Abstract
Objectives
The objective of the present study was to translate our laboratory investigations to establish safety and efficacy of 8 weeks of chronic SQ BNP administration in human Stage C HF.
Background
B-Type natriuretic peptide (BNP) is a cardiac hormone with vasodilating, natriuretic, renin-angiotensin (RAS) inhibiting and lusitropic properties. We have previously demonstrated that chronic cardiac hormone replacement with subcutaneous (SQ) administration of BNP in experimental heart failure (HF) resulted in improved cardiovascular function.
Methods
We performed a randomized double-blind placebo-controlled proof of concept study comparing eight weeks of SQ BNP (10 μg/Kg bid) (n=20) with Placebo (n=20) in patients with EF<35% and NYHA class II–III HF. Primary outcomes were LV volumes and LV mass determined by cardiac MRI. Secondary outcomes include LV filling pressure by Doppler echo, humoral function and renal function.
Results
Eight weeks of chronic SQ BNP resulted in a greater reduction of LV systolic and diastolic volume index and LV mass index as compared to placebo. There was a significantly greater improvement of Minnesota Living with Heart Failure (MLHF) score, LV filling pressure as demonstrated by the reductions of E/e′ ratio and decrease in LA volume index as compared to placebo. GFR was preserved with SQ BNP, as was the ability to activate plasma cGMP. (p<0.05 vs placebo)
Conclusion
In this pilot proof of concept study, chronic protein therapy with SQ BNP improved LV remodeling, LV filling pressure and MLHF score in patients with stable systolic HF on optimal therapy. RAS was suppressed and GFR preserved. SQ BNP represents a novel, safe and efficacious protein therapeutic strategy in human HF. Further studies are warranted to determine if these physiologic observations can be translated into improved clinical outcomes and ultimately delay the progression of HF.
Keywords: Natriuretic peptides, Kidney, cGMP
INTRODUCTION
The AHA/ACC classification of heart failure (HF) Stage A to D emphasizes the need to develop therapeutic strategies to prevent the progression of HF(1). B-type natriuretic peptide (BNP) is a 32-amino acid peptide produced mainly by cardiomyocytes and plays an important role in cardiorenal homeostasis. Specifically, BNP binds to the natriuretic peptide-A receptor (NPR-A), which via 3′,5′-cyclic guanosine monophosphate (cGMP), mediates natriuresis, vasodilatation, renin-angiotensin-aldosterone (RAAS) inhibition, anti-hypertrophic, anti-fibrotic, and positive lusitropic properties (2). Despite activation of both plasma and cardiac BNP in HF, exogenous administration of BNP resulted in activation of its second messenger cGMP with favorable cardiac unloading actions(3). In both experimental and human HF, investigations have supported the hypothesis that the synthetic capacity of the cardiomyocytes may be overwhelmed in severe HF relative to the demands of the system, leading to a state of relative deficiency of BNP(4). Recent investigations have demonstrated that in severe HF, despite high levels of immunoreactive BNP detected in the plasma, there was very little biologically active mature BNP1–32, suggesting the presence of abnormal molecular forms with reduced biological actions(5–7).
Intravenous infusion of human BNP (Nesiritide) is approved by the FDA for the in-patient management of acute decompensated congestive heart failure (ADHF) (3). The FUSION I and II studies evaluated the use of outpatient serial intravenous (IV) infusions of BNP in patients with Stage D HF(8,9). Most recently, findings of the 7,000 patient ASCEND Trial (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) reported that 1 to 7 day infusion of BNP was safe with no adverse renal or mortality properties and was associated with modest improvement in dyspnea(10).
We have previously reported that subcutaneous (SQ) administration of BNP in experimental HF resulted in improved cardiac output with reduced systemic vascular resistance and cardiac filling pressures(11). Importantly, we translated these studies to humans and completed a dosing finding study which reported that acute SQ administration of BNP at a dose of 10 μg/kg in human Stage C HF resulted in increased plasma BNP, and cGMP and urinary cGMP excretion, associated with favorable cardiorenal and humoral responses(12).
The objective of the present study was to further translate our laboratory investigations to establish safety and efficacy of 8 weeks of chronic SQ BNP administration in human Stage C HF.
METHODS
We performed a randomized double-blind placebo-controlled proof of concept study comparing eight weeks of SQ BNP (10 μg/Kg) to SQ Placebo twice a day, in patients with EF<35% and NYHA class II-III stable HF. Primary outcomes were left ventricular (LV) volumes and LV mass determined by cardiac MRI. Secondary outcomes include LV filling pressure by Doppler echo, humoral function and renal function. The protocol was approved by the Mayo Clinic Institutional Review Board, and informed consent was obtained from all participants.
Study Protocol
From May 2003 to May 2008, recruitment was limited to subjects aged 18 years and above with a resting LVEF of 35% or less and stable, mild symptoms of HF (NYHA Class II and III), as defined by the criteria outlined by the New York Heart Association (NYHA) classification. Subjects were admitted to the Clinic Research Unit (CRU) at St. Mary’s Hospital, Mayo Clinic CTSA, Rochester, MN, the afternoon before the scheduled active renal clearance study day. Cardiac MRI was performed, 6 minute walk and Minnesota Living with Heart Failure Questionnaire (MLHFQ) was assessed. On the following morning, the patients underwent a short renal clearance test using Lothalamate Meglumine (Conray 60%) given subcutaneously to measure the glomerular filtration rate. Echocardiography was performed for determination of baseline LV filling pressure. Venous blood samples were also collected for determination of BNP, cGMP and plasma renin activity. At the completion of the short renal clearance test, the patients were randomized to SQ BNP or placebo group. The patients were then instructed about the proper technique for diluting the BNP or placebo and the subcutaneous injection was given in the anterior abdominal wall. Thirty minutes after the first dose, a venous blood sample for determination of BNP and cGMP was drawn. The patients then self-administered the 2nd dose of subcutaneous BNP or placebo 12 hours after the 1st dose. Subsequently, the third dose was self-administered under supervision 12 hours later. The patients were then dismissed with instructions and enough supplies for eight weeks of subcutaneous administration twice a day.
Participants who experienced symptomatic hypotension as defined by systolic BP< 85 mmHg or had symptoms of lightheadedness or visual disturbances during the baseline visit in the CRU were discontinued from the study.
At the end of the eight-week study period, +/− 3 days, the patients were admitted to the CRU in the afternoon prior to the final renal clearance study. In our previous pilot dose finding study (12), we demonstrated that the blood pressure returned to baseline 2 hours after the administration of SQ BNP. Hence, in the current study, patients received their self-administered SQ injection at least 2 hours before the cardiac MRI, 6 minute walk and MLHFQ assessment. The next morning, echocardiography was performed, after which, a short renal clearance test and humeral determination were carried out before and after a final dose of SQ injection in the same manner as the baseline study. The randomization table was provided by the statistician and it was created to allow for a final n=20 in each group with completed primary end point data.
Exclusion Criteria Specification
Exclusion criteria include: MI within 3 months of screening; Unstable angina within 14 days of screening, or any evidence of myocardial ischemia; Valvular stenosis, hypertrophic, restrictive or obstructive cardiomyopathy, constrictive pericarditis, primary pulmonary hypertension, or biopsy proven active myocarditis; Sustained VT or V-fib within 14 days of screening; Persistent atrial fibrillation; Second or third degree AV block without a permanent cardiac pacemaker; CVA within 3 months of screening, or other evidence of significantly compromised CNS perfusion; Total bilirubin of > 1.5 mg/dL or an AST and ALT 1.5 times the upper limit of normal; Serum creatinine of >3.0 mg/dL; Serum sodium of <125 mEq/dL or > 160 mEq/dL; Serum potassium of < 3.5 mEq/dL or > 5.0 mEq/dL; Serum digoxin level of > 2.0 ng/ml; Systolic pressure of <85 mmHg; LVEF > 35% within 24 months of screening; Unable to self-administer subcutaneous injection twice a day; Diagnosed with AIDS or known positive HIV titer; Other acute or chronic medical conditions or laboratory abnormality which may increase the risks associated with study participation or may interfere with interpretation of the data; Received an investigational drug within 1 month prior to dosing; Unable to undergo Cardiac MRI; In the opinion of the investigator, is unlikely to comply with the study protocol or is unsuitable for any reasons.
Analytic Methods
The concentration of Iothalamate in the plasma and urine collected during the short renal clearance test was determined with capillary electrophoresis in the Mayo Renal laboratory for the determination of GFR. Specific plasma radioimmunoassays include BNP, cGMP and renin were performed based upon previously published methods by the co-investigator.(12)
Assessment of LV filling pressure and left atrium volume by Echocardiography
Pulsed wave Doppler examination of mitral (before and with Valsalva maneuver) and pulmonary venous inflow as well as Doppler tissue imaging of the mitral annulus were performed. Left atrial volume was calculated from 2-D measurements and was indexed to body surface area as previously described(13).
Cardiac magnetic resonance imaging (MRI)
Patients underwent cardiac MRI for measurement of left ventricular (LV) ejection fraction, LV end diastolic volume, LV end systolic volume and LV mass. All examinations were performed using a 1.5 Tesla MRI system (Signa Twin Speed Excite, GE Healthcare, Waukesha, WI). After initial localization scans, short axis cine images were obtained at 8 mm intervals from base to apex using an EKG-gated balanced steady state. Two-chamber, three-chamber, and four-chamber cine bSSFP acquisitions were then acquired using the short axis images for prescription. Images were transferred to a workstation (GE Advantage Windows, GE Healthcare) and a software analysis package (Mass Analysis, version 6, Medis, Leiden, The Netherlands) was used to trace endocardial and epicardial contours around each end-diastolic slice, and endocardial contours around each end-systolic slice. Ejection fraction, end-systolic volume, end-diastolic volume and LV mass were then computed by adding the contribution of the individual slices. All cardiac MR images were reviewed by Dr Glockner in a blinded fashion.
Data Analysis
Continuous measurements that are normally distributed are expressed as mean ± standard deviation and those that are skewed are expressed as median and quartiles, while categorical variables are reported as a number (percent). Chi-square tests or Fisher’s exact tests were used to compare categorical variables between BNP group and placebo group, while two sample t-tests or rank sum tests were used to compare the continuous variables between the two groups. Comparisons from baseline to 8 weeks within groups were completed with paired t-tests for normally distributed variables, signed-rank tests for non-normal differences. A p-value less than 0.05 was considered significant in this study.
RESULTS
Participants
We randomized 45 patients to receive SQ BNP (n=24) or SQ placebo (n=21) injections twice a day for 8 weeks. Three subjects in the BNP group developed symptomatic hypotension as defined by systolic BP< 85 mmHg or had symptoms of lightheadedness or visual disturbances during the baseline visit in the CRU and were excluded from the study as per protocol. One subject in the BNP group withdrew from the study for personal reasons. One subject in the placebo group had worsening HF symptoms during the 8 week period and withdrew from the study. Hence, in the final analysis, we had N=20 in the BNP group and N=20 in the placebo group.
The baseline demographic and clinical characteristics between the study groups are similar except for a higher prevalence of history of hypertension in the placebo group as compared to the BNP group. (Table 1) However, despite this difference, the baseline systolic and diastolic blood pressure and heart rate were similar between the 2 groups. The mean ejection fraction (EF), plasma BNP levels and distribution of NYHA Class 2 versus Class 3 patients were similar between the placebo and BNP groups. The majority (95%) of patients were treated with either an angiotensin converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (Ang II) or nitrates, in combination with a beta-blocker and a diuretic. Approximately, 20% were also treated with spironolactone in each group. 85% of the subjects in the BNP group and 80% of the subjects in the placebo group are on target doses of ACEI/AngII/Nitrates while 80% of the subjects in the BNP group and 85% of the subjects in the placebo group are on target doses of beta blockers.
Table 1.
Baseline Characteristics
| Baseline characteristics | Placebo (n=20) | BNP (n=20) | P value |
|---|---|---|---|
| Female, no. (%) | 8 (40) | 4 (20) | 0.17 |
| NYHA class II, no. (%) | 10 (50) | 12 (60) | 0.53 |
| NYHA class III, no. (%) | 10 (50) | 8 (40) | 0.55 |
| LV EF (%) | 31±7 | 32±9 | 0.79 |
| Systolic blood pressure (mm Hg) | 125±16 | 133±17 | 0.14 |
| Diastolic blood pressure (mm Hg) | 70±11 | 73±11 | 0.34 |
| Heart rate (bpm) | 69±12 | 66±13 | 0.59 |
| Plasma BNP (pg/mL) | 57(20;250) | 51(15;121) | 0.67 |
| Glomerular Filtration Rate, ml/1.73m2 | 78±21 | 74±23 | 0.59 |
| History of PVD, no. (%) | 1 (5) | 2 (10) | 0.55 |
| History of COPD, no. (%) | 1 (5) | 2 (10) | 0.55 |
| Ischaemic cardiomyopathy, no. (%) | 10 (50) | 10 (50) | 1.00 |
| History of hypertension, no. (%) | 16 (80) | 8 (40) | 0.010 |
| History of arrhythmia, no. (%) | 4 (20) | 5 (25) | 0.70 |
| History of smoking, no. (%) | 7 (35) | 9 (45) | 0.52 |
| History of diabetes, no. (%) | 7 (35) | 9 (45) | 0.52 |
| History of high cholesterol, no. (%) | 16 (80) | 13 (65) | 0.29 |
| ACE1 or Angio II, or nitrates, no. (%) | 19 (95) | 20 (100) | 0.31 |
| Digoxin, no. (%) | 8 (42) | 7 (35) | 0.65 |
| Beta blocker, no. (%) | 19 (95) | 20 (100) | 0.31 |
| Diuretic (loop), no. (%) | 14 (70) | 15 (75) | 0.72 |
| Coumadin, no. (%) | 1 (6) | 2 (13) | 0.48 |
| Spironolactone, no. (%) | 4 (20) | 4 (21) | 0.94 |
| Diuretic (thiazide), no. (%) | 3 (16) | 4 (20) | 0.73 |
| Antiarrhythmics, no. (%) | 0 (0) | 5 (25) | 0.017 |
Normally distributed variables are expressed as Mean ± SD, while variables with a skewed distribution are expressed as median and quartiles; NYHA: New York heart association; LV EF: left ventricular ejection fraction; BNP; B-type natriuretic peptide; PVD: peripheral vascular diseases; COPD: chronic obstructive pulmonary disease; ACEI: angiotensin converting enzyme inhibitor; Angio II: angiotensin II receptor blocker.
The Effects of SQ BNP on Left Ventricular Structure and Function
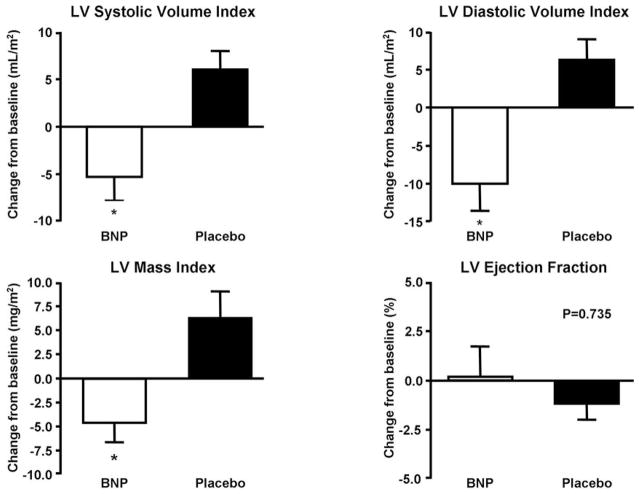
Figure 1 illustrates the change from baseline to 8 weeks of left ventricular structure and function as assessed by MRI. Eight weeks of chronic SQ BNP administered twice daily resulted in a significantly greater reduction in left ventricular (LV) end-systolic volume index as compared to placebo (BNP group: −5 ±13 ml/m2 versus Placebo group: +6 ±10 ml/m2, p=0.004). Similarly, there was a greater reduction in LV end-diastolic volume (−10 ±15 ml/m2) in the BNP group as compared to the placebo group (+6 ±12 ml/m2) at 8 weeks compared to baseline (P=0.001). These reductions in LV volumes were associated with a greater decrease in LV mass index in the BNP group (−4 ±10 mg/m2) as compared to the placebo group (+6 ±13 mg/m2) (P=0.006). LVEF was unchanged in both the BNP and placebo groups. The above changes in LV structure occurred in the absence of significant changes from baseline to 8 weeks; in heart rate (BNP group: 67±12 to 65±12 bpm; placebo group: 74±12 to 73±14 bpm); systolic blood pressure (BNP group: 140±20 to 135±25 mmHg; Placebo group: 121±17 to 126±15 mmHg) and diastolic blood pressure (BNP group: 79±12 to 77±13 mmHg; Placebo group: 74±12 to 75±12 mmHg) measured when the MRI was performed.
Figure 1. Changes in BNP and Placebo Groups.
Changes in left ventricular (LV) end –systolic volume Index (* P=0.004), left ventricular (LV) end – diastolic volume Index (*P=0.001), LV mass index (*P=0.006) and LV ejection fraction from baseline to 8 weeks in BNP and Placebo groups
*P value BNP versus placebo
Figure 2 illustrates echocardiographic assessment of LV filling pressure. SQ BNP improved LV filling pressure as demonstrated by a greater reduction of Doppler mitral inflow velocity (E) to mitral annulus tissue Doppler velocity (e′) ratio E/e′ and the greater decrease in LA volume index compared to placebo. The above changes occurred in the absence of significant changes from baseline to 8 weeks; in heart rate (BNP group: 62±15 to 66±13 bpm; placebo group: 66±11 to 70±11 bpm); systolic blood pressure (BNP group: 133±20 to 129±36 mmHg; Placebo group: 123±16 to 123±15 mmHg) and diastolic blood pressure (BNP group: 72±13 to 74±12 mmHg; Placebo group: 69±11 to 69±12 mmHg) measured at the time when the echo was performed.
Figure 2. Change in Left ventricular Doppler E/e.

′ ratio (*P=0.001) and left atrium (LA) volume index.
The dot within each box represents the mean value while the horizontal line within each box represents the median value and quartiles outside the box (*P=0.003) from baseline to 8 weeks in BNP and Placebo groups
*P value BNP versus placebo
The Effects of SQ BNP on Clinical Well-being
Minnesota Living with Heart Failure (MLHF) score was assessed at baseline and at 8 weeks. Chronic SQ BNP administration resulted in a greater improvement of MLHF as compared to the placebo group at 8 weeks. Specifically, there was a greater decrease of the MLHF score in the BNP group (−9±12) as compared to the placebo group (+1 ±14) (BNP vs. placebo p=0.013). There was a trend for a greater increase in 6 minute walk distance in the BNP group versus placebo (2.2±70 meters vs 0.49±37 meters, p=0.091).
The Effects of SQ BNP on Neurohumoral Parameters
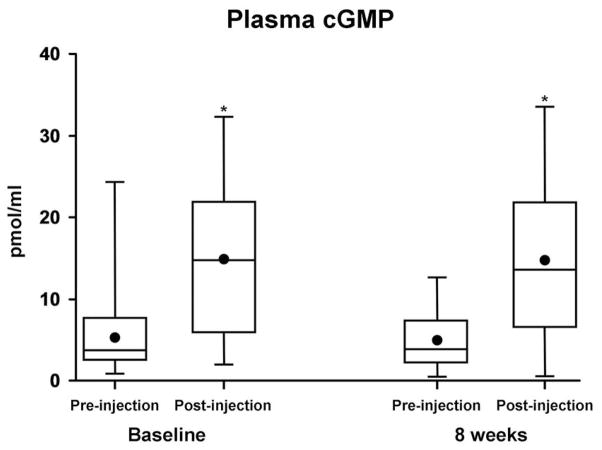
SQ BNP administration resulted in the increase of the second messenger, plasma cGMP levels both at time of first dosing and with the last dosing at 8 weeks (Figure 3). Thus, 8 weeks of twice daily administration of SQ BNP did not result in the development of tolerance to BNP as demonstrated by a similar activation of the second messenger cGMP, after 8 weeks as compared to baseline. There was a greater decrease in plasma renin activity with SQ BNP compared to placebo at 8 weeks (−4±7 vs +2±5, ng/ml/hr, p=0.005).
Figure 3. Plasma cGMP in the BNP group at baseline (*P=0.001) and 8 weeks (*P=0.001), pre-injection and 30 minutes post injection.
The dot within each box represents the mean value while the horizontal line within each box represents the median value and quartiles outside the box. *P value pre-injection versus post-injection
The Effects of SQ BNP on Renal Function
Glomerular filtration rate (GFR) as determined by iothalmate clearance was preserved with SQ BNP (+6.9±14, ml/1.73m2) with a trend towards a greater increase at 8 weeks as compared to placebo (−2.8±25, ml/1.73m2) (P=0.14 BNP vs placebo). In addition, plasma cystatin C trended to decrease in the BNP group (−0.04±0.2 mg/dL, n=16) at 8 weeks while it trended to increase in the placebo group (+0.09±0.2, mg/dL, n=18). (p=0.1 BNP vs placebo.)
Serious Adverse Events and Adverse Events
Serious Adverse Events
Three subjects in the BNP group developed hypotension as defined by systolic BP< 85 mmHg or had symptoms of lightheadedness or visual disturbances during the baseline visit in the CRU and were excluded from the study as per protocol. All subjects recovered with placement at Trendelenburg position and fluid administration without any clinical consequences. One subject in the placebo group had worsening HF symptoms during the 8 week period and withdrew from the study.
Adverse events are reported in Table 2. There was a strong trend for higher incidences of lightheadedness in the BNP group as compared to the placebo group. However, it must be noted that in these patients, it did not result in the discontinuation of the study drug. In the placebo group, more patients developed fatigue and shortness of breath as compared to the BNP group. However, this did not achieve statistical significance.
Table 2.
Adverse Events
| BNP (n = 20) | Placebo ( n = 20) | P value | |
|---|---|---|---|
| Lightheadedness | 3 (15%) | 0 (0%) | 0.07 |
| SOB and Fatigue | 3 (15%) | 7 (35%) | 0.14 |
| Injection site sting/itchy | 4 (20%) | 3 (15%) | 0.68 |
| Flu-like symptoms | 1 (5%) | 2 (10%) | 0.55 |
| Flushing | 3 (15%) | 1 (5%) | 0.29 |
| Loose stools | 2 (10%) | 0 (0%) | 0.15 |
| Atrial Fibrillation | 0 (0%) | 1 (5%) | 0.31 |
| Hyperkalemia | 1 (5%) | 1 (5%) | 1.00 |
| Edema | 1 (5%) | 0 (0%) | 0.31 |
| Other symptoms | 2 (10%) | 2 (10%) | 1.00 |
DISCUSSION
In this proof of concept human study, 8 weeks of protein therapy with SQ BNP administered twice daily improved LV remodeling, diastolic function and clinical symptoms (i.e., MLHF score) in patients with stage C systolic HF on optimal medical therapy. Furthermore, plasma renin activity was suppressed and GFR preserved. Importantly, there was no development of tolerance to chronic SQ BNP administration as evident by a preserved activation of cGMP, its second messenger after 8 weeks of therapy.
Despite recent advances in the treatment of overt symptomatic (Stage C/D) HF, mortality and morbidity among patients with HF remains high. Our study addresses the concept of reverse LV remodeling with chronic administration of the cardiac peptide BNP as novel protein therapy for stage C systolic HF. The rationale for using BNP as a novel protein therapeutic for reducing LV remodeling was also strengthened by our pilot study which reported that a three day infusion of BNP in humans with first time acute anterior acute myocardial infarction (AMI) was associated with reduced LV systolic and diastolic volumes one month following AMI(14).
The current study is the first to report the potential favorable LV remodeling actions of chronic BNP administration in human stage C systolic HF. The degree of LV remodeling seen in the current study is comparable to a previous MRI sub-study of the Metoprolol CR/XL Randomized Intervention Trial in Heart Failure (MERIT-HF). In that sub-study (n=41), Groenning et al reported a reduction in LV end-systolic volume index of −9.7 ml/m2 and LV end-diastolic volume index of −10 ml/m2 at 5–7 weeks after therapy.(15) In the current study, we report a reduction in left LV end-systolic volume index of −5 ±13 ml/m2 and a reduction in LV end-diastolic volume (−10 ±15 ml/m2) in the BNP group after 8 weeks of therapy. In a recent meta-analysis by Kramer et al, they reported that a decrease of 10 ml in the mean change of EDV was associated with a 1.9-fold (95% CI: 1.2 to 3.2, p < 0.012) increased odds that an intervention would have significantly favorable effects on mortality. Furthermore, a decrease of 10 ml in the mean ESV change corresponded to a relative OR of 0.96 for mortality (95% CI: 0.93 to 0.98, p < 0.01). (16) Plasma renin activity was suppressed to a greater extent in the BNP group as compared to the placebo group. The effects of BNP on LV structure may be due to the indirect unloading and diuretic effects of BNP. In the current study, we did not have to reduce the dose of diuretics in any patients. However; we did not have to increase the dose of the diuretics as well. The diuretic and natriuretic actions of BNP may have resulted in decreased filling pressure in the left ventricle contributing to the improved remodeling. Unfortunately, we did not measure the plasma volume of the patients. These findings confirm and extend previous preclinical studies which reported that BNP acting via the natriuretic peptide A receptor (NPR-A) has potent anti-hypertrophic and anti fibrotic properties. Specifically, NPR-A is present on cardiomyocytes (17,18) and incubation with BNP suppresses the response to hypertrophic factors in isolated cardiomyoctyes(19). Further, we and others have demonstrated that cardiomyocyte specific disruption of NPR-A results in exaggerated hypertrophy and fibrosis in response to cardiac stress(20). These studies suggest that the cardiac hormone BNP regulates myocardial structure by direct anti-hypertrophic effects on the cardiomyocyte which complement their effects to reduce load and suppress pro-hypertrophic humoral factors. Further, transcripts for NPR-A are also present in isolated fibroblasts (18)and in-vitro studies have demonstrated that BNP provides broad opposition to fibroblast activation and collagen production(21). Thus, direct actions of BNP on both the cardiomyocyte and the fibroblast may contribute to the anti-hypertrophic and anti-fibrotic effects of BNP. However, without a washout time for the SQ BNP, we cannot exclude the fact that the acute unloading effects of BNP may account for the changes in LV systolic and diastolic volumes. However, assessment of LV Mass index by MRI is not affected by acute loading conditions. Hence, the greater decrease in LV mass index in the BNP group as compared to the placebo group may suggest that SQ BNP has a favorable effect in the LV mass. There was no significant correlation between baseline systolic blood pressure and the changes in LV systolic and diastolic volumes and mass index at 8 weeks.
In the current study, 8 weeks of chronic BNP administration also resulted in an improvement of LV filling pressure as measured by a greater decrease in Doppler E/e′ ratios and LA volume index in the BNP group as compared to the placebo group. Studies in isolated cardiomyocytes preparations report improvement in the speed of myocyte/myocardial relaxation (22) and increases in resting cell length after BNP or cGMP exposure. In in vivo studies in experimental canine HF, we have described the pro-lusitropic effects of BNP (23). Additionally, a key integrated physiological study demonstrated that in patients with isolated diastolic dysfunction, infusion of BNP significantly attenuated the increase in pulmonary capillary wedge pressure during exercise, without an effect on resting hemodynamics (24). These findings suggest possible direct and indirect effects to improve operant diastolic function. Hence, the greater improvement of LV structure and filling pressure in the BNP group may account for the improvement in MLHF scores as compared to the placebo group. The findings of the current study are different from the ASCEND and FUSION studies.(9,10) However, there are major differences in the patient population, route, dose and duration of administration of BNP in the 3 studies. The current study recruited stable NYHA Class 2–3 patients, while the patients in both FUSION and ASCEND were NYHA class 3–4. In the FUSION study, the patients received once a week infusions while in the ASCEND study, they received up to 6 days of infusion. In our current pilot study, the patients received twice a day SQ administration for 8 weeks.
There was no development of tolerance to 8 weeks of twice daily SQ BNP administration as evident by a preserved activation of cGMP, its second messenger. This is consistent with our previous findings in a canine model of HF where we demonstrated that 10 days of SQ BNP administration did not result in the development of tolerance(25). In the current study, we demonstrated that 8 weeks of SQ BNP preserved GFR with a strong trend for GFR to improve and plasma cystatin C to decrease as compared to placebo.
Three (12.5%) of the subjects did not tolerate 10 μ/Kg bolus SQ BNP injections and were excluded from the study as per study protocol. Another 3 (12.5%) subjects developed symptoms of lightheadedness during the 8 week period, which resolved without withdrawal of study drug. One subject in the placebo group withdrew from the study due to worsening HF symptoms and there was a trend for more subjects in the placebo group developing symptoms of worsening shortness of breath or fatigue as compared to the BNP group. Indeed, in the ASCEND trial, there was significantly greater symptomatic and asymptomatic hypotension in the BNP treated group compared to placebo. Therefore, a limiting factor of the use of BNP in our study and others is hypotension. Further studies are required to test lower doses of SQ BNP.
Limitations
This study was designed as a translational proof of concept investigation of the chronic use of twice daily SQ BNP administration in patients with stage C systolic HF and not as a definitive clinical trial as the sample size is small. Furthermore, we only tested a single dose at 10 μ/Kg. Further studies that are adequately powered to evaluate potential clinical efficacy in HF are warranted. Due to limited resources and funding, we were not able to include an active drug run-in period before randomization of the subjects to the treatment groups and hence, we were not able to do an intention to treat analysis which is a limitation of the study design. Because of the use of MRI, none of our patients had a defibrillator or biventricular pacing. With the results from the recently published EMPHASIS Trial(26), our patient population should be treated with spironolactone antagonist; however, only 20% of them were taking a spironolactone antagonist.
In conclusion, this proof of concept pilot study is the first to demonstrate that SQ BNP administration represents a novel, safe and efficacious protein therapeutic strategy in human stage C systolic HF with favorable actions of myocardial structure and function and patient well-being. Further studies are warranted to determine if these functional responses can be translated into improved clinical outcomes contributing to delaying the progression of HF.
ABBREVIATIONS
- BNP
B-Type natriuretic peptide
- SQ
subcutaneous
- MLHF
Minnesota Living with Heart Failure score
- RAAS
renin-angiotensin-aldosterone
- cGMP
3′,5′-cyclic guanosine monophosphate
- ASCEND Trial
Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure
- Cardiac MRI
Cardiac magnetic resonance imaging
Footnotes
ClinicalTrials.gov Identifier: NCT00252187
Disclosures: There are no financial or other relations that could lead to a conflict of interest in the context of this study except for the following: This study, which was principally funded by the American Heart Association, also received some funding from Scios Inc. Mayo Clinic and Drs. Chen, Simari and Burnett have patented and licensed designer natriuretic peptides other than BNP. This research was supported by grants RO1 HL 36634, R01 HL 84155, PO1 HL 76611 and NIH/NCRR CTSA Grant Number UL1 RR024150 from the National Institutes of Health, American Heart Association Scientist Development Grant awarded to Dr Chen and a research grant from Scios Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Chen HH, Burnett JC., Jr Therapeutic potential for existing and novel forms of natriuretic peptides. Heart Fail Clin. 2006;2:365–73. doi: 10.1016/j.hfc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. Jama. 2002;287:1531–40. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 4.Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both! J Am Coll Cardiol. 2007;49:1089–91. doi: 10.1016/j.jacc.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102:17442–7. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang F, O’Rear J, Schellenberger U, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–8. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu H, Masuta K, Aono K, et al. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316:129–35. doi: 10.1016/s0009-8981(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Saltzberg MT, Berkowitz RL, et al. Safety and feasibility of using serial infusions of nesiritide for heart failure in an outpatient setting (from the FUSION I trial) Am J Cardiol. 2004;94:595–601. doi: 10.1016/j.amjcard.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Krum H, Massie BM, et al. Safety and efficacy of outpatient nesiritide in patients with advanced heart failure: results of the Second Follow-Up Serial Infusions of Nesiritide (FUSION II) trial. Circ Heart Fail. 2008;1:9–16. doi: 10.1161/CIRCHEARTFAILURE.108.767483. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 11.Chen HH, Grantham JA, Schirger JA, Jougasaki M, Redfield MM, Burnett JC., Jr Subcutaneous administration of brain natriuretic peptide in experimental heart failure. J Am Coll Cardiol. 2000;36:1706–12. doi: 10.1016/s0735-1097(00)00911-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen HH, Redfield MM, Nordstrom LJ, Horton DP, Burnett JC., Jr Subcutaneous administration of the cardiac hormone BNP in symptomatic human heart failure. J Card Fail. 2004;10:115–9. doi: 10.1016/j.cardfail.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Chen HH, Martin FL, Gibbons RJ, et al. Low-dose nesiritide in human anterior myocardial infarction suppresses aldosterone and preserves ventricular function and structure: a proof of concept study. Heart. 2009;95:1315–9. doi: 10.1136/hrt.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000;36:2072–80. doi: 10.1016/s0735-1097(00)01006-8. [DOI] [PubMed] [Google Scholar]
- 16.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown LA, Nunez DJ, Wilkins MR. Differential regulation of natriuretic peptide receptor messenger RNAs during the development of cardiac hypertrophy in the rat. J Clin Invest. 1993;92:2702–12. doi: 10.1172/JCI116887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin X, Hanze J, Heese F, Sodmann R, Lang RE. Gene expression of natriuretic peptide receptors in myocardial cells. Circ Res. 1995;77:750–8. doi: 10.1161/01.res.77.4.750. [DOI] [PubMed] [Google Scholar]
- 19.Silberbach M, Roberts CT., Jr Natriuretic peptide signalling: molecular and cellular pathways to growth regulation. Cell Signal. 2001;13:221–31. doi: 10.1016/s0898-6568(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 20.Patel JB, Valencik ML, Pritchett AM, Burnett JC, Jr, McDonald JA, Redfield MM. Cardiac-specific attenuation of natriuretic peptide A receptor activity accentuates adverse cardiac remodeling and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2005;289:H777–84. doi: 10.1152/ajpheart.00117.2005. [DOI] [PubMed] [Google Scholar]
- 21.Tsuruda T, Boerrigter G, Huntley BK, et al. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91:1127–34. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 22.Shah AM, Spurgeon HA, Sollott SJ, Talo A, Lakatta EG. 8-bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ Res. 1994;74:970–8. doi: 10.1161/01.res.74.5.970. [DOI] [PubMed] [Google Scholar]
- 23.Lainchbury JG, Burnett JC, Jr, Meyer D, Redfield MM. Effects of natriuretic peptides on load and myocardial function in normal and heart failure dogs. Am J Physiol Heart Circ Physiol. 2000;278:H33–40. doi: 10.1152/ajpheart.2000.278.1.H33. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson PB, Wheeldon NM, MacFadyen RJ, Pringle SD, MacDonald TM. Effects of brain natriuretic peptide on exercise hemodynamics and neurohormones in isolated diastolic heart failure. Circulation. 1996;93:2037–42. doi: 10.1161/01.cir.93.11.2037. [DOI] [PubMed] [Google Scholar]
- 25.Chen HH, Schirger JA, Cataliotti A, Burnett JC., Jr Intact acute cardiorenal and humoral responsiveness following chronic subcutaneous administration of the cardiac peptide BNP in experimental heart failure. Eur J Heart Fail. 2006;8:681–6. doi: 10.1016/j.ejheart.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]