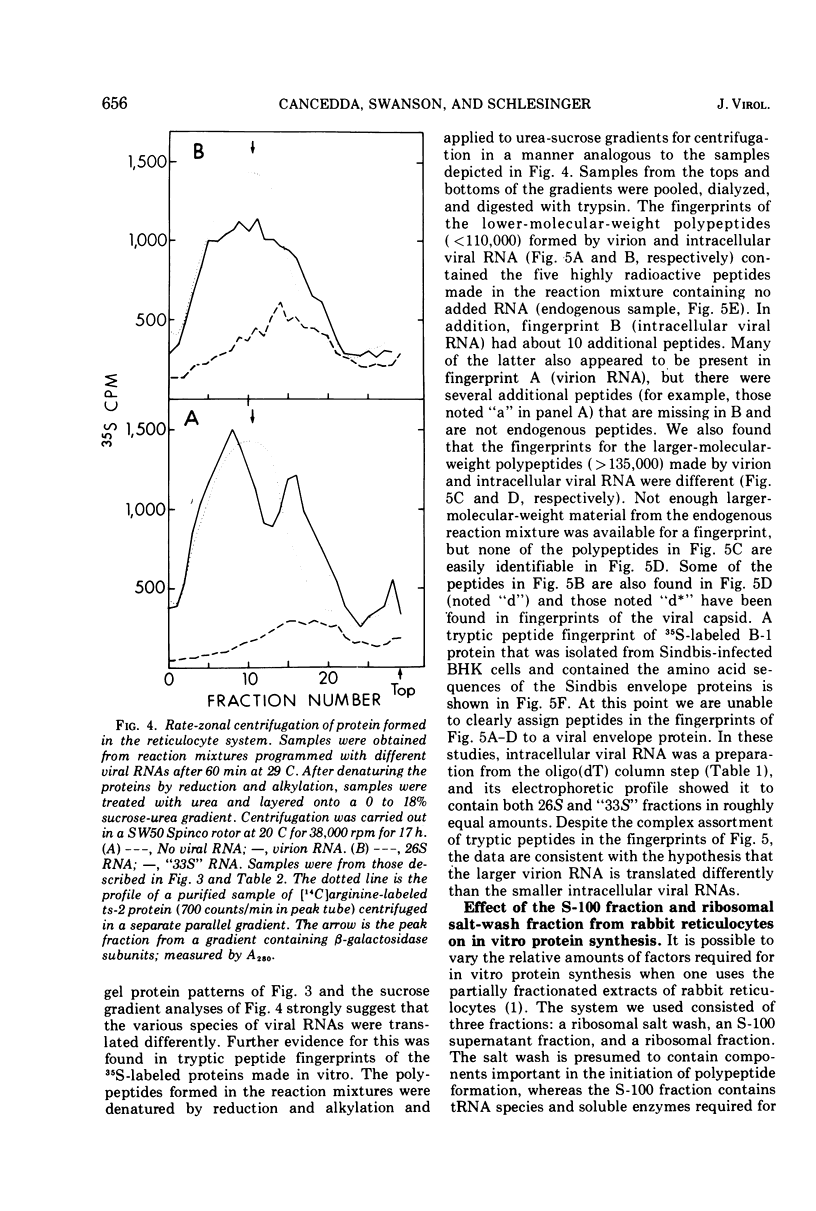
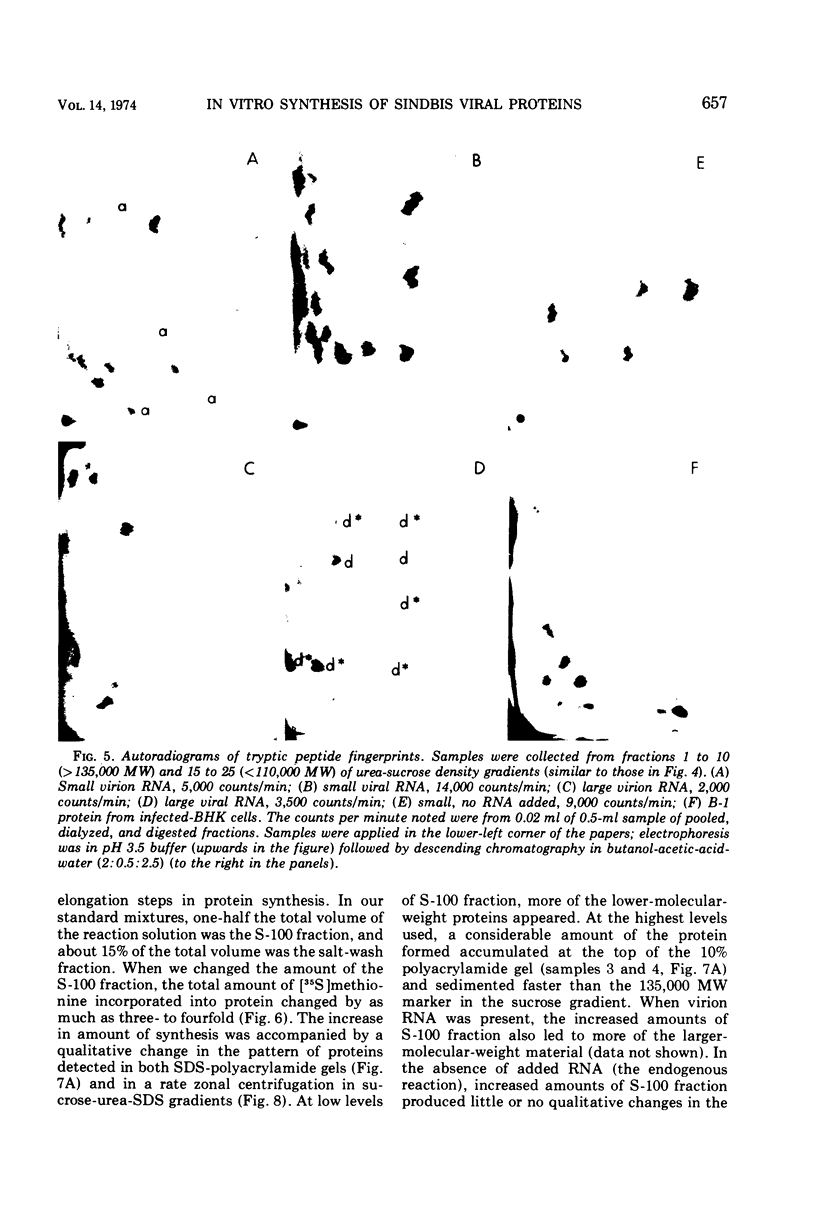
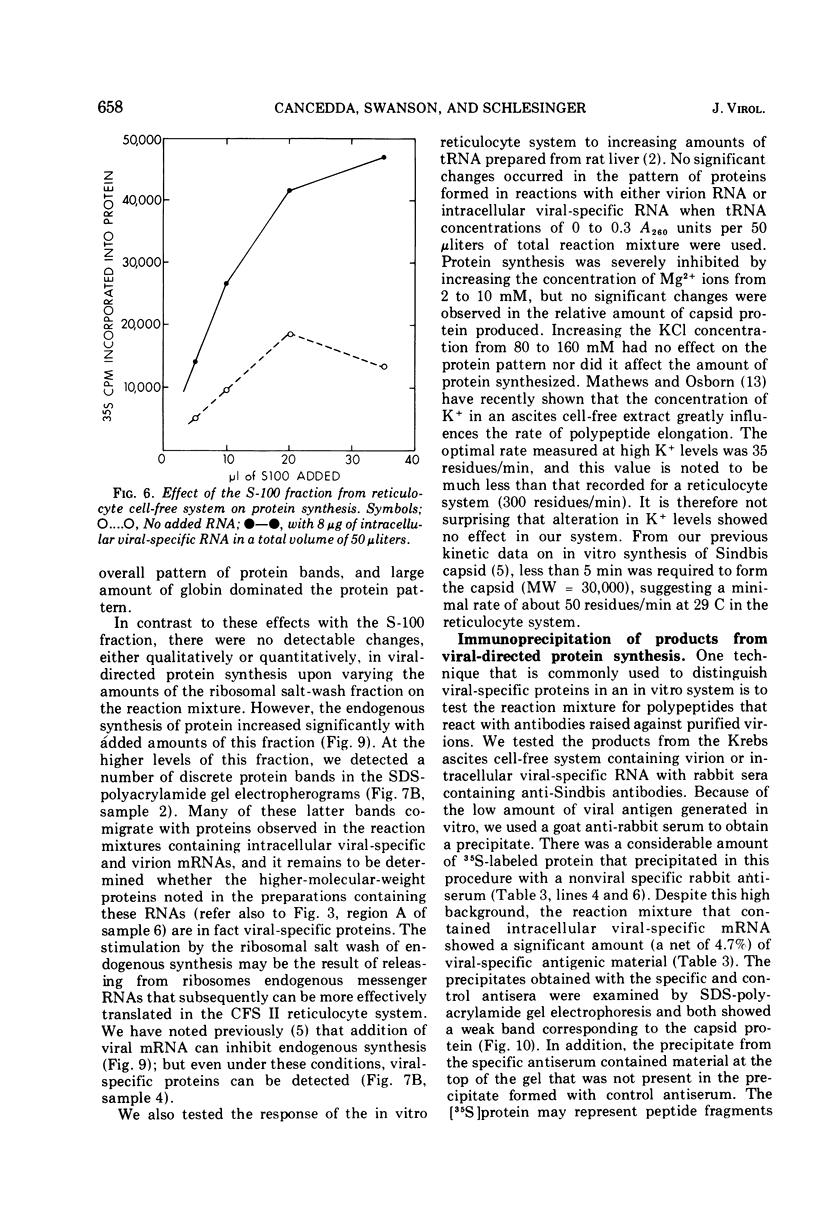
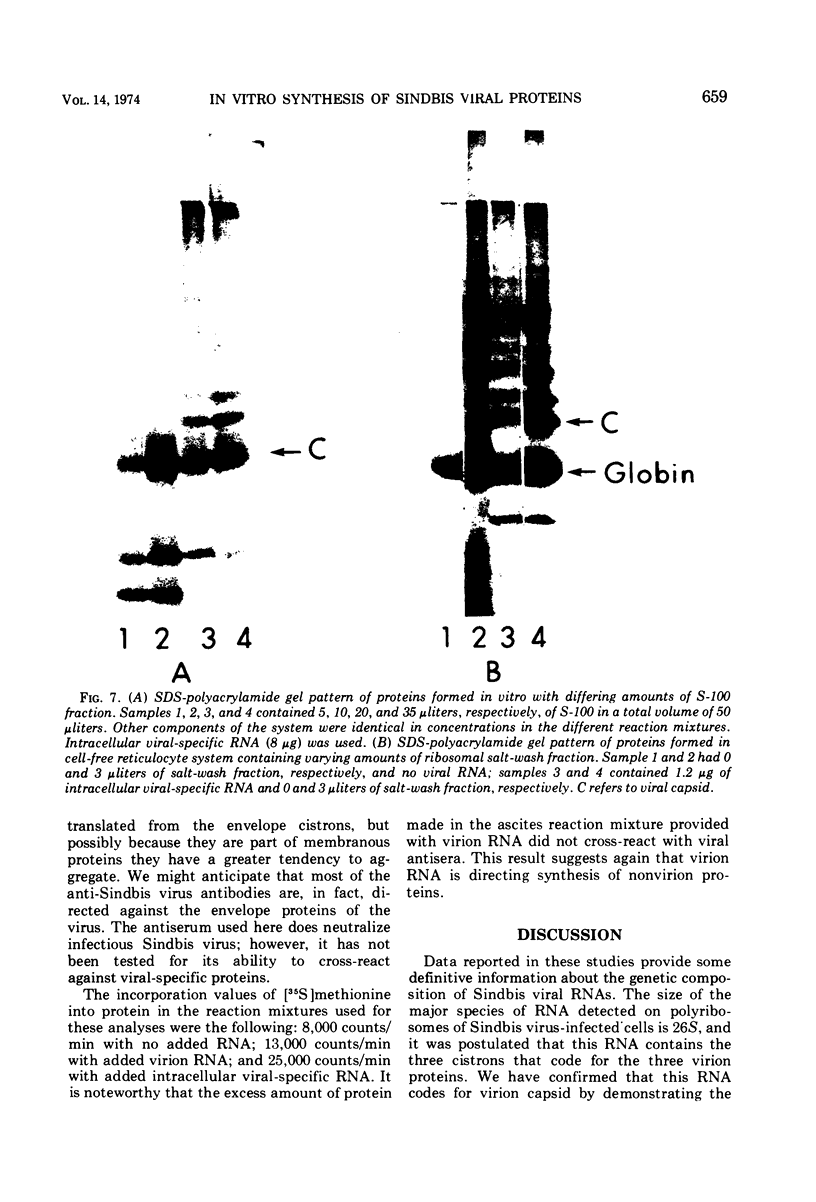
Abstract
Cell-free extracts from Krebs ascites cells and rabbit reticulocytes synthesized a variety of viral-specific proteins when programmed with several different kinds of Sindbis viral RNAs. The RNAs included purified virion RNA (42S) and two species (26S and “33S”) of purified intracellular viral messenger RNAs from viral-infected BHK cells. Proteins formed in vitro were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, rate-zonal centrifugation in urea-sucrose gradients, two-dimensional tryptic peptide fingerprints, and immunoprecipitation with rabbit anti-Sindbis virus serum. The only major identifiable protein formed in vitro was viral capsid, but the relative amount of capsid produced was determined by the mRNA, the source of cell-free extract, and the components of the cell-free system. Virion RNA directed synthesis of larger-molecular-weight proteins than did intracellular viral RNAs, and some of this protein was distinct from that formed by the smaller viral RNAs. Indirect evidence is presented for in vitro synthesis of viral envelope proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Yau P. M., Herbert E., Zucker W. V. Involvement of hemin, a stimulatory fraction from ribosomes and a protein synthesis inhibitor in the regulation of hemoglobin synthesis. J Mol Biol. 1972 Jan 28;63(2):247–264. doi: 10.1016/0022-2836(72)90373-7. [DOI] [PubMed] [Google Scholar]
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Formation of Sindbis virus capsid protein in mammalian cell-free extracts programmed with viral messenger RNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1843–1847. doi: 10.1073/pnas.71.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
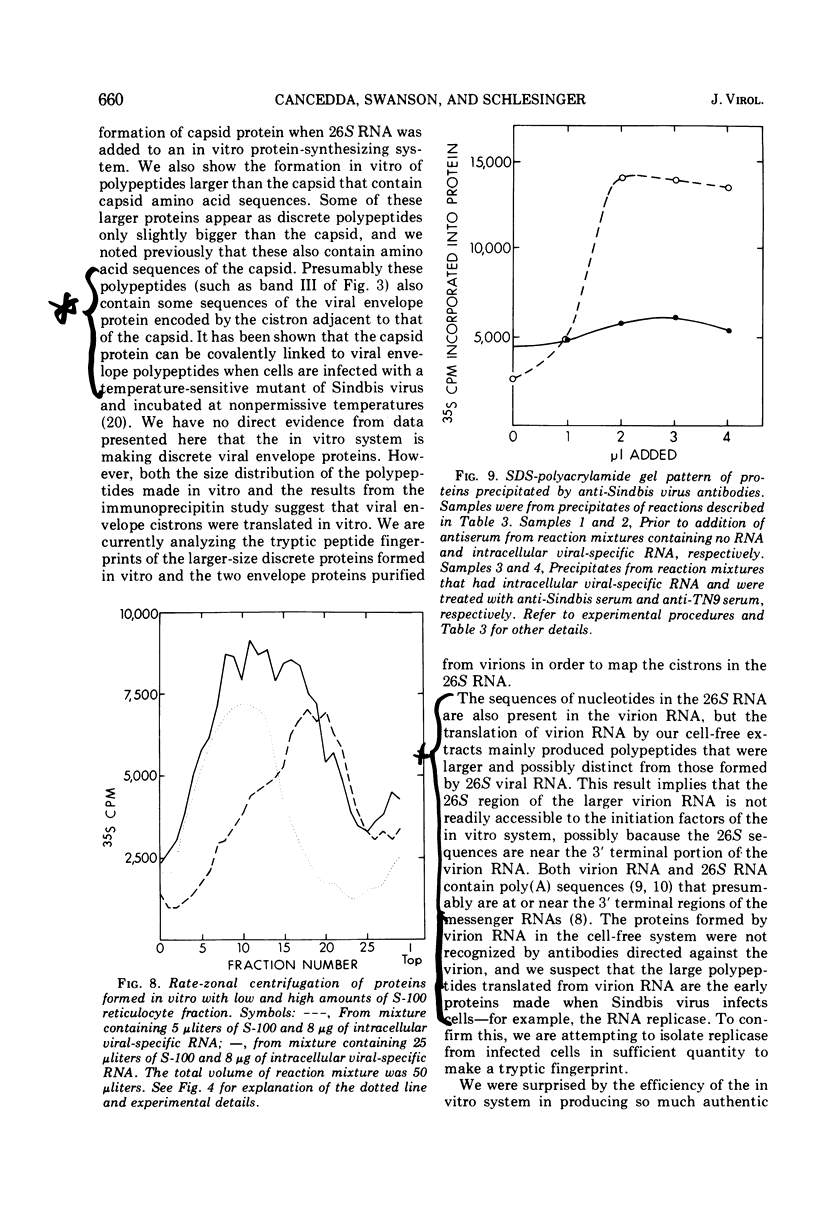
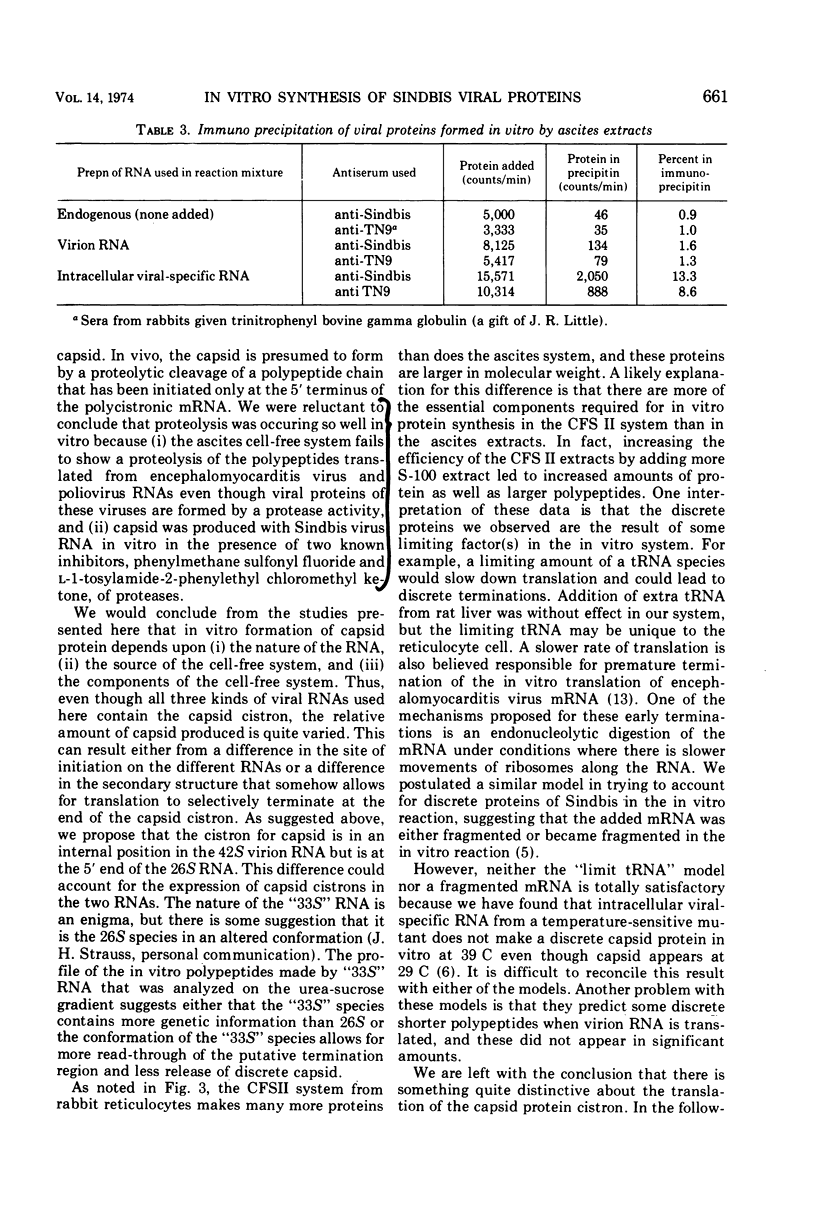
- Cancedda R., Swanson R., Schlesinger M. J. Viral proteins formed in a cell-free rabbit reticulocyte system programmed with RNA from a temperature-sensitive mutant of Sindbis virus. J Virol. 1974 Sep;14(3):664–671. doi: 10.1128/jvi.14.3.664-671.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghue T. P., Faulkner P. Characterisation of the 3'-terminus of Sindbis virion RNA. Nat New Biol. 1973 Dec 12;246(154):168–170. doi: 10.1038/newbio246168a0. [DOI] [PubMed] [Google Scholar]
- Eaton B. T., Donaghue T. P., Faulkner P. Presence of poly (A) in the polyribosome-associated RNA of Sindbis-infected BHK cells. Nat New Biol. 1972 Jul 26;238(82):109–111. doi: 10.1038/newbio238109a0. [DOI] [PubMed] [Google Scholar]
- Eaton B. T., Faulkner P. Heterogeneity in the poly(A) content of the genome of Sindbis virus. Virology. 1972 Dec;50(3):865–873. doi: 10.1016/0042-6822(72)90440-0. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Osborn M. The rate of polypeptide chain elongation in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1974 Mar 8;340(2):147–152. doi: 10.1016/0005-2787(74)90107-5. [DOI] [PubMed] [Google Scholar]
- Mowshowitz D. Identification of polysomal RNA in BHK cells infected by sindbis virus. J Virol. 1973 Apr;11(4):535–543. doi: 10.1128/jvi.11.4.535-543.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Boyle M. K. Selective inhibition of the synthesis of Sindbis virion proteins by an inhibitor of chymotrypsin. J Virol. 1972 Jan;9(1):187–188. doi: 10.1128/jvi.9.1.187-188.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Burge B. W., Coady H. M. Intracellular conversion of the RNA of sindbis virus to a double-stranded form. Virology. 1967 Oct;33(2):239–249. doi: 10.1016/0042-6822(67)90143-2. [DOI] [PubMed] [Google Scholar]
- Rosemond H., Sreevalsan T. Viral RNAs associated with ribosomes in Sindbis virus-infected HeLa cells. J Virol. 1973 Mar;11(3):399–415. doi: 10.1128/jvi.11.3.399-415.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Inhibition of interjacent ribonucleic acid (26S) synthesis in cells infected by Sindbis virus. J Virol. 1969 Aug;4(2):117–122. doi: 10.1128/jvi.4.2.117-122.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Virus-specific proteins synthesized in cells infected with RNA+ temperature-sensitive mutants of Sindbis virus. J Virol. 1970 Mar;5(3):329–337. doi: 10.1128/jvi.5.3.329-337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: chemical composition, biological activity, and mode of interference. J Virol. 1973 Oct;12(4):862–871. doi: 10.1128/jvi.12.4.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]