExBID treatment over 3 years.15
An extended-release formulation of exenatide, exenatide once weekly (ExQW), was developed to provide uninterrupted exenatide exposure to maintain continuous glycemic control with once-weekly subcutaneous administration. This formulation encapsulates exenatide in poly-(D,L-lactide-co-glycolide) microspheres which release the drug slowly, allowing for steady therapeutic levels.16 In the Effects of Exenatide Long-Acting Release on Glucose Control and Safety in Subjects With T2DM (DURATION-1) trial, the safety and efficacy of this formulation was compared to ExBID.17 After the 30-week randomized and controlled phase, patients continued into an open-label, open-ended extension phase where all patients were treated with ExQW. Results from the 30-week, 52-week, and 2-year assessment periods have been presented previously.17–19 Herein are the results of the 3-year assessment, examining the durability of glycemic control and other cardiometabolic benefits and the safety and tolerability of ExQW treatment of patients with T2DM.
Methods
Study design
The study design of DURATION-1 has been described previously.17–19 Briefly, patients were at least 16 years old, with T2DM treated for at least 2 months before screening, and treated with background therapies of diet modification and exercise or pharmacological treatment with metformin, a sulfonylurea, a thiazolidinedione, or a combination of two of these agents. Patients received 5 μg of ExBID for 3 days prior to randomization. Patients were then randomized to receive either weekly subcutaneous injections of 2 mg ExQW or subcutaneous injections of 5 μg ExBID for the first 4 weeks followed by injections of 10 μg ExBID through week 30. At 30 weeks, patients entered an uncontrolled, open-label, open-ended phase of the study in which all patients received ExQW (2 mg) regardless of initial randomization. Dosages of concomitant antihypertensive, lipid lowering, or antidiabetes medications were generally to remain stable. However, since the primary purpose of the extension was to collect safety information, changes were allowed if instructed by the investigator or primary care physician. A common clinical protocol was approved for each study site by the appropriate Institutional Review Board. Patients provided written informed consent prior to participation. The study was conducted in accordance with the principles described in the Declaration of Helsinki, including all amendments through to the South Africa revision of 1996.20
As patients originally receiving ExBID were switched to ExQW at 30 weeks, the results presented here – examining data at 3 years of treatment in the intention-to-treat (ITT) and completer populations – were not separated based on the initial randomization to either ExQW or ExBID and instead presented as a single population.
Outcomes
The outcomes measured in this study included HbA1c, fasting plasma glucose (FPG), body weight, fasting lipids, blood pressure, HOMA-B, fasting insulin, safety, and tolerability. Plasma analytes and HbA1c were quantitated by Quintiles Laboratories Ltd, (Smyrna, GA, USA) using standard methods.17
Statistical analysis
The ITT population was defined as all randomized patients who received at least one injection of exenatide in the initial 30-week phase of the study. The 3-year completer population consisted of patients from the ITT population who completed at least 152 weeks of treatment in compliance with the protocol, regardless of initial randomization to either ExBID or ExQW. Descriptive statistics on demographics and analysis of primary glycemic endpoints, body weight, blood pressure, fasting lipid concentrations, and HOMA-B values were provided for both the 3-year completer and ITT populations. Safety analyses were provided for the ITT population. For ITT analyses, the last observation carried forward method was applied to account for missing data from patients who withdrew early. The analyses of changes in HbA1c from baseline were based on a general linear model (analysis of variance) including original treatment assignment, baseline HbA1c strata (<9% or ≥9%), and concomitant sulfonylurea use at screening as factors. Baseline FPG, body weight, and blood pressure were added in the model (analysis of covariance) as a covariate for the parametric analysis of the change from baseline of each respective parameter. Efficacy endpoint results of changes from the baseline are expressed as least squares (LS) means ± standard error (SE) unless otherwise stated; confidence intervals (95% CI) were also calculated. Statistical analysis was performed using SAS® 9.2 (SAS Institute Inc, Cary, NC, USA).
Adverse events (AEs)
Treatment-emergent AEs were defined as events that occurred during or after the first injection of randomized medication and were reported until the end of the trial. Hypoglycemic episodes were classified as major if the event (1) resulted in loss of consciousness, seizure, coma, or other change in mental status consistent with neuroglycopenia (as judged by the investigator or physician), in which symptoms resolved after administration of intramuscular glucagon or intravenous glucose or (2) required third-party assistance because of severe impairment in consciousness or behavior and was accompanied by a blood glucose concentration of <3.0 mmol/L (54 mg/dL). Minor hypoglycemia was defined as a report of symptoms consistent with hypoglycemia and a glucose value of <3.0 mmol/L prior to treatment of the episode.
Nausea, diarrhea, and vomiting events were attributed to a defined period according to the event onset date. Incidence was divided into 30-week intervals. Within each 30-week interval, the percent of patients with events was calculated using the total number of patients remaining in the trial during the defined period.
Results
Patient disposition and baseline characteristics
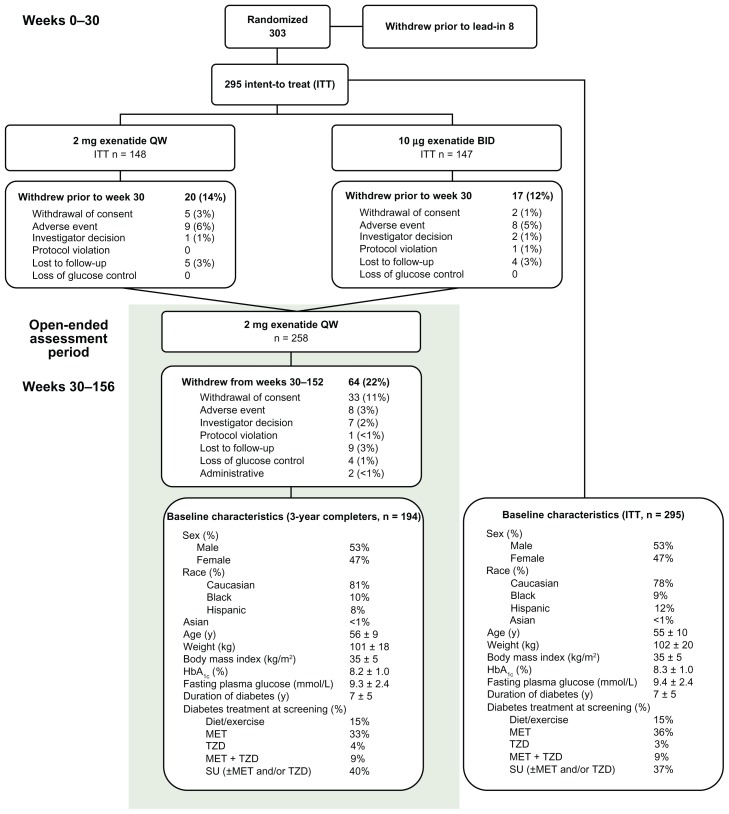
The ITT population of the 30-week controlled portion of the study included 295 patients. Of these, 194 patients (66%) completed at least 3 years of treatment. The baseline characteristics of the ITT and 3-year completer populations were similar (Figure 1). Thirty-seven and 64 patients withdrew from the study during the initial treatment period (weeks 1–30) and subsequent long-term extension (weeks 30–152), respectively. Prior to week 30, the most common reason for withdrawal from either study arm was AEs (n = 9, ExQW; n = 8, ExBID). From weeks 30–152, patients withdrew primarily due to withdrawal of consent (n = 33), with eight patients (3.0%) withdrawing due to an AE (Figure 1).
Figure 1.
Enrollment, patient disposition, and baseline characteristics.
Abbreviations: BID, twice daily; HbA1c, hemoglobin A1c; ITT, intention-to-treat; MET, metformin; QW, once weekly; SU, sulfonylurea; TZD, thiazolidinedione.
Glycemic control, beta-cell function, and effects on body weight
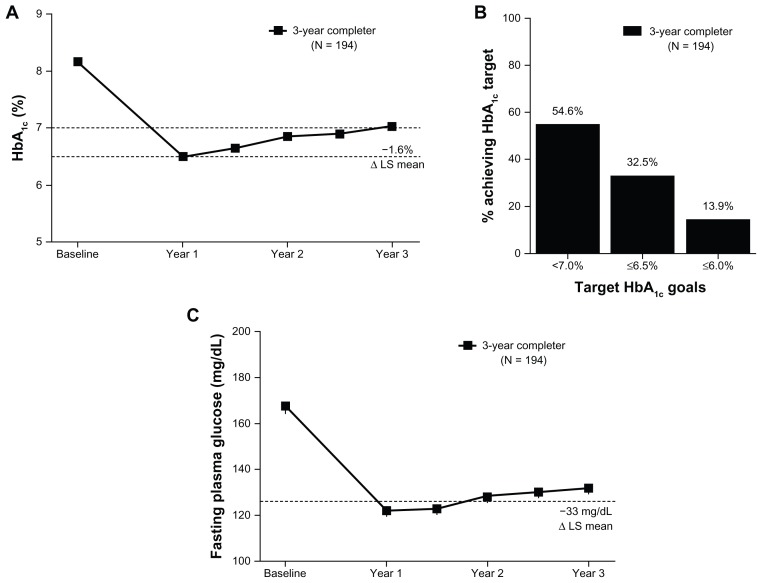
At baseline, the mean ± SE HbA1c for patients in the 3-year completer population completing 3 years of exenatide treatment was 8.2% ± 0.96%. After 3 years of treatment, the observed mean HbA1c for the completer population was 7.0% ± 0.95%, with a significant LS mean ± SE (95% CI) reduction from baseline of −1.6% ± 0.08% (−1.74%, −1.41%) (Figure 2A). Similar improvements were observed in the ITT population (−1.4% ± 0.08% [−1.51%, −1.21%]). At the end of the 3-year assessment period, 86% of patients achieved some reduction in HbA1c; 55% of patients achieved HbA1c < 7% (3.1% at baseline), and 33% achieved HbA1c ≤ 6.5% (0% at baseline) (Figure 2B). A similar percentage of ITT patients achieved these glycemic goals (50% achieved HbA1c < 7% and 31% achieved HbA1c ≤ 6.5%).
Figure 2.
Glycemic control over 3 years. (A) The mean ± standard error HbA1c (%) over 3 years for the completer population. LS mean change from baseline to 3 years for the completer population. Dotted lines at 7% and 6.5% represent target HbA1c goals. (B) The proportion of patients reaching indicated HbA1c target goals in the completer population. 3.1% of the completer population was at HbA1c < 7% at baseline. (C) The mean ± standard error fasting plasma glucose over 3 years for the completer population.
Notes: LS mean change from baseline to 3 years for the completer population are indicated. The dotted line at 7 mmol/L represents the threshold at which diabetes is diagnosed.
Abbreviations: HbA1c, hemoglobin A1c; LS, least squares.
Consistent with the HbA1c reduction, FPG was also significantly reduced relative to baseline, with improvements of −1.8 ± 0.2 mmol/L (−2.1, −1.5) and −1.5 ± 0.2 mmol/L (−1.8, −1.2) in the 3-year completer and ITT populations, respectively (Figure 2C). Across 3 years of treatment, the changes from baseline in both HbA1c and in FPG followed a similar pattern with the largest reductions observed after the first year of treatment and generally maintained thereafter (Figure 2A and C). While the majority of patients did not alter their medication regimen, new additions and dose modifications of glucose-lowering medications occurred during the extension phase of the study. Medication additions or dose increases occurred in 33 of the 194 patients (17%) completing 3 years of the study. The remaining 161 patients (83%) had no change, a decrease, or discontinuation of glucose-lowering medications.
Despite reductions in fasting glucose, no change in fasting insulin secretion was observed over the 3 years of treatment (mean ± SE; 125 ± 5.6 pmol/L at baseline; 123 ± 5.8 pmol/L at 3 years). Improvements in beta-cell function (as measured using HOMA-B) in the completer population were observed after the first year of treatment (mean ± SE change from baseline, 39.5% ± 4.9%) and were generally maintained through 3 years (mean ± SE change from baseline, 25.1% ± 4.4%).
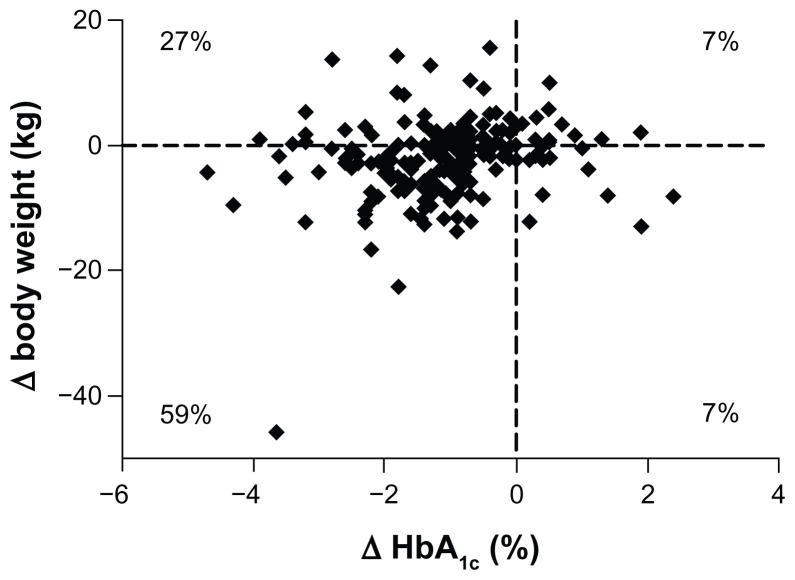
Patients completing 3 years of ExQW treatment showed a significant reduction in body weight of −2.3 ± 0.6 kg (−3.4, −1.2) from baseline. Similar body weight reductions were also observed in the ITT population. The change from baseline was smaller at 3 years compared to 1 year. However, decreases in body weight were achieved in the majority (66%) of the completer population after 3 years of treatment and 59% of the population experienced both weight loss and reductions in HbA1c (Figure 3).
Figure 3.
Change in body weight over 3 years.
Notes: Scatter plot (individual subject data, n = 194) of change in HbA1c versus change in body weight in the completer population.
Abbreviation: HbA1c, hemoglobin A1c.
Effects on blood pressure and fasting lipids
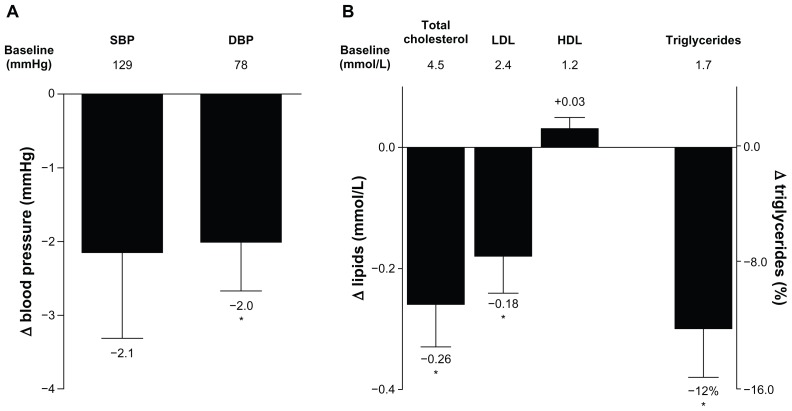
Improvements in systolic blood pressure that were observed in the 3-year completer population after 2 years were also observed after 3 years of treatment, but did not reach statistical significance (−2.14 ± 1.2 mmHg [−4.46, 0.17]). Improvements in diastolic blood pressure at 3 years were significant, with a reduction from baseline of −2.0 ± 0.7 mmHg (−3.3, −0.7) (Figure 4A).
Figure 4.
Change in blood pressure and serum lipids. (A) LS mean ± standard error change from baseline in SBP and DBP after 3 years of treatment in the completer population (n = 194). Mean baseline values are indicated below the bars. (B) LS mean ± standard error change from baseline in serum lipids, and geometric LS mean change ± standard error from baseline in triglycerides in the completer population (n = 194).
Notes: Mean baseline values are indicated; median is provided for triglycerides; *P < 0.05.
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; LS, least squares; SBP, systolic blood pressure.
Similar to the changes observed after 2 years of ExQW treatment,21 improvements in serum lipid profiles after 3 years were maintained in patients completing 3 years of ExQW treatment, with significant reductions in total cholesterol, low-density lipoprotein cholesterol, and triglycerides (Figure 4B). The LS mean ± SE (95% CI) change from baseline was −0.26 ± 0.07 mmol/L (−0.40, −0.11) for total cholesterol and −0.18 ± 0.06 mmol/L (−0.31, −0.05) for low-density lipoprotein cholesterol. The geometric LS mean percent change ± SE (95% CI) from baseline was −12% ± 3.2% (−18%, −6%) for triglycerides (LS mean ± SE [95% CI] change was −0.34 ± 0.07 mmol/L [−0.48, −0.19]). Increases in high-density lipoprotein cholesterol were also noted but these changes did not reach statistical significance.
During the course of the study, 29% and 32% of patients in the 3-year completer population added or increased their dose of antihypertensive or lipid-lowering medications, respectively, with 48% and 49% of patients having no change, a decrease, or discontinuation of antihypertensive or lipid-lowering medications. Twenty-three percent and 19% of patients were not using antihypertensive or lipid-lowering medications, respectively, and did not add these medications during the study.
Safety and tolerability
ExQW was generally well-tolerated across 3 years of therapy. Treatment emergent AEs with an incidence of ≥5% during weeks 0–156 are listed in Table 1. The AEs for ExBID treatment during the initial 30 weeks of the trial have been reported previously.17 Over the 3 years of the study (weeks 0–156) the AEs reported most frequently with ExQW treatment included upper respiratory tract infection, nausea, nasopharyngitis, diarrhea, sinusitis, and vomiting. With the exception of sinusitis, which was predominantly assessed as moderate in intensity, these AEs were mostly assessed as mild in intensity. Only two AEs of nausea led to withdrawal during the open-ended assessment period (weeks 30–156).
Table 1.
Treatment-emergent adverse events with an incidence of ≥5% and annual event rates
| Adverse event | Weeks 0 to 30 (n = 145) (Incidence %) |
Weeks 0 to 156 (n = 278) (Incidence %) |
Weeks 0 to 30 (n = 145) (Annual event rate) |
Weeks 0 to 156 (n = 278) (Annual event rate) |
|---|---|---|---|---|
| Upper respiratory tract infection | 8.1 | 33.5 | 16.2 | 18.7 |
| Nausea | 27.0 | 25.9 | 84.6 | 18.6 |
| Nasopharyngitis | 6.8 | 23.7 | 18.7 | 17.5 |
| Diarrhea | 16.2 | 23.0 | 37.3 | 15.0 |
| Sinusitis | 4.7 | 17.6 | 8.7 | 11.5 |
| Vomiting | 10.8 | 15.8 | 36.1 | 12.0 |
| Urinary tract infection | 10.1 | 14.0 | 22.4 | 8.7 |
| Back pain | 4.7 | 14.0 | 8.7 | 6.3 |
| Injection site pruritus | 18.2 | 13.7 | 51.0 | 7.5 |
| Constipation | 10.1 | 11.9 | 19.9 | 5.2 |
| Arthralgia | 4.7 | 11.9 | 12.4 | 5.7 |
| Pain in extremity | 0.7 | 11.9 | 2.5 | 5.2 |
| Gastroenteritis viral | 8.8 | 11.5 | 16.2 | 6.0 |
| Gastroesophageal reflux disease | 7.4 | 10.4 | 14.9 | 4.1 |
| Headache | 6.1 | 10.1 | 26.1 | 6.3 |
| Hypertension | 3.4 | 9.7 | 6.2 | 3.8 |
| Fatigue | 6.1 | 9.4 | 11.2 | 3.8 |
| Bronchitis | 2.7 | 9.4 | 5.0 | 4.4 |
| Dyspepsia | 7.4 | 8.3 | 14.9 | 4.2 |
| Cough | 3.4 | 8.3 | 6.2 | 3.5 |
| Musculoskeletal pain | 1.4 | 8.3 | 2.5 | 3.5 |
| Injection site erythema | 7.4 | 7.2 | 13.7 | 3.1 |
| Injection site bruising | 4.7 | 7.2 | 10.0 | 3.3 |
| Muscle spasms | 2.0 | 7.2 | 6.2 | 3.3 |
| Fall | 1.4 | 7.2 | 2.5 | 3.1 |
| Influenza | 1.4 | 7.2 | 2.5 | 2.9 |
| Dizziness | 3.4 | 6.8 | 8.7 | 4.1 |
| Rash | 0.0 | 6.1 | 0.0 | 2.6 |
| Contusion | 2.0 | 5.8 | 3.7 | 2.6 |
| Pharyngolaryngeal pain | 1.4 | 5.8 | 2.5 | 3.1 |
| Insomnia | 2.7 | 5.4 | 7.5 | 2.3 |
| Nephrolithiasis | 1.4 | 5.4 | 2.5 | 2.9 |
| Abdominal pain | 3.4 | 5.0 | 6.2 | 2.3 |
| Depression | 1.4 | 5.0 | 3.7 | 2.5 |
| Oedema peripheral | 0.7 | 5.0 | 1.2 | 2.2 |
Notes: Data are in descending order based on incidence from 0 to 156 weeks. Annual Event Rates are represented as events per 100 years of patient exposure.
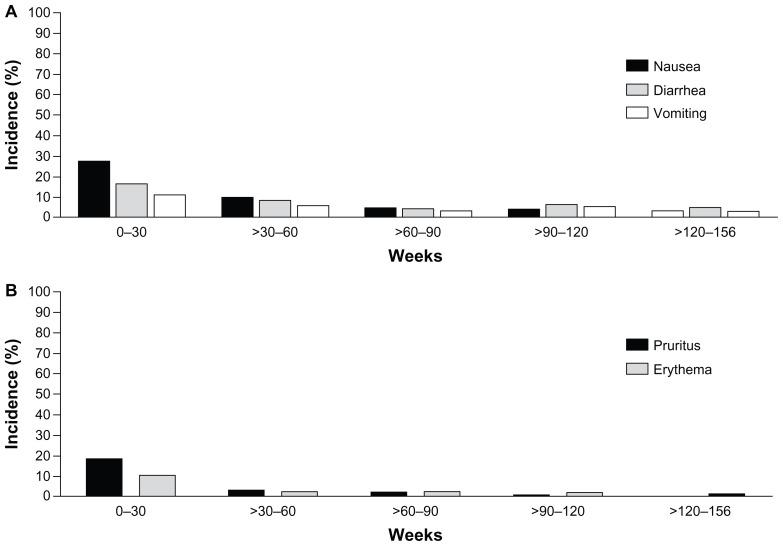
Given the length of the trial, the cumulative incidence of AEs, and the absence of a comparator arm during the extension, annual event rates were calculated. The annual event rates (events per 100 years of patient exposure) for the most common AEs during the first 30 weeks – nausea, injection site pruritus, diarrhea, and vomiting – declined when assessed over 3 years of ExQW exposure (Table 1). This decrease in the annual event rate is consistent with the finding that the highest incidence of nausea, diarrhea, and vomiting occurred during the first 12 weeks of ExQW exposure with a decreased incidence thereafter (Figure 5A). The incidence of nausea, diarrhea, and vomiting occurred in 25%, 14.9%, and 9.5% of patients treated with ExQW in the ITT population, respectively, during the first 12 weeks and in 1% of patients (any of the three AEs) during weeks 144–156. Likewise, the highest incidence of the two most frequently occurring injection site-related AEs – pruritus and erythema – occurred during the first 12 weeks and also decreased over time (Figure 5B). The incidence of injection site pruritus and erythema occurred in 18.2% and 7.4% of patients, respectively, during the first 12 weeks and no patients experienced either AE during weeks 144–156. The annual event rates for AEs such as upper respiratory tract infection, nasopharyngitis, and sinusitis remained generally constant during the initial 30 weeks of the trial (the controlled period) and for the entire 3 years of the study (Table 1).
Figure 5.
Incidence of adverse events over time. From 0–30 weeks, only the incidence of AEs occurring in subjects in the ExQW group is included (n = 148). After week 30, all subjects remaining in the trial during a given period are included (n = 295). Events are attributed to a defined period according to the event onset date; periods are grouped into 30-week intervals. (A) Incidence of nausea, diarrhea, and vomiting through week 156. (B) Incidence of injection site pruritus and erythema through week 156.
Abbreviations: AEs, adverse events; ExQW, exenatide once-weekly.
No major hypoglycemia was reported during the 3 years of the study. Minor hypoglycemia occurred in 21 (19.3%) of the 109 patients using concomitant sulfonylurea at screening and in two (1.0%) of the 186 patients not using sulfonylurea background therapy over the 3-year trial period. The annual event rate per 100 years of patient exposure in subjects treated with exenatide with or without sulfonylurea over 3 years of exposure was 35.7 and 1.2, respectively. A total of 48 (17.3%) of 278 patients treated with ExQW experienced a serious AE at some point during the 3-year trial period. However, there was no observable pattern of reported serious AEs. The System Organ Class designation with the highest incidence of serious AEs was cardiac disorders, with eleven patients (4.0%) treated with ExQW experiencing a serious cardiac event. No cases of pancreatitis, pancreatic cancer, or thyroid cancer were reported in the 3 years of the trial. Furthermore, there were no cardiovascular, pulmonary, hepatic, or renal toxicity events specifically associated with long-term ExQW use.
Discussion
Diabetes is a progressive disease that necessitates long-term pharmacological intervention. The current treatment paradigm suggests a stepwise and additive approach starting with monotherapy using the commonly prescribed agent metformin. As glycemic control fails with disease progression, dual and triple therapy is recommended. Additional oral agents, such as sulfonylureas, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, or injectable therapies such as insulin or GLP-1 receptor agonists are initiated.21,22 Given the sequential addition of therapies, some agents can be used for many years. As such, there is significant value associated with the characterization of a given agent’s efficacy and safety beyond the typical length of time needed to establish treatment efficacy. The evaluation of long-term efficacy includes not only the durability of glycemic control, but also an assessment of sustained or newly observed improvements in other biomarkers of patient health. Several long-term studies of antidiabetes drugs have assessed their continued efficacy and safety over time.2,3,15,23–26 Previously, results from the extension of the DURATION-1 trial demonstrated that improvements in glycemic control and certain cardiovascular risk markers were maintained for 2 years in patients treated with ExQW.19 The current analysis extends these observations to 3 years and characterizes the safety and tolerability of ExQW with sustained use. Further, the longer duration of ExQW treatment in this study allows for an examination of the possible benefits of ExQW on beta-cell health.
Efficacy
Long-term treatment with ExBID has shown sustained improvements in glycemic control and cardiovascular risk factors such as body weight, blood pressure, and fasting lipids over 3 years of treatment.15 Likewise, previous assessments of ExQW in the current trial at 30 weeks, 1 year, and 2 years have shown a consistent pattern of sustained improvement in these risk factors.17–19 The current analysis showed that glycemic control and improvement in several cardiometabolic parameters persisted with 3 years of ExQW treatment.
Significant improvements in HbA1c and FPG were observed. The majority of patients observed reductions in HbA1c over baseline and achieved the American Diabetes Association recommended goal of <7%. While the majority of the initial glycemic effect was maintained over the 3 years of treatment, a gradual upward trend in HbA1c and FPG concentrations was observed that is difficult to interpret in the absence of a comparator arm. This trend could represent reduced compliance with diabetes treatment, diminished response to therapy including concomitant oral antidiabetes therapies, or progression of the diabetes disease state. In the comparator-controlled extension of the DURATION-3 study, a similar upward trend was noted at 84 weeks in both the ExQW and the insulin glargine groups. Individual patient analysis found no obvious differences between patients who lost glycemic control over time and patients who had continued improvements over time. Indeed, the rate of HbA1c increase in each group was similar, suggesting disease progression might be the cause of the upward drift in HbA1c over time.27
The apparent decline in glycemic control is a common finding among longer-term studies.2,3 The ADOPT study compared the glycemic durability of rosiglitazone, metformin, and a sulfonylurea by measuring the time to monotherapy failure as defined by an FPG level of >180 mg/dL.2 Results of the ADOPT study showed a gradual loss of glycemic control in all treatment arms, similar to that observed in the current analysis. The study investigators suggested that the superior durability of rosiglitazone could be due to a prevention of beta-cell deterioration that is common in T2DM.2 The results of the current analysis, showing an improvement in HOMA-B, suggest that long-term ExQW treatment may likewise promote beta-cell health. However, the caveats associated with HOMA necessitate additional clinical data, such as results from glucose clamp or glucose tolerance tests, in order to more conclusively determine if ExQW treatment improves beta-cell function.
Other observed benefits of ExQW therapy included improvements in certain cardiometabolic parameters. Significant improvements in body weight were maintained after 3 years of treatment, with 66% of the population maintaining or newly achieving weight loss relative to baseline. Blood pressure and fasting lipid improvements (total cholesterol, low-density lipoprotein cholesterol, and triglycerides) were also maintained at 3 years, with the exception of sitting systolic blood pressure, which remained reduced but did not reach statistical significance.
Long-term improvements in parameters beyond glycemic control, such as those noted above, have been demonstrated to be clinically valuable. In the Action in Diabetes and Vascular Disease: Preterax® and Diamicron MR® Controlled Evaluation (ADVANCE) trial, patients observing small reductions in systolic (5.6 mmHg) and diastolic (2.2 mmHg) blood pressure reduced their relative risk of major microvascular or macrovascular events by 9%. The major contributor to this composite risk reduction was an 18% reduction in the risk of death from cardiovascular disease.28 In addition, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) lipid study, patients with diabetes and dyslipidemia (high triglycerides and low high-density lipoprotein cholesterol) who were treated with the cholesterol-lowering drug fenofibrate had a small reduction in adverse cardiovascular outcomes.29 While studies targeting the individual factors of hypertension or dyslipidemia have shown some reduction in cardiovascular risk,29–33 studies targeting multifactorial interventions have observed greater reductions in cardiovascular risk.34 The results of the Steno-2 study revealed that long-term, intensified treatment targeted to improve several modifiable cardiovascular risk factors including HbA1c, cholesterol, triglycerides, and systolic and diastolic blood pressure significantly reduced the risk of cardiovascular and microvascular AEs by as much as 50%.35 Thus, therapies with long-term improvements in both glycemic control and cardiovascular risk factors may confer a long-term health advantage in diabetic patients.
Safety and tolerability
Long-term safety and tolerability is also a critical concern among therapies with the potential for extended use, especially as tolerability concerns relate to treatment adherence. Exenatide therapy for 3 years was generally well-tolerated and the incidence of several gastrointestinal-related (nausea, diarrhea, and vomiting) and injection-site-related AEs decreased over time. As opposed to gastrointestinal- and injection site-related AEs, the annualized event rates for AEs accumulating over the 3-year observation period such as upper respiratory tract infections, nasopharyngitis, and sinusitis were largely consistent with annualized event rates observed during the 30-week controlled period, suggesting an equal likelihood of incidence with either short-term or long-term therapy.
The safety profile over 3 years was generally consistent with previously published reports on ExQW treatment for 30 weeks.17 A similar number of ExQW patients withdrew from the trial due to an AE during the initial 30 weeks of the trial as in the subsequent 2.5 years. No major hypoglycemia was reported over 3 years and minor hypoglycemia occurred rarely but more frequently in patients receiving concomitant sulfonylurea. The rates of serious AEs per System Organ Class designation were generally low. Although an analysis using the Food and Drug Administration AE reporting database suggested that GLP-1 receptor agonists might have side effects including pancreatitis, pancreatic cancer, and thyroid cancer,36 no instances of these events were reported during 3 years of ExQW use.
Study strengths and limitations
This analysis provides a long-term assessment of the efficacy, safety, and tolerability of exenatide use over 3 years. The long duration of this clinical study allows the observation of yearly efficacy changes and a more thorough safety/tolerability picture than a real-world analysis of the common events associated with ExQW use over 3 years.
Primary efficacy data are summarized using both the completer population as well as the ITT population. Use of the completer population allows for a normalization of treatment exposure, an important consideration with longer-term observations. The ITT analysis allows for inclusion of all subjects who received ExQW regardless of exposure. There are important caveats to both methods, which require consideration when interpreting efficacy. For instance, the normalization of exposure achieved with completer populations comes with a natural bias towards subjects who tolerated the treatment well and observed satisfactory efficacy to opt to continue with the treatment. Conversely, the ITT last observation carried forward approach introduces exposure as a variable which becomes an increasingly significant issue as trial duration increases (eg, the relevance of summarizing efficacy at extremes of the exposure range is questionable).
Given the duration of the trial, some care providers chose to adjust antihyperglycemic, hypertension, and lipid therapies. However, for all three therapies, the majority of patients did not change, decrease, or discontinue their medications. The observed improvements in HbA1c, FPG, blood pressure, and fasting lipids may have been influenced by these changes. However, the results are more typical of real world clinical practice where additional therapies are added to a patient’s therapeutic regimen to attain a desirable target. The lack of a control arm to contextualize what therapy additions would have looked like in the absence of ExQW warrants caution.
Conclusion
The 3-year assessment of subjects in the DURATION-1 trial shows that longer-term treatment with ExQW resulted in sustained improvements in glycemic control and several clinically important cardiometabolic parameters. In addition, exenatide was well-tolerated, with noted reductions in the occurrence of gastrointestinal- and injection site-related events over time. No major hypoglycemia occurred and minor hypoglycemia was observed at a low rate, especially in patients not using concomitant sulfonylurea. No cases of pancreatitis, pancreatic, or thyroid cancer were reported over the 3 years and the observed safety profile was consistent with that described previously for ExQW.
Acknowledgments
The authors thank Courtney Van Biene for assistance with computer programming and Alison R Meloni for editorial assistance. The study was sponsored by Amylin Pharmaceuticals, LLC.
Footnotes
Disclosure
All authors are employees and shareholders of Amylin Pharmaceuticals, LLC.
References
- 1.International Expert Committee. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 3.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 4.BYETTA® exenatide injection [prescribing information] San Diego, CA: Amylin Pharmaceuticals, Inc; 2011. [Google Scholar]
- 5.Apovian CM, Bergenstal RM, Cuddihy RM, et al. Effects of exenatide combined with lifestyle modification in patients with type 2 diabetes. Am J Med. 2010;123(5):468.e9–468.e17. doi: 10.1016/j.amjmed.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab. 2008;294(5):E846–E852. doi: 10.1152/ajpendo.00030.2008. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24(10):2943–2952. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 9.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 10.Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281(1):E155–E161. doi: 10.1152/ajpendo.2001.281.1.E155. [DOI] [PubMed] [Google Scholar]
- 11.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Bunck MC, Corner A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011;34(9):2041–2047. doi: 10.2337/dc11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control and safety over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 14.Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther. 2007;29(1):139–153. doi: 10.1016/j.clinthera.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24(1):275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 16.DeYoung MB, MacConell L, Sarin V, Trautmann M, Herbert P. Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol Ther. 2011;13(11):1145–1154. doi: 10.1089/dia.2011.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 18.Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33(6):1255–1261. doi: 10.2337/dc09-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor K, Gurney K, Han J, Pencek R, Walsh B, Trautmann M. Exenatide once weekly treatment maintained improvements in glycemic control and weight loss over 2 years. BMC Endocr Disord. 2011;11:9. doi: 10.1186/1472-6823-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277(11):925–926. [PubMed] [Google Scholar]
- 21.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540–559. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Ahrén B, Foley JE, Ferrannini E, et al. Changes in prandial glucagon levels after a 2-year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2010;33(4):730–732. doi: 10.2337/dc09-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169(6):616–625. doi: 10.1001/archinternmed.2009.20. [DOI] [PubMed] [Google Scholar]
- 25.Seck T, Nauck M, Sheng D, et al. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. Int J Clin Pract. 2010;64(5):562–576. doi: 10.1111/j.1742-1241.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 26.Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(5):442–451. doi: 10.1111/j.1463-1326.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 27.Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35(4):683–689. doi: 10.2337/dc11-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel A ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 29.Ginsberg HN. The ACCORD (Action to Control Cardiovascular Risk in Diabetes) lipid trial: what we learn from subgroup analyses. Diabetes Care. 2011;34(Suppl 2):S107–S108. doi: 10.2337/dc11-s203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curb JD, Pressel SL, Cutler JA, et al. Systolic Hypertension in the Elderly Program Cooperative Research Group. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276(23):1886–1892. [PubMed] [Google Scholar]
- 31.Aalbers J. ACCORD lipid study results strengthen guideline approach of adding fenofibrate to therapy of dyslipidaemic type 2 diabetic patients. Cardiovasc J Afr. 2010;21(2):118–119. [PubMed] [Google Scholar]
- 32.Elam M, Lovato LC, Ginsberg H. Role of fibrates in cardiovascular disease prevention, the ACCORD-Lipid perspective. Curr Opin Lipidol. 2011;22(1):55–61. doi: 10.1097/MOL.0b013e328341a5a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 34.Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353(9153):617–622. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 35.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 36.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]