Abstract
Transgenic mice are used increasingly to model brain amyloidosis, mimicking the pathogenic processes involved in Alzheimer's disease (AD). In this chapter, an in vivo strategy is described that has been successfully used to map amyloid-β deposits in transgenic mouse models of AD with magnetic resonance imaging (MRI), utilizing both the endogenous contrast induced by the plaques attributed to their iron content and by selectively enhancing the signal from amyloid-β plaques using molecular-targeting vectors labeled with MRI contrast agents. To obtain sufficient spatial resolution for effective and sensitive mouse brain imaging, magnetic fields of 7-Tesla (T) or more are required. These are higher than the 1.5-T field strength routinely used for human brain imaging. The higher magnetic fields affect contrast agent efficiency and dictate the choice of pulse sequence parameters for in vivo MRI, all addressed in this chapter. Two-dimensional (2D) multi-slice and three-dimensional (3D) MRI acquisitions are described and their advantages and limitations are discussed. The experimental setup required for mouse brain imaging is explained in detail, including anesthesia, immobilization of the mouse's head to reduce motion artifacts, and anatomical landmarks to use for the slice alignment procedure to improve image co-registration during longitudinal studies and for subsequent matching of MRI with histology.
Keywords: MRI, Amyloid-β deposits, Susceptibility, Amyloid-β burden, Amyloidosis, Molecular imaging, Contrast agent, Mouse, Transgenic, 2D, 3D
1. Introduction
Based on the hypothesis that amyloidosis plays a major role in the pathogenesis of Alzheimer's disease (AD), a large number of transgenic mouse lines have been developed to model this aspect of AD (1–6). These transgenic mice are currently being used to study the amyloidosis process in vivo, and to test experimental approaches for clearing amyloid-β (Aβ). Noninvasive, in vivo imaging methods to map the distribution of CNS Aβ deposits in these mice would be very valuable for monitoring Aβ plaque formation and progression and to directly assess the efficacy of plaque clearance therapies. Considerable effort has, therefore, gone toward developing imaging approaches using such diverse methods as positron emission tomography (PET), multiphoton optical microscopy, and magnetic resonance imaging (MRI). MRI provides some distinct advantages for whole brain assessment of Aβ burden, including higher spatial resolution than PET and much greater penetration than multiphoton microscopy. MRI is also much more widely available for future clinical-imaging studies in AD patients. As 7-Tesla (T) human scanners have become widely available, the mouse imaging methods described in this chapter at the same field strength are highly relevant to clinical imaging.
MRI methods to image Aβ plaques in human AD patients are not currently available. It has been suggested that iron concentrated in AD plaques may enable their MRI detection using ultra high magnetic field strength (>7-T) and 40-μm × 40-μm × 40-μm spatial resolution that could only be achieved in postmortem brain tissue from AD patients over several hours of scans (7). This was accomplished using an MR pulse sequence optimized for dark contrast enhancement of lesions that were difficult to reproduce subsequently by Dhenain et al. at 11.7-T (8); an inconsistency likely due to the variable nature in chemical constituents of these plaques. Imaging Aβ lesions in transgenic mouse models of AD, on the other hand, was successfully demonstrated by several groups both ex vivo (9–14) and in vivo (14–24). Results from our laboratories (14) indicate that although some large plaques can be observed in old transgenic mice using susceptibility-induced contrast in T2*-weighted MR images (Fig. 1), the vast majority of plaques in these animals cannot be detected without contrast enhancement with magnetically labeled ligands targeted to Aβ (9, 14, 15, 18, 25, 26).
Fig. 1.
Large Aβ plaques (arrowhead) can be seen in vivo with T2*-weighted 2D multi-slice gradient-echo MRI through subtle signal enhancement (a). Injection of PUT-Gd-DTPA-Aβ1-40 in the same mouse signifi cantly enhances the Aβ plaque after 6 h (b). The matched immunostained histological section confi rms these fi ndings (c, arrowhead).
This chapter describes a method that has been successfully used to map brain Aβ plaques (9, 14, 15, 18, 25, 26) involving high field (7-T) MRI of transgenic mice, injected with magnetically labeled peptides for susceptibility-induced contrast-enhanced mapping of plaques. Details are provided on the peptides and magnetic labeling methods. Although iron-oxide nanoparticles have also been used for magnetic labeling (15, 26), the protocol provided in this chapter is focused on paramagnetic labeling with a chelated form of gadolinium (GdDTPA) which is the smallest (~1 nm in size) and clinically the most widely used contrast agent (27, 28). Nonetheless, the same approach can easily be extended to larger nanoparticles (29–32), such as iron-oxide particles (15, 26).
The experimental setup and protocols required for optimal in vivo detection of Aβ plaques in the mouse brain are detailed using both 2D and 3D MRI acquisition schemes (33) in less than 2 h.
2. Materials
2.1. Labeling of Amyloid-β Peptide
2.1.1. Equipment
ABI 430A peptide synthesizer (AME Bioscience, Chicago, IL).
Vydac C18 preparative column, 2.5 × 30 cm (Vydac Separations, Hesperia, CA).
2.1.2. Supplies
All reagents available from Sigma unless otherwise noted.
Aβ1-40.
K6Aβ1-30-NH2 (Yale University, Keck Peptide Synthesis Facility).
Diethylenetriaminepentaacetic acid (DTPA).
Hydrofluoric acid.
Acetonitrile.
Trifluoroacetic acid.
Gd (III) chloride hexahydrate (Aldrich, Milwaukee, WI).
1 N NaOH.
0.4 M Putrescine in water.
EDC-coupling agent (Pierce Biotechnology, Rockford, IL).
Dialysis membrane (molecular weight cutoff: 2,000 g/mol).
Mannitol.
Phosphate-buffered saline (PBS).
2.2. MR Microimaging System
2.2.1. Equipment
1. MR microimaging (μ MRI) scanner
Preferably, mouse brain imaging experiments should be performed at a magnetic field strength of at least 7-T (see Note 1). The experiments described in this chapter were initially performed with a SMIS console (MRRS, Guildford, UK) that was subsequently upgraded to a Bruker Biospec Avance 2 console interfaced to a 7-T 200-mm horizontal bore magnet (Magnex Scientific, Abingdon, UK) equipped with actively shielded gradient coils (initially Magnex: inner diameter, ID = 120 mm, 250-mT/m gradient strength, 200-μs rise time and then Bruker BGA-9S: ID = 90-mm, 750-mT/m gradient strength, 100-μs rise time). Scanners with very similar performances are also made by other manufacturers such as Agilent Technologies (Varian, Santa Clara, CA, USA).
2. MRI probe
A radiofrequency (RF) coil fitting closely around the mouse's head should be used for brain imaging. In these experiments, we used RF coils dedicated for mouse head imaging that were developed in-house or are commercially available (Doty Scientific, Columbia, SC, USA; M2M Imaging Corp., Cleveland, OH, USA; Rapid MR International, Columbus, OH, USA). The results described in this chapter were produced with coils chosen to resonate at a proton frequency of 300 MHz with their inner diameter (ID) designed to fit closely around the mouse's head. The length (L) along the magnet bore axis for each coil was selected to compromise between high coil sensitivity and magnetic field homogeneity over the mouse brain (see Note 2). For the homemade cylindrically shaped Helmholtz coil shown in Fig. 2c, also referred to as a saddle coil (34), the dimensions were as follow: ID = 22 mm and L = 20 mm. We also designed in-house a quadrature birdcage coil (35, 36) tailored to cover the mouse head and that one can be seen in Fig. 2d (ID = 21.5 mm and L = 29 mm). A circularly polarized Litz coil available commercially (Doty Scientific, Columbia, SC, USA) (37) was also used (ID = 25 mm and L = 22 mm, Fig. 2e).
Fig. 2.
Overview of mouse positioning/handling in the custom holder. (a) Position the mouse head in the bite bar and maintain the head immobilized with hand until taping the tail to stabilize the animal. (b) Insert the nosecone to ensure confi ned anesthetic delivery. (c) The mouse holding platform incorporates the RF coil (saddle coil shown) with tooth bar, a nosecone for isofl urane delivery via a vaporizer/scavenger system and a monitoring system measuring rectal temperature, blood pulse, and respiration rate. Multi-rung coils that have in general more homogenous RF fi eld can also be considered such as (d) the birdcage coil (here designed in-house) or (e) the litzcage coil, in this example purchased from Doty Scientifi c (Columbia, SC, USA).
3. Mouse holder
The rf coil should be incorporated into a holder that stabilizes the mouse's head during MRI and can be fitted with devices for gas anesthesia delivery and physiological monitoring. MR compatible mouse holders are becoming available from commercial vendors of small animal MRI systems as well as rf coils, but most reports to date have used custom-holding devices. We have developed our own holder, incorporating the mouse head coil, a nose cone for isoflurane anesthesia, and several physiological-monitoring devices (Fig. 2; see Note 3). The main design goal of the mouse holder should be to hold the head in a stationary and reproducible position during the 2–3 h that the animal must be maintained inside the magnet. Predictably, the design closely resembles a stereotaxic injection device, but is fabricated from nonmetallic MRI compatible materials (see Note 4). The head holder should be equipped with a calibrated tooth bar allowing enough vertical and horizontal range (5–10 mm) to center any brain region of interest within the RF coil. Ear bars would be helpful to further stabilize the mouse head, but most RF coil designs are not open structures, and it is difficult to incorporate ear bars within the close-fitting head coil.
4. Gas anesthesia
Isoflurane vaporizer/anesthesia machine (VMS Matrix Medical, Orchard Park, NY).
5. Surgical microscope
(M650, Wild, Heerbrugg, Switzerland or M320F12, Leica Microsystems, Wetzlar, Germany).
6. Syringe pump
PHD2000 computer-controlled syringe pump (Harvard Apparatus, Hollison, MA).
2.2.2. Supplies
Isoflurane (Aerane, Baxter, Deerfield, IL).
Surgical supplies (available from any surgical supplies vendor): small sharp dissection scissors; two pairs of #5 Dumont forceps; 5-0 silk suture; 30-gauge needle; 70% ethanol for cleaning instruments.
Cannulae for infusing magnetically labeled peptides: Polyethylene tubing PE-10 (Intramedic, Becton Dickinson, Parsippany, NJ), Inner diameter ID = 0.28 mm (0.011″) and Outer diameter OD = 0.61 mm (0.024″).
3. Methods
3.1. Labeling of Amyloid-β Peptide
The MRI ligands described in this chapter are experimental probes that can be used as control compounds for subsequent animal studies using novel probes. Intact Aβ1-40 is unlikely to be used in humans because of its well-documented intrinsic toxicity that may not be noticeable in acute studies. We are currently developing more soluble nontoxic Aβ derivatives, such as K6Aβ1-30, that are likely to be more suitable as MRI probes for determining brain Aβ load in patients (14).
3.1.1. Peptide Synthesis
MR imaging ligand based on Aβ1-40 or K6Aβ1-30 is synthesized on a ABI 430A peptide synthesizer using standard protocols for tBOC (tert-butyloxycarbonyl) chemistry, attaching DTPA to the amino terminus of the peptide as the final step of synthesis.
The peptides are cleaved from the resins using hydrofluoric acid and purification is performed by high pressure liquid chromatography (HPLC) on a Vydac C18 preparative column, using linear gradients from 0 to 70% of acetonitrile in 0.1% trifluoroacetic acid.
3.1.2. Gadolinium Chelation
Gadolinium (Gd) is chelated to DTPA-Aβ1-40 or DTPA-K6Aβ1-30 by incubating the peptide in water or acetonitrile solution at pH 7.0 for 24 h with threefold molar excess of Gd, derived from Gd (III) chloride hexahydrate. For example, if 5 mg of peptide is labeled, the reaction can be performed in 1 ml total volume of 10% acetonitrile (see Note 5). The pH of the solution can be adjusted with a few microliter of 1 N NaOH and monitored with pH test strips. Mass spectroscopy of the lyophilized end-product, Gd-DTPA-Aβ1-40, can be used to verify the expected molecular weight (4,976.6 g/mol).
3.1.3. Putrescine Labeling
For attaching putrescine to Gd-DTPA-Aβ1-40 to increase its blood–brain barrier (BBB) permeability, we have followed the protocol of Poduslo et al. (9) with some minor modifications.
Gd-DTPA-Aβ1-40 (10 mg) is dissolved in 1 ml of 0.4 M putrescine, pH 4.7, and added to a solution of 1.4 g EDC in 1 ml of 0.4 M putrescine, pH 4.7.
This solution is mixed at room temperature for 4 h and subsequently dialyzed (molecular weight cut off: 2,000 g/mol) at 4°C for 2 days to remove excess putrescine and EDC (see Note 6).
Following dialysis, the peptide is relabeled with Gd, as described above for DTPA-Aβ1-40. The resulting Put-Gd-DTPA-Aβ1-40 can be injected intravenously to label amyloid plaques (9).
3.1.4. Mannitol Co-injection
Gd-DTPA-Aβ1-40 does not cross the BBB alone, and must either be modified with compounds such as putrescine (see above) or co-injected into the carotid artery with mannitol. For mannitol co-injection, 400 μg of Gd-DTPA-Aβ1-40 should be suspended in 100 μl of water, and then dissolved in 600 μl of 15% mannitol in PBS immediately before infusion.
3.2. Mouse Preparation
- Catheter construction
- Heat the PE10 polyethylene tubing using either a heat gun or heated oil and stretch to further reduce its diameter from OD = 0.61 mm to an approximate OD of 0.25 mm.
- Cut the tubing to the length required for the syringe pump (see Note 7). The tapered end of the catheter will be inserted into either the artery or the vein during surgery.
Anesthetize the mouse with isoflurane: 5% isoflurane in air for 3 min to induce anesthesia, followed by 1–1.5% isoflurane in air to maintain anesthesia.
The skin is shaved and cleaned with 70% ethanol.
Cut with fine scissors, either on the neck to expose the common carotid artery or on the inside of the thigh to expose the femoral vein.
Under a surgical microscope, the vessel of interest is identified and a small section freed from the overlying muscle tissue with two pairs of fine forceps.
For injection into the common carotid artery (CCA), a 5-0 silk suture is tied loosely at the cephalic end of the right common artery and an identical suture is ligated at its central portion. Between the ligations, a puncture is made with a 30-gauge needle. A modified PE-10 tubing, attached to a 1-cc syringe filled with labeled peptide, is introduced into the right CCA through the small puncture. The suture at the cephalic CCA is then tightened around the intraluminal catheter to prevent bleeding. During injection, the left CCA is temporarily clamped with a microvascular clip.
For injection into the femoral vein, a small hole is made in the vein with a 30-gauge needle, and the modified PE-10 tubing subsequently inserted.
The Gd-DTPA-Aβ1-40/mannitol mixture (600–700 μl) is injected into the carotid artery at a rate of 60 μl/min, while a similar volume of Put-Gd-DTPA-Aβ1-40 is injected at the same rate into the femoral vein. In either case, the injection takes approximately 10 min.
After injection into the right CCA, the microvascular clip is removed, the catheter is withdrawn from the right CCA and the cephalic CCA is ligated. The puncture is subsequently sealed with Krazy® glue. The CCA can then be unligated and with the blood flow restored the wound is closed with suture.
After injection into the femoral vein, the PE-10 tubing is withdrawn and the puncture is temporarily sealed with a small cotton ball to stop bleeding and the overlying skin then sutured.
Mice recover consciousness immediately after removal from anesthesia and should be kept warm until regaining full mobility.
3.3. In Vivo Two-Dimensional Interleaved Multi-slice MR Brain Imaging
2D MRI schemes by their nature are more easily implementable to both gradient echo (GE)- and spin-echo (SE)-based sequences due to the flexibility and ease of adjustment of the parameters. Their use became quickly widespread with the introduction of multiplexing (38, 39) rendering them equally sensitive to their 3D counterpart while offering more possibilities on the tradeoffs between the slice thickness, slice coverage, and the minimum imaging time required (40, 41). In fact, 2D MRI is almost indispensible when a conventional spin-echo sequence is considered due to its slower efficiency (15–17, 42).
- Mouse setup:
- After locking the upper inscissors in the tooth bar, press gently with the index finger just above the nose to avoid unhooking the teeth while pulling the tail taut and immobilizing it with tape (Fig. 2; see Note 9). This simple approach has proved to be very efficient for reducing motion artifacts and has provided high-quality MR brain images (Figs. 1 and 4) (15, 18).
- Slice alignment:
- Three single 1-mm thick slices are acquired simultaneously as orthogonal pilot orientations to achieve accurate image alignment after few iterations, ensuring accurate matching to histology and reproducibility between imaging sessions during longitudinal studies (Fig. 3). Pilot scans are low resolution MR images, acquired within several minutes at most, and providing anatomical landmarks adequate to specify the final slice alignment for the high resolution image acquisition. For Aβ plaque imaging in transgenic mice, we acquire multi-slice MR images in the coronal orientation. The mid-sagittal pilot image is used to align the final slices, checking first to ensure that the pilot is well aligned by verifying that the midline blood vessels are obvious throughout the image.
- Two “notches” are then identified, anteriorly between the olfactory bulb and the frontal cortex, and posteriorly at the junction of the cerebellum and midbrain (Fig. 3).
- The final image slices are placed perpendicular to the line between these two notches, checking for symmetric orientation also on the horizontal pilot image.
High-resolution MRI acquisition: We determined that Aβ plaques could be better detected with T2*-weighted MRI, 4–6 h after injection of Gd-DTPA-Aβ1-40 and mannitol (15, 18). For each brain, 31 contiguous coronal image slices are acquired from the frontal cortex to the cerebellum, with 78 μm × 78 μm in-plane resolution using a gradient-echo sequence (TE = 15 ms; TR = 1.5 s; flip angle = 55°; slice thickness = 250 μm; total imaging time = 59 min). This sequence provided good anatomical detail and soft-tissue contrast, with sufficient susceptibility-induced contrast to detect Gd accumulation in plaques in the brains of the transgenic mice (Fig. 4) (15, 18).
Fig. 4.
Aβ plaques were detected with in vivo μMRI, 6 h after injection of Gd-DTPA-Aβ1-40 and 15% mannitol. In vivo T2*-weighted 2D multi-slice gradient-echo coronal μMR images show control (a) and transgenic (b) mouse brains. Many μMRI lesions could be matched to immunostained Aβ plaques (c, arrowheads), seen more clearly in the higher-magnification insets. High-power microscopic examination of the immunostained regions revealed that they were indeed parenchymal Aβ plaques, and not blood vessels or vascular amyloid (inset, corresponding to the region marked by an asterisk). bv Blood vessel (reprinted with permission from Wadghiri et al., Magn Reson Med. 2003;50(2):293–302, Wiley InterScience).
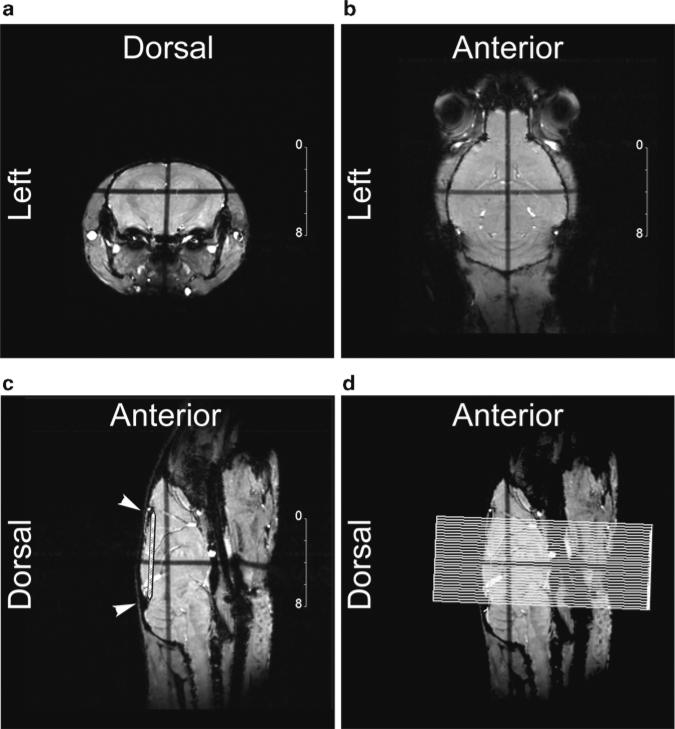
Fig. 3.
Orthogonal pilot images (1 mm thick) are acquired corresponding to the coronal (a), horizontal (b), and sagittal (c) orientations (in-plane resolution ranging from 100 to 120 μm), obtained over several minutes using an iterative alignment process. The crossed dark stripes seen in all three images are induced by the saturation effect of the slice inter-crossing acquired simultaneously, and help in slice positioning. The sagittal pilot orientation (c) provides easily identifi able landmarks for the alignment of 2D multi-slice coronal images. A baseline (white striped segment) is drawn from the two anatomical notches indicated by the white arrowheads in (c). Image (d) shows the resulting coronal slice grid, placed orthogonally to the baseline and overlaid on the sagittal pilot.
3.4. In Vivo Three-Dimensional MR Brain Imaging with 100 μm Isotropic Acquisition
As previously described, 2D sequences provide some distinct advantages in their flexibility between the choice of slice thickness, slice coverage, and the minimum imaging time required (40, 41). However, when narrow slices are needed, they become quickly limited by the gradient strength available.
Additionally, cross-talk due to the multi-slice overlap can be another source of potential artifacts or a loss of signal-to-noise (SNR) likely more noticeable in spin-echo sequence and these issues may require the introduction of a slice gap. In contrast, in 3D sequences each acquisition represents an average of the entire sampled volume acquired through a slab that is less demanding in gradient strength. When combined with fast gradient-echo imaging sequences such as fast low-angle shot (FLASH) (3), whole 3D mouse head datasets can be practically implemented, with a 100 μm isotropic resolution, in less than 2 h as described below. This eases both the co-registration of serial MRI scans and with histologically stained sections for retrospective realignment using either the public domain program ImageJ (NIH Image, NIH, Bethesda, Maryland, USA) or commercially available imaging packages such as Analyze (AnalyzeDirect, Inc., Overland Park, KS, USA) or Amira (Visage Imaging GmbH, Berlin, Germany).
The mouse setup and slice alignment is identical to the 2D protocol in Subheading 3.3. Briefly, anesthetize the mouse with isoflurane and then proceed with identical care as described in the 2D protocol to properly secure the mouse in the holder before MRI to avoid potential motion artifact (Fig. 2; see Note 8). This will ease the co-registration during serial scans and with histology. At the final stage of the high resolution image acquisition for Aβ plaque imaging in transgenic mice, the slab of the 3D sequence is oriented along the dorso-ventral direction to minimize the wrap around by acquiring the horizontal orientation. Although the same two “notches” can be used as landmark to achieve a straightforward co-registration right after the acquisition when alignment is properly done, this stage becomes less critical since it can be replaced by virtual realignment retrospectively (Fig. 5).
- High-resolution MRI acquisition: 4–6 h after injection of Gd-DTPA-Aβ1-40 and mannitol (15, 18) or Gd-DTPA-K6Aβ1-30 and mannitol (14), a 3D Spoiled Gradient Recalled (3D-SPGR) sequence is acquired with 100 μm × 100 μm × 100 μm isotropic resolution using either a single or a four echo train as follow:
- Single echo: FOV: 2.56 × 2.56 × 2.56 cm, Matrix: 256 × 256 × 256, TE: 15 ms, TR: 50 ms, FA: 20, NA: 2, BW: 195.312 Hz/pixel (50 KHz total bandwidth) resulting in an effective scan time: 1 h 50 min.
- Multi-echo: FOV: 2.56 × 2.56 × 2.56 cm, Matrix: 256 × 256 × 256, TE: 4.07 ms, echo spacing (ES): 6.7 ms, TR: 65 ms, FA: 20, NA: 2, BW: 195.312 Hz/pixel (50 KHz total bandwidth). Scan time: 2 h 21 min.
Fig. 5.
The three orthogonal brain orientations in the mouse are shown as follow (a) coronal, (b) sagittal, and (c) horizontal. All three orientations were obtained from the same mouse head dataset acquired using a 3D MRI sequence (voxel resolution = 100 μm × 100 μm × 100 μm) shown in (d) as a 3D volume rendering in which both eyeballs can be seen. The bregma and lambda are two points that are easily identifi able on the dorsal surface of the mouse skull. Both landmarks are generally used as reference points in mouse brain atlases in which the bregma is the zero coordinate (Paxinos G. and Franklin K.B.J, the mouse brain in Stereotaxic corordinates, Elsevier, ISBN). The acquisition of 3D dataset with 100 μm isotropic resolution enables retrospective realignment of the slice sections in any desired orientation without compromising the quality of the resulting images. The co-registration with subsequent histology from the same mouse brain can then be easily achieved.
Both sequences provide good anatomical detail and soft-tissue contrast, with sufficient susceptibility-induced contrast to detect Gd accumulation in plaques in the brains of the transgenic mice (Fig. 6) (14). The multi-echo sequence can be very advantageous for objectively quantifying the Aβ burden as we previously demonstrated (25, 26).
-
2
Following in vivo MRI, the mice should be intra-cardially or intra-aortically perfused with PBS followed by 4% paraformaldehyde in PBS.
-
3
Extract brain and submit to fixation by immersion in 4% paraformaldehyde in PBS overnight at 4°C before considering sectioning for subsequent co-registration and validation. See Chapter 28 for details on tissue processing and staining.
Fig. 6.
In vivo Aβ plaque visualization is possible through endogenous detection. (a) A few large plaques can be identifi ed in this 20-month-old Tg2576 mouse (see arrows) and can most likely be attributed to higher iron content especially in the larger and more mature plaques. The same transgenic mouse was subsequently imaged few weeks later to insure safe recovery and reduce the anesthetic burden. (b) Following intracarotid infusion of Gd-DTPA-K6Aβ1-30 co-injected with mannitol, the number of visualized plaques is substantially increased. These plaques by MRI co-register very well with plaques detected with 6E10/4G8 antibody on histological sections as depicted in (c) (reprinted with permission from Sigurdsson et al., Neurobiology of Aging 2008;29(6):836–847, Elsevier).
3.5. Co-registration of MRI and Histology
Using the slice alignment procedure described above, excellent co-registration of MRI and histology can be obtained by blocking the sample, dorsal side down, and cutting coronal sections vertically across the block in the same orientation as the MR image slices. Take care to align the brain in the same manner as for MRI before sectioning.
Acknowledgments
The research described in this chapter was supported by grants from the NIH (AG020197 and AG032611 to EMS; AG20245, and AG008051 to TW, the Alzheimer's Association (IIRG-08-91618 to YZW, ZEN-08-91006 to EMS), the American Health Assistance Foundation (ADR-A2008-155 to YZW). We thank Yongsheng, Li and Jeffrey A. Blind and Amr Morsi for assistance with the surgical protocols. We also thank Dr Florence Janody for artistic help with Fig. 2.
Footnotes
Static field strength: The diversity of magnet strengths available for MRI studies raises questions about which field strength is optimal. In most centers, MRI studies will be performed on whatever magnet is available for small animal imaging. However, if there is a choice of field strength, the following factors should be considered. MRI sensitivity, generally measured as the SNR ratio, is linearly proportional to the magnetic field strength. Therefore, increasing the field strength will enable imaging with increased spatial resolution and/or a reduction in the MRI acquisition time. Currently for mouse brain imaging, magnetic field strengths ≥7-T enable in-plane resolution better than 100 μm and slice thickness lower than 500 μm using acquisition times of 1–2 h. MR image contrast, usually a function of relaxation times T1 and T2, also depends on magnetic field strength. Although a full discussion of MR image contrast is beyond the scope of this chapter, relevant to Aβ plaque MRI is the fact that T1 differences are reduced at higher fields, and as a consequence T1-weighted MRI is less efficient and requires longer acquisition times. On the other hand, T2- and T2*-weighted sequences are more sensitive to small changes in magnetic properties of tissues at high field, and we and others have demonstrated more sensitive detection of magnetically tagged amyloid using T2- and T2*-weighted sequences, compared to T1-weighted MRI (14, 15, 18, 25).
Rf coil: For the saddle coil, we employed a linear coil design with a relatively simple tuning and impedance matching circuitry to facilitate rapid optimization of the coil sensitivity during the MRI setup. Although this design did not take advantage of the theoretical gain from a quadrature mode (36), it proved very efficient at high fields thanks to its simplified structure. Both the birdcage designed in-house and the commercial Litzcage coil are circularly polarized providing a quadrature detection.
Physiological monitoring: We have incorporated several devices, available in a computer-controlled system (MP100, Biopac Systems, Inc., Goleta, CA) in our mouse holder, including a rectal temperature probe, an infrared plethysmograph placed around the tail to monitor the blood pulse rate, and an airfilled cushion/pressure transducer placed under the mouse body to measure respiration rate. A respiration monitor is required to implement respiration-gated acquisition, although we have found that motion artifacts can be avoided with the mouse setup described in this chapter, without the need for gated acquisition. A similar MRI compatible setup from Small Animal instruments, Inc. (Stony Brook, NY, USA) can also be considered for effective physiological monitoring in combination with the various sensors described previously.
MR compatible materials: Plastics are generally used to construct animal holders for MRI: acrylic, polycarbonate, nylon, PVC, or delrin are all good choices. Metallic materials should be avoided except in the coil itself and must be limited to non-ferromagnetic metals, e.g., copper, aluminum, silver, and platinum.
Performing this reaction in a buffer should be avoided because ions may interfere with the labeling. Removal of the excess Gd by various means is likely to be accompanied by a loss of chelator bound Gd. Although free Gd can be toxic to mice, the animals seemed to tolerate well Gd-DTPA-Aβ1-40 that was not purified following peptide labeling with threefold molar excess of Gd. The solubility of DTPA-Aβ1-40 depends on the lot of peptide, and the chelation can be performed in 10–20% acetonitrile in water if needed. Some precipitation can occur when the Gd solution is added, and/or following overnight labeling at 4°C. We have not analyzed the composition of the precipitate, but it can be removed by centrifugation prior to lyophilization or injection. DTPA-K6Aβ1-30 is much more soluble than DTPA-Aβ1-40, and its chelation to Gd can be performed in water.
In the original procedure by Poduslo et al. (9), the pH is maintained at pH 4.7 during the incubation period. This should promote putrescine coupling but we have obtained similar mass spectroscopy profile when the pH has not been kept constant during the reaction. The disadvantage of this method is that numerous peaks of similar molecular weight are obtained, indicating a mixture of DTPA-Aβ1-40 peptides with different numbers of attached putrescine molecules. We have also noticed that with one lot of DTPA-Aβ1-40, substantial precipitate formed during the reaction that reduced its yield. To avoid these issues, it may be better to label amino acids prior to peptide synthesis with putrescine or any other ligand that enhances brain uptake.
When cutting the polyethylene tubing, use a sharp scalpel blade or razor blade to avoid distorting/crushing the material. Do not use a pair of scissors.
Noticeable image artifacts (blurring) can arise from breathing motion in the neck and caudal head, which will make Aβ plaque detection impossible if not corrected. These artifacts can be greatly reduced if the mouse is properly secured in the holder as described in Subheading 3 (Fig. 2).
The whole body should remain lightly stretched during MRI, enabling the mouse to breathe freely while greatly reducing head motion from breathing.
References
- 1.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science (New York, NY) 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 3.McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 2006;22:281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Ashe KH, Zahs KR. Probing the biology of Alzheimer's disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obulesu M, Rao DM. Animal models of Alzheimer's disease: an understanding of pathology and therapeutic avenues. The International Journal of Neuroscience. 2010;120:531–537. doi: 10.3109/00207451003760080. [DOI] [PubMed] [Google Scholar]
- 6.Wisniewski T, Sigurdsson EM. Murine models of Alzheimer's disease and their use in developing immunotherapies. Biochimica et Biophysica Acta. 2010;1802:847–859. doi: 10.1016/j.bbadis.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benveniste H, Einstein G, Kim KR, Hulette C, Johnson GA. Detection of neuritic plaques in Alzheimer's disease by magnetic resonance microscopy. Proc. Natl. Acad. Sci. of the United States of America. 1999;96:14079–14084. doi: 10.1073/pnas.96.24.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhenain M, Privat N, Duyckaerts C, Jacobs RE. Senile plaques do not induce susceptibility effects in T2*-weighted MR microscopic images. NMR Biomed. 2002;15:197–203. doi: 10.1002/nbm.760. [DOI] [PubMed] [Google Scholar]
- 9.Poduslo JF, Wengenack TM, Curran GL, Wisniewski T, Sigurdsson EM, Macura SI, Borowski BJ, et al. Molecular targeting of Alzheimer's amyloid plaques for contrast-enhanced magnetic resonance imaging. Neurobiol Dis. 2002;11:315–329. doi: 10.1006/nbdi.2002.0550. [DOI] [PubMed] [Google Scholar]
- 10.Helpern JA, Lee SP, Falangola MF, Dyakin VV, Bogart A, Ardekani B, Duff K, et al. MRI assessment of neuropathology in a transgenic mouse model of Alzheimer's disease. Magn Reson Med. 2004;51:794–798. doi: 10.1002/mrm.20038. [DOI] [PubMed] [Google Scholar]
- 11.Lee SP, Falangola MF, Nixon RA, Duff K, Helpern JA. Visualization of beta-amyloid plaques in a transgenic mouse model of Alzheimer's disease using MR microscopy without contrast reagents. Magn Reson Med. 2004;52:538–544. doi: 10.1002/mrm.20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Yarowsky P, Gordon MN, Di Carlo G, Munireddy S, van Zijl PC, Mori S. Detection of amyloid plaques in mouse models of Alzheimer's disease by magnetic resonance imaging. Magn Reson Med. 2004;51:452–457. doi: 10.1002/mrm.10730. [DOI] [PubMed] [Google Scholar]
- 13.Dhenain M, Delatour B, Walczak C, Volk A. Passive staining: a novel ex vivo MRI protocol to detect amyloid deposits in mouse models of Alzheimer's disease. Magn Reson Med. 2006;55:687–693. doi: 10.1002/mrm.20810. [DOI] [PubMed] [Google Scholar]
- 14.Sigurdsson EM, Wadghiri YZ, Mosconi L, Blind JA, Knudsen E, Asuni A, Scholtzova H, et al. A non-toxic ligand for voxel-based MRI analysis of plaques in AD transgenic mice. Neurobiol Aging. 2008;29:836–847. doi: 10.1016/j.neurobiolaging.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadghiri YZ, Sigurdsson EM, Sadowski M, Elliott JI, Li Y, Scholtzova H, Tang CY, et al. Detection of Alzheimer's amyloid in transgenic mice using magnetic resonance microimaging. Magn Reson Med. 2003;50:293–302. doi: 10.1002/mrm.10529. [DOI] [PubMed] [Google Scholar]
- 16.Jack CR, Jr., Garwood M, Wengenack TM, Borowski B, Curran GL, Lin J, Adriany G, et al. In vivo visualization of Alzheimer's amyloid plaques by magnetic resonance imaging in transgenic mice without a contrast agent. Magn Reson Med. 2004;52:1263–1271. doi: 10.1002/mrm.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Jr., Wengenack TM, Reyes DA, Garwood M, Curran GL, Borowski BJ, Lin J, et al. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer's transgenic mice. J Neurosci. 2005;25:10041–10048. doi: 10.1523/JNEUROSCI.2588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigurdsson EM, Wadghiri YZ, Sadowski M, Elliott JI, Li Y, Scholtzova H, Tang CY, et al. In vivo magnetic resonance of amyloid plaques in Alzheimer's disease model mice. In: Hyman B, Demonet JF, Christen Y, editors. The living brain and Alzheimer's disease. Hardcover ed. Springer Verlag; Berlin: 2004. pp. 47–59. [Google Scholar]
- 19.Higuchi M, Iwata N, Matsuba Y, Sato K, Sasamoto K, Saido TC. 19F and 1H MRI detection of amyloid beta plaques in vivo. Nat Neurosci. 2005;8:527–533. doi: 10.1038/nn1422. [DOI] [PubMed] [Google Scholar]
- 20.Vanhoutte G, Dewachter I, Borghgraef P, Van Leuven F, Van der Linden A. Noninvasive in vivo MRI detection of neuritic plaques associated with iron in APP(V717I) transgenic mice, a model for Alzheimer's disease. Magn Reson Med. 2005;53:607–613. doi: 10.1002/mrm.20385. [DOI] [PubMed] [Google Scholar]
- 21.Braakman N, Matysik J, van Duinen SG, Verbeek F, Schliebs R, de Groot HJ, Alia A. Longitudinal assessment of Alzheimer's beta-amyloid plaque development in transgenic mice monitored by in vivo magnetic resonance microimaging. J Magn Reson Imaging. 2006;24:530–536. doi: 10.1002/jmri.20675. [DOI] [PubMed] [Google Scholar]
- 22.Borthakur A, Gur T, Wheaton AJ, Corbo M, Trojanowski JQ, Lee VM, Reddy R. In vivo measurement of plaque burden in a mouse model of Alzheimer's disease. J Magn Reson Imaging. 2006;24:1011–1017. doi: 10.1002/jmri.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faber C, Zahneisen B, Tippmann F, Schroeder A, Fahrenholz F. Gradient-echo and CRAZED imaging for minute detection of Alzheimer plaques in an APPV717I × ADAM10-dn mouse model. Magn Reson Med. 2007;57:696–703. doi: 10.1002/mrm.21201. [DOI] [PubMed] [Google Scholar]
- 24.Muskulus M, Scheenstra AE, Braakman N, Dijkstra J, Verduyn-Lunel S, Alia A, de Groot HJ, et al. Prospects for early detection of Alzheimer's disease from serial MR images in transgenic mice. Current Alzheimer Research. 2009;6:503–518. doi: 10.2174/156720509790147089. [DOI] [PubMed] [Google Scholar]
- 25.Scholtzova H, Wadghiri YZ, Douadi M, Sigurdsson EM, Li YS, Quartermain D, Banerjee P, et al. Memantine leads to behavioral improvement and amyloid reduction in Alzheimer's-disease-model transgenic mice shown as by micromagnetic resonance imaging. Journal of Neuroscience Research. 2008;86:2784–2791. doi: 10.1002/jnr.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Zaim Wadghiri Y, Minh Hoang D, Tsui W, Sun Y, Chung E, Li Y, et al. Detection of amyloid plaques targeted by USPIO-Abeta1–42 in Alzheimer's disease transgenic mice using magnetic resonance microimaging. Neuroimage. 2011;55(4):1600–1609. doi: 10.1016/j.neuroimage.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinmann HJ, Brasch RC, Press WR, Wesbey GE. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol. 1984b;142:619–624. doi: 10.2214/ajr.142.3.619. [DOI] [PubMed] [Google Scholar]
- 28.Weinmann HJ, Laniado M, Mutzel W. Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiological chemistry and physics and medical NMR. 1984a;16:167–172. [PubMed] [Google Scholar]
- 29.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 30.Mulder WJ, Griffioen AW, Strijkers GJ, Cormode DP, Nicolay K, Fayad ZA. Magnetic and fluorescent nanoparticles for multimodality imaging. Nanomedicine (London, England) 2007;2:307–324. doi: 10.2217/17435889.2.3.307. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui TS, Jani A, Williams F, Muller RN, Vander Elst L, Laurent S, Yao F, et al. Lanthanide complexes on Ag nanoparticles: designing contrast agents for magnetic resonance imaging. Journal of Colloid and Interface Science. 2009;337:88–96. doi: 10.1016/j.jcis.2009.04.096. [DOI] [PubMed] [Google Scholar]
- 32.Wadghiri YZ, Briley-Saebo K. Nanobiomaterials for Preclinical Studies and Clinical Diagnostic. In: Sitharaman B, editor. Nanobiomaterials Handbook. Hardback ed. CRC Press; New York: 2011. pp. 1–24. [Google Scholar]
- 33.Johnson G, Zaim Wadghiri Y, Turnbull DH. Sensitivity in 2D multislice and 3D MR imaging. Magn Reson Med. 2003;49(5):848–855. [Google Scholar]
- 34.Hoult DI, Richards RE. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J Magn Reson. 1976:71–85. doi: 10.1016/j.jmr.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Hayes CE, Edelstein WA, Schenck JF, O.M. M, Eash M. An efficient, highly homogeneous radiofrequency coil for whole-body NMR imaging at 1.5T. J Mag Reson. 1985;63:622–628. [Google Scholar]
- 36.Glover GH, Hayes CE, Pelc NJ, Edelstein WA, Mueller OM, Hart HR, O'Donnell M, et al. Comparison of linear and circular polarization for magnetic resonance imaging. J Mag Reson. 1985;64:255–270. [Google Scholar]
- 37.Doty FD, Entzminger G, Jr., Hauck CD. Errortolerant RF litz coils for NMR/MRI. J Magn Reson. 1999;140:17–31. doi: 10.1006/jmre.1999.1828. [DOI] [PubMed] [Google Scholar]
- 38.Crooks LE, Ortendahl DA, Kaufman L, Hoenninger J, Arakawa M, Watts J, Cannon CR, et al. Clinical efficiency of nuclear magnetic resonance imaging. Radiology. 1983;146:123–128. doi: 10.1148/radiology.146.1.6849032. [DOI] [PubMed] [Google Scholar]
- 39.Crooks L, Arakawa M, Hoenninger J, Watts J, McRee R, Kaufman L, Davis PL, et al. Nuclear magnetic resonance whole-body imager operating at 3.5 KGauss. Radiology. 1982;143:169–174. doi: 10.1148/radiology.143.1.7063722. [DOI] [PubMed] [Google Scholar]
- 40.Brunner P, Ernst RR. Sensitivity and performance time in NMR imaging. J Magn Reson. 1979:83–106. [Google Scholar]
- 41.Johnson G, Wadghiri YZ, Turnbull DH. 2D multislice and 3D MRI sequences are often equally sensitive. Magn Reson Med. 1999;41:824–828. doi: 10.1002/(sici)1522-2594(199904)41:4<824::aid-mrm23>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Jack CR, Jr., Marjanska M, Wengenack TM, Reyes DA, Curran GL, Lin J, Preboske GM, et al. Magnetic resonance imaging of Alzheimer's pathology in the brains of living transgenic mice: a new tool in Alzheimer's disease research. Neuroscientist. 2007;13:38–48. doi: 10.1177/1073858406295610. [DOI] [PubMed] [Google Scholar]