Abstract
Assessment of the dielectrophoresis (DEP) cross-over frequency (fxo), cell diameter, and derivative membrane capacitance (Cm) values for a group of undifferentiated human embryonic stem cell (hESC) lines (H1, H9, RCM1, RH1), and for a transgenic subclone of H1 (T8) revealed that hESC lines could not be discriminated on their mean fxo and Cm values, the latter of which ranged from 14 to 20 mF/m2. Differentiation of H1 and H9 to a mesenchymal stem cell-like phenotype resulted in similar significant increases in mean Cm values to 41–49 mF/m2 in both lines (p < 0.0001). BMP4-induced differentiation of RCM1 to a trophoblast cell-like phenotype also resulted in a distinct and significant increase in mean Cm value to 28 mF/m2 (p < 0.0001). The progressive transition to a higher membrane capacitance was also evident after each passage of cell culture as H9 cells transitioned to a mesenchymal stem cell-like state induced by growth on a substrate of hyaluronan. These findings confirm the existence of distinctive parameters between undifferentiated and differentiating cells on which future application of dielectrophoresis in the context of hESC manufacturing can be based.
INTRODUCTION
Human embryonic stem cells (hESCs) isolated from early blastocyst stage embryos1, 2 and comparably pluripotent stem cells induced from adult cells by gene transduction3 (iPSCs) constitute a promising resource for disease modeling, drug screening, and cell therapies for regenerative medicine. This is found on their immortality and pluripotency, as compared to the restricted growth potential and repertoires of adult tissue-sourced stem cells. However, the breadth of this capacity also makes controlling cell behavior in vitro more difficult, potentially undermining their utility due to variable and heterogeneous cell production and the difficulty of distinguishing cells of a desired phenotype from other contaminating populations. Thus, to achieve standardized production of the quantity and type of cells desired for particular applications, there is a need for sensitive and non-invasive methods to discriminate and ultimately segregate live cell populations.
Dielectrophoresis (DEP) is a well studied electrokinetic technique4, 5, 6, 7 which has been used to discriminate between distinct cellular identities in heterogeneous populations, notably haematopoietic stem cells and differentiated derivatives in bone marrow and blood,8, 9 mesenchymal stem cells in adipose tissue,10 and neural stem cell populations.11, 12 A DEP device for antibody-independent capture of viable circulating cancer cells in blood has already been developed,13 and theoretically DEP could provide a simple and non-invasive way to positively or negatively select for target or contaminating cell types in a cell preparation intended for transplantation. The DEP response of a cell involves either movement up a field gradient towards an electrode (positive DEP) or down a field gradient away from an electrode (negative DEP). A transition between these two responses occurs where the effective polarisability of the cell matches that of the surrounding medium, and this can occur at both a low frequency (kHz range) or high frequency (>100 MHz). In the kHz range explored in this work, the corresponding DEP cross-over frequency fxo for a spherical cell of radius r is given to good approximation by
| (1) |
where Cm is the specific capacitance of the cell membrane and σm is the conductivity of the suspending medium.7
Measurement of fxo and Cm has been shown to provide a sensitive, non-invasive way to monitor changes in cell states associated with activation and clonal expansion, apoptosis, necrosis, and responses to chemical and physical agents.14, 15, 16, 17 As such, DEP could be applied in the context of stem cell processing as a means for real-time characterisation of the identity and viability of manufactured cell preparations.
Recently, the application of DEP to neural stem cell populations suggested that the differentiation fate of neural stem cells could be predicted by distinct changes in their fxo and Cm values before the presence of specific cell-surface proteins (antigens) could be detected.12, 13 In the present study, our objective was to evaluate DEP as a means to discriminate and characterize undifferentiated hESCs and differentiating progeny. This is an important prerequisite to an ambition to use DEP in the context of manufacturing hESC derived cell products given variation which can exist between cell lines in the course of adaptation to culture or genetic modification. Using the aforementioned Eq. 1, we measured fxo and derive Cm values in several undifferentiated hESC lines (H1, H9, RCM1, RH1),1, 18, 19, 20 a transgenic clonal derivative (T8; a subclone of H1 expressing a genome-integrated green fluorescent protein (GFP) reporter under the control of the OCT4-pluripotency associated transcription factor promoter),20 and derivatives of these cell lines to multipotent-mesenchymal-stem cell like cells21 and to trophoblast.22 Our findings confirm the existence of distinctive parameters of undifferentiated and differentiating cells on which future application of DEP in hESC manufacturing can be based.
MATERIALS AND METHODS
Cell culture
The use of hESC lines in this project was approved by the MRC steering committee overseeing the UK Stem Cell Bank. RCM1 and RH1 were derived at the Roslin Institute under a license from the Human Fertilisation and Embryology Authority to P. De Sousa and licensed from Roslin Cells and Geron, respectively. Both cell lines have been deposited in the UKSCB (www.ukstemcellbank.org.uk). H1 and H9 were derived first at Wisconsin Regional Primate Research Center, University of Wisconsin and licensed from WiCell. They are available from the Wisconsin International Stem Cell Bank (www.wicell.org). The T8 cell line was derived at the Roslin Institute by transfecting the H1 hESC cell line with an octamer-binding transcription factor 4-GFP (OCT4-GFP) reporter.
RCM1,18 RH1,19 H1 and H9,1 and T8 (Ref. 20) hESCs were cultured on plastic tissue culture plates (Corning Inc., NY) coated with growth factor-reduced MatrigelTM (Becton Dickinson) at 37 °C, 5% CO2. T8 hESCs were cultured in mTeSR1 medium (Stemcell Technologies) supplemented with 100 μg ml−1 G418 (Gibco).20 RCM1, RH1, H1, and H9 hESCs were cultured in mTeSR1 alone. Cells were routinely passaged using 200 units/ml of collagenase in Knockout Dulbecco's modified Eagle medium (KO-DMEM, Invitrogen) followed by manual scraping.
H1 and H9 hESCs were differentiated to a mesenchymal stem cell like phenotype using an established protocol involving successive passaging on a substrate of hyaluronan (HA), also known as hyaluronic acid or hyaluronate,21 in a human dermal fibroblast conditioned medium (HDF-CM) composed of KO-DMEM, 20% knockout serum replacement (KOSR, Invitrogen), 1 mM glutamine, 0.1 mM β-mercaptoethanol, and 4 ng/ml bFGF, prepared as described previously.17 Mesenchymal stem cell-like lines originating from H1 and H9 were designated H1-MSC and H9-MSC, respectively. A stock solution of hyaluronan (1200 kD, Proud.385908, Merck-Calbiochem, Nottingham, UK) was produced by solubilising it in physiological strength phosphate buffered saline (PBS) at 10 mg/ml and used for substrate coating. Tissue culture wells were coated with HA stock solution applied at 0.1 ml/cm2 for 30 min at 4 °C before warming to room temperature and grown in 80% KO-DMEM and 20% KOSR (Gibco). For trophoblast cell differentiation, RCM1 hESCs were cultured on MatrigelTM (Becton Dickinson) coated plastic tissue culture plates in HDF-CM (Ref. 18) further supplemented with bone morphogenetic protein (BMP4) at 100 ng/ml for 7 days as described previously.22 These cells were designated RCM1-trophoblast.
For immunolabeling, both hESCs and differentiated cells were washed in PBS buffer and fixed with 4% paraformaldehyde (PFA), permeabilised with 0.2% igepal (Sigma), and blocked in 10% rabbit serum (Millipore). Fixed and permeabilised hESCs were incubated with mouse anti-OCT4 (dilution 1:50, Santa Cruz Biotechnology). Primary antibody binding was detected by incubating with rabbit anti-mouse Alexa-Fluor®-488 (dilution 1:200, Invitrogen); nuclei were counterstained with DAPI23 (Roche). Fixed and permeabilised HA-differentiated cells were washed in PBS and stained to detect mesenchymal markers by incubating with goat anti-human CD105 (1:50 dilution, R&D Systems), and mouse anti-human STRO-1 (1:200 dilution, Millipore). Secondary antibodies were donkey anti-goat Alexa-Fluor-555 (1:400 dilution, Invitrogen), and rabbit anti-mouse Alexa-Fluor-488 (1:200 dilution, Invitrogen), respectively. Fixed and permeabilised RCM1-trophoblast cells were washed in PBS and incubated with mouse anti-human CDX2 (1:50 dilution, Vector labs) and mouse anti-hCG-β (1:25 dilution, Abcam). Primary antibodies were detected by incubating with goat anti-mouse Alexa-Fluor-488 (dilution 1:200, Invitrogen) and nuclei were counterstained with DAPI23 (Roche). Images were captured with a Zeiss Observer microscope and Nikon camera.
Osteoblast formation
Osteogenic differentiation was induced as previously described by Sottile et al.24 An osteogenic (OS) supplement consisting of 50 μM ascorbic acid phosphate (Wako, Neuss, Germany), 10 mM β-glycerophosphate (Sigma-Aldrich, Dorset, UK), and 100 nM dexamethasone (Sigma-Aldrich, Dorset, UK) was added to DMEM medium. Cells grown previously on HA (H1) for 10 passages were plated on 0.1% gelatin and treated with embryoid body differentiation (EBD) medium consisting of 80% KO-DMEM (Invitrogen), 20% fetal bovine serum (FBS) (PAA Laboratories, Pasching, Austria), 1 mM L-glutamine, 0.1 mM μ-mercaptoethanol, 1% nonessential amino acids (all Invitrogen) +/− OS that was changed every 2 days over the indicated intervals.
Mineralisation analysis
For mineralized nodule detection, fixed wells of cells were washed twice with calcium and magnesium-free (CMF)-PBS before incubation with a 1% Alizarin-Red S (Sigma-Aldrich, Dorset, UK) solution in CMF-PBS for 10 min. This was followed by washing twice with water and then fixation in 95% methanol. Cell matrix-associated calcium deposition was determined in 96-well plates using the Sigma calcium assay kit (Sigma-Aldrich, Dorset, UK), in triplicate wells.
Flow cytometry
Single cell suspensions of cultured cells were prepared by treatment with Trypsin-EDTA (Gibco Invitrogen, Paisley, UK) for 5–10 min at 37 °C followed by re-suspension in FACS PBS (PBS with 0.1% bovine serum albumin [BSA]). Cells were incubated for 20 min at 4 °C with pre-conjugated MSC markers CD73-PE (BD Biosciences), CD105-FITC, CD90-FITC, Stro1-APC, CD34-PE (BD Biolegend), and CD14-PE (Becton Dickson). To remove unbound antibody, 2 ml of FACS PBS were added per aliquot, and decanted after centrifugation at (1300 rpm) for 5 min. Cell samples finally resuspended in 250 μl FACS PBS and analyzed using a flow cytometer (FACSCalibur Becton Dickinson) equipped with 488 nm and 633 nm lasers and standard filter set. FACS data were analyzed and plotted using fcs express software.
DEP studies
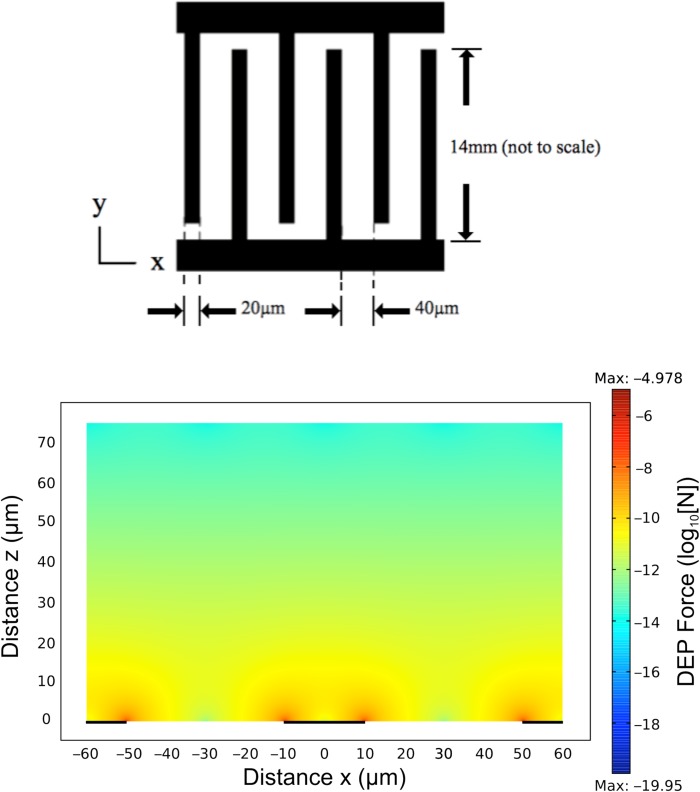
DEP cross-over frequencies fxo for individual cells were obtained using the electrode design shown in Figure 1. The electrodes were fabricated using standard photolithography and liftoff techniques, and consisted of gold (150 nm) thermally evaporated onto a chrome adhesion layer (5 nm) and patterned into an interdigitated array on a glass substrate. The electrode substrate formed the floor of an open chamber, of internal length 10 mm, width 10 mm, and height 80 μm, constructed from polydimethylsiloxane (PDMS) bonded onto the glass substrate containing the electrodes. The spatial variation of the field non-uniformity generated by this interdigitated electrode design, characterized by the factor , was modeled using comsol (Multiphysics 4.3) finite element software. This information was used to derive the magnitude of the DEP force acting on typical cells as a function of the applied sinusoidal voltage and location above the electrode plane. An example of this modelling is shown in Figure 1. The maximum Brownian diffusional force (kT/2r) acting on a cell of radius 5 μm at room temperature is 4 × 10−16 N, and from Figure 1, the corresponding DEP force for an applied voltage of 3 V(pk) greatly exceeded this value. However, the sedimentation force acting on a 5 μm radius cell can be estimated as 2 × 10−13 N (assuming a specific density difference of 40 kg/m3 between the medium and cell). To ensure that sedimentation forces did not influence the assessment of the DEP force when close to fxo, studies were limited to those cells located no higher than 20 μm above the electrode plane.
Figure 1.
(Top): Interdigitated electrode geometry used to determine the DEP cross-over frequency fxo. (Bottom): comsol Multiphysics simulation of the DEP force (log10[N]) acting on a 10 μm diameter cell (Clausius-Mossotti factor = 0.5) with an applied voltage of 3 V (pk), as a function of distance (x μm) along and height above (z μm) the electrode plane.
Before DEP characterization the cells were centrifuged (100× g, 5 min) and washed twice in 10 ml of the DEP medium, before final suspension in this medium at a density of ∼107 cells/ml. The DEP isotonic medium consisted of 8.5% (w/w) sucrose, 0.5% (w/w) glucose, at pH 7.4 (adjusted with NaOH) and conductivity 33 mS/m, adjusted with PBS. Osmolality (310 mOsm) and conductivity values were measured using an osmometer (Advanced Instruments Inc., Model 3300) and a conductivity meter (Oakton CON 510), respectively. Cell suspensions were pipetted onto the electrodes, the chamber was closed with a cover slip and then mounted in an inverted microscope (Meiji TC5100). Sinusoidal voltages of 3 V (pk) were applied to the electrodes in continuous incremental steps of 10 kHz, commencing at 10 kHz and finishing at 200 kHz, using an Agilent/HP 3325 A signal generator. By focusing on the electrode plane discrimination between cells undergoing negative DEP and positive DEP was possible, and an estimation of fxo was made for each cell by analysis of video images captured during this procedure. The diameter of each characterized cell was measured using Free Ruler (www.pascal.com/software/freeruler).
Statistical analysis
Statistical analyses (unpaired t-tests or one-way ANOVA with Tukey's post-hoc test, as appropriate) were performed in GraphPad Prism, version 6.
RESULTS AND DISCUSSION
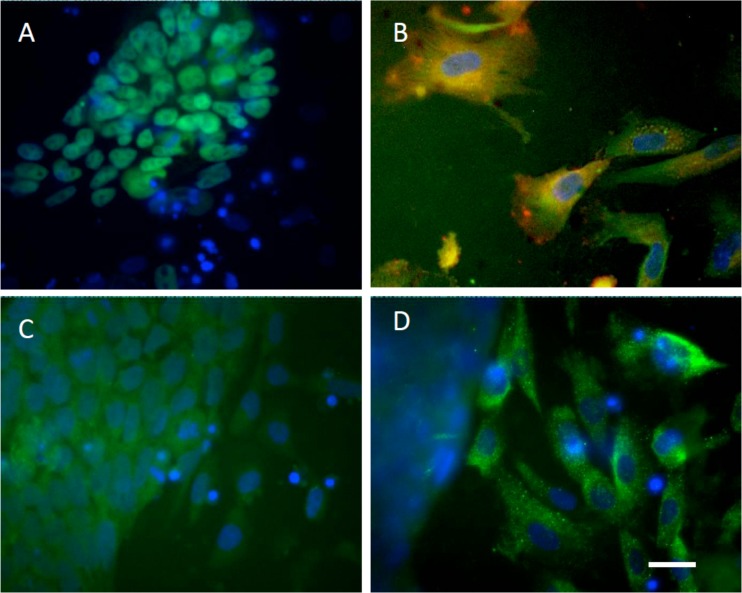
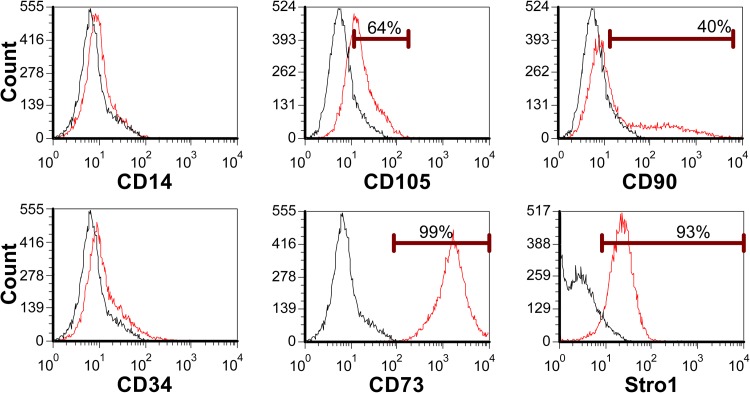
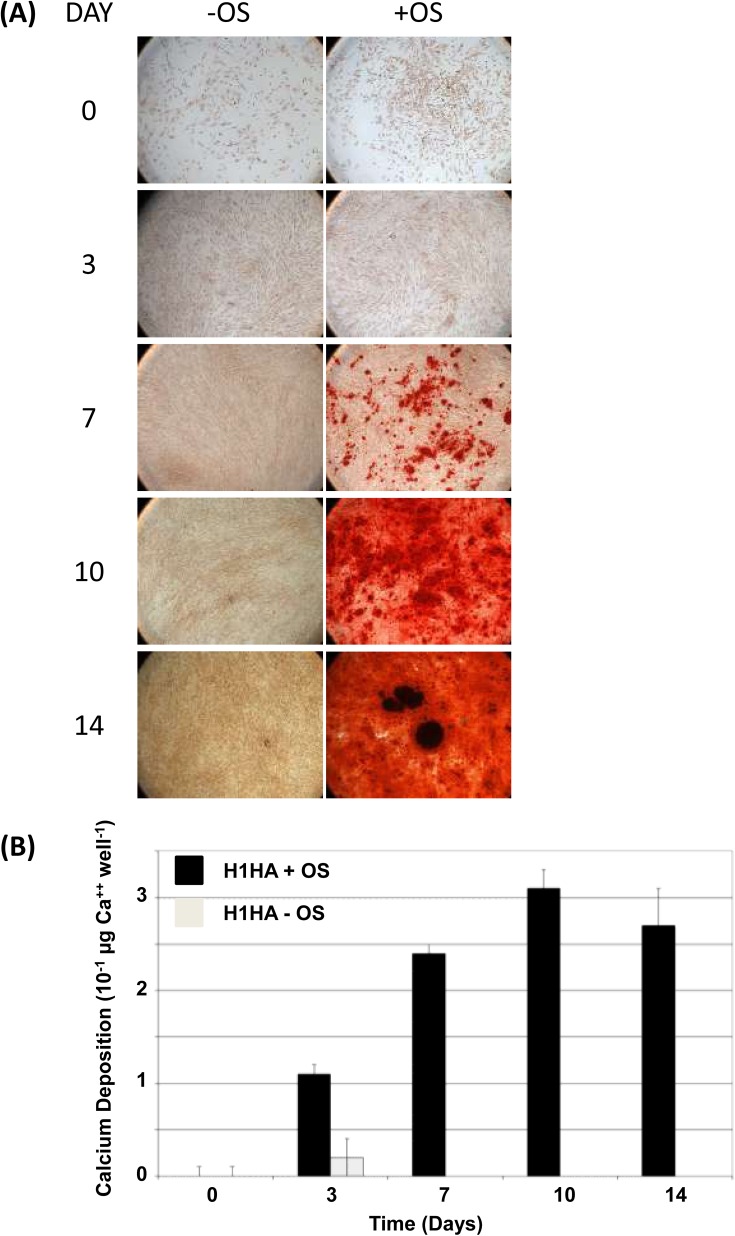
The undifferentiated phenotype and pluripotency of hESC lines used in this study has been reported in previous studies.1, 18, 19, 20 Flow cytometry confirmed all lines were positive for undifferentiated glycolipids SSEA-4 and TRA-1-60, and negative for SSEA-1 (Table TABLE I.). The former were lower in the transgenic GFP reporter-expressing T8 line. By in situ immunocytochemistry undifferentiated cell colonies in all lines were positive for pluripotency associated transcription factors, Oct4 and Nanog (Oct4 shown for H1, Figure 2a). H1 and H9 hESC's were differentiated to MSC-like lineages as previously reported in Harkness et al.21 Consistent with an adult tissue derived MSC flow cytometry confirmed cells were CD73+, CD105+, CD90+, and Stro1+ and CD14−, CD34− (shown for H1, Figure 3). CD105 and Stro1 expression was confirmed by immunocytochemistry (Figure 2b). To confirm a MSC-like potency, cells were induced to undergo mineralization in vitro in response to an osteogenic stimulus (OS) as previously described by Sottille et al.24 H1-MSC grown on HA for 10 passages underwent OS dependent mineralization characteristic of osteoblast-like cells. This was reflected by Alazarin Red staining and calcium deposition (Figure 4), both of which increased over successive days of stimulation and were maximal by 10 days of treatment respectively. RCM1 cells were non-specifically differentiated to yield heterogeneous populations of trophoblast like cells using another established protocol Xu et al.22 This was verified by immunostaining for CDX2 and hCG-β markers (Figures 2c, 2d).
TABLE I.
Flow cytometry analysis data for five undifferentiated hESC lines used in the study confirmed that comparable and high level of detection of SSEA-4 and TRA-1-60 and low levels of SSEA-1, as expected for this phenotype. Passage number at which cells assessed denoted by “p.”
| % marker positive | |||
|---|---|---|---|
| hESC lines | SSEA-1 | SSEA-4 | TRA1-60 |
| H1-p62 | 4 | 69 | 63 |
| H9-p73 | 2 | 85 | 75 |
| RCM1-p65 | 5 | 97 | 97 |
| RH1-p53 | 5 | 60 | 71 |
| T8-p98 | 3 | 32 | 66 |
Figure 2.
Immunocytochemical confirmation of hESC and derivative lineages. Undifferentiated H1 hESCs (a) and MSC-like cells (b), and RCM-1 trophoblast-like cells ((c), (d)) stained for OCT4 (a), MSC lineage markers CD105 (red) and Stro-1 (green) (b) and trophoblast markers CDX2 (green, (c)) and hCG-β (green, (d)). Nuclei are counterstained with DAPI (blue) in all panels. Scale bar equals to 20 μm.
Figure 3.
Flow cytometric analysis of H1-MSC-like cells confirmed they were negative for CD14, CD34, and positive for CD105 (64%), CD90 (40%), CD73 (99%), and Stro1 (58%).
Figure 4.
Differentiation potential of hyaluronan-generated hES-derived MSC-like cells. (a) H1HA cells differentiate along the ostoeogenic lineage to form mineralised bone when cultured in conditions conducive to osteogenesis (+OS) but not control conditions (−OS), as detected by alizarin red staining (red staining detectable from day 7 onwards). (b) Calcium deposition quantified by Sigma calcium assay kit from the experiment in (a) shows that mineralised calcium only accumulates to detectable levels in osteogenic conditions (+OS, black bars).
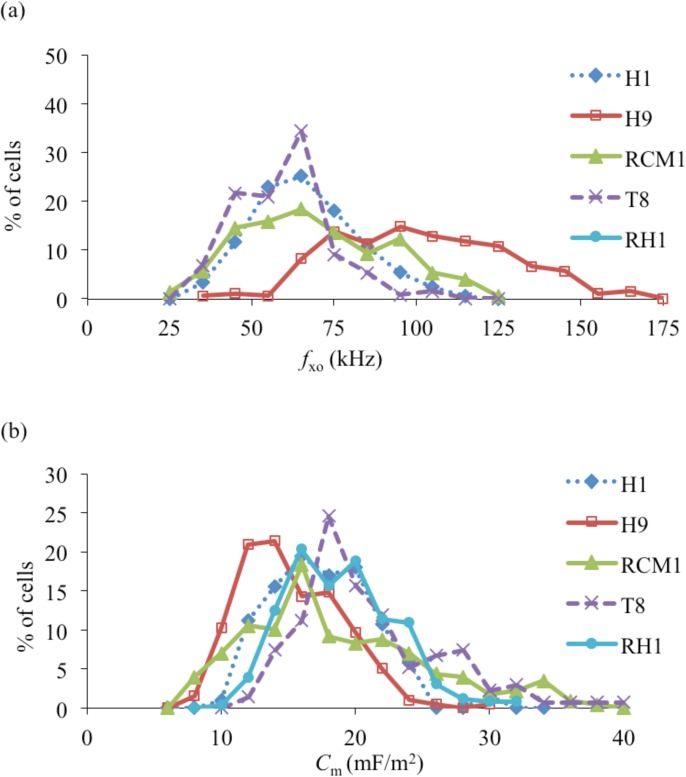
A summary of the experimentally measured cell diameters and DEP cross-over frequencies (fxo) for the various hESCs and their differentiated progeny is given in Table TABLE II., together with their membrane capacitances derived using Eq. 1. The standard deviation (σ = 1) values given are based on approximating the fxo and Cm distributions shown in Figure 5 as normal distributions. The results presented in Table TABLE II. and Figure 5 indicate that there is a significant overlap of the membrane capacitance values for the different hESC lines, with an overall mean value of 17.6 ± 4.7 mF/m2. The overlap of the fxo distributions shown in Figure 5a indicates that (apart from with H9) it would not be possible to obtain pure samples of different hESC lines from a mixed population using DEP as a separation tool. High purity separation of H9 cells from a mixture of H1 and H9 cells, for example, could be obtained using the DEP device described by Gupta et al.13 at 120 kHz with a cell medium conductivity of 35 mS/m, but with a recovery of less than 50% for the H9 cells.
TABLE II.
Summary of the data (cell diameter, DEP cross-over frequency (fxo), and membrane capacitance (Cm)) obtained for the human embryonic stem cells and their differentiated progeny.
| Cell type | Diameter (μm) | fxo (kHz) | Cm (mF/m2) |
|---|---|---|---|
| H1 (n = 206) | 14.5 ± 2.8 | 68.4 ± 16.2 | 16.1 ± 3.9 |
| H9 (n = 196) | 11.1 ± 1.7 | 101.6 ± 25.8 | 14.2 ± 3.7 |
| RCM1 (n = 229) | 14.2 ± 2.3 | 68.2 ± 22 | 17.6 ± 6.7 |
| RH1 (n = 254) | 13.7 ± 1.6 | 63.8 ± 14.7 | 17.9 ± 3.9 |
| T8 (n = 134) | 14.3 ± 2.0 | 55.6 ± 13.1 | 20.0 ± 5.3 |
| H1-MSC (n = 98) | 15.0 ± 3.1 | 26.1 ± 7.4 | 41.6 ± 11.0 |
| H9-MSC (n = 185) | 12.5 ± 2.2 | 26.2 ± 7.6 | 49.4 ± 12.2 |
| RCM1-trophoblast (n = 221) | 12.8 ± 1.6 | 44.4 ± 11.8 | 28.3 ± 7.3 |
Figure 5.
(a) Percentage distributions of the DEP cross-over frequencies (fxo) measured for the different human embryonic stem cell lines. (b) The derived membrane capacitance (Cm) values.
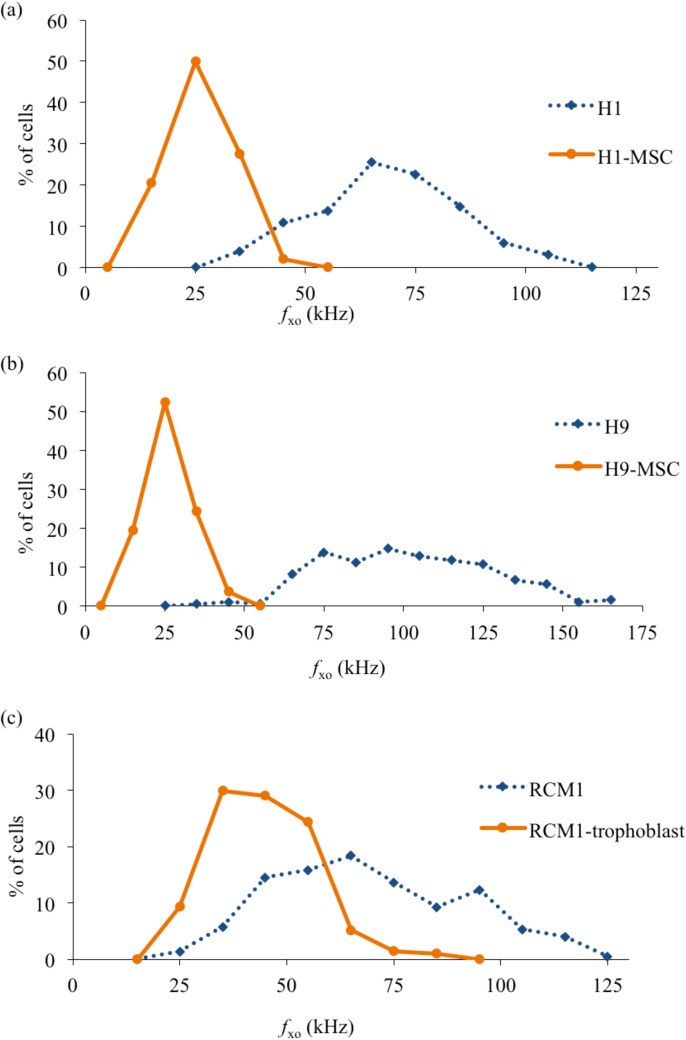
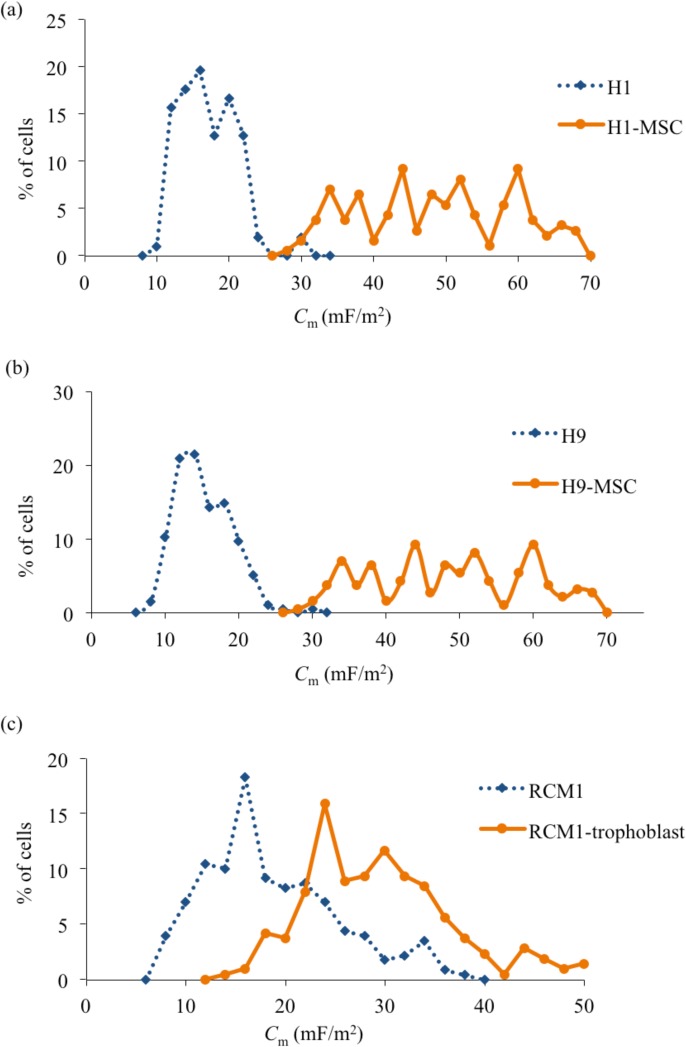
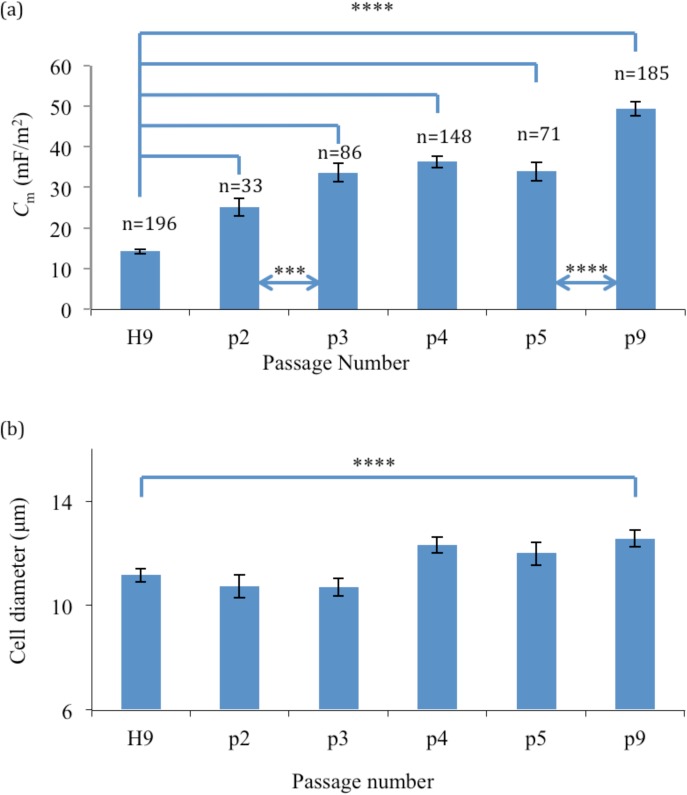
From Figure 6, it is also clear that H1 and H9 cells both exhibited well-separated distributions of their fxo values, with little overlap, compared to those exhibited by their differentiated progeny (H1-MSC and H9-MSC, respectively). Separation of pure populations of differentiated cells from their progenitor H1 or H9 cells, with relatively high yields, should therefore be possible using DEP. However, DEP is unlikely to provide a sensitive method for distinguishing populations of RCM1-trophoblast from RCM1 ES cells. Coupled with the distinct separation of fxo distributions after differentiation, the results shown in Figures 67 indicate that there is also a distinct increase of cellular membrane capacitance values (p < 0.0001). H1-MSCs and H9-MSCs exhibit a broad distribution of Cm values, extending from around 30 to 70 mF/m2, compared to the more narrow ranges centered near 17 and 14 mF/m2 exhibited by the H1 and H9 cells, respectively. Although the RCM1 and RCM1-trophoblast exhibit a clear difference in their mean Cm value (∼18 mF/m2 and 28 mF/m2, respectively), there is a significant overlap of their Cm distributions. Finally, the changes in membrane capacitance and cell diameter for H9 cells as they are differentiated into H9-MSCs are shown in Figure 8 as a function of passage number. There is no statistical difference in cell diameter between H9 and H9-MSCs at passage 2 and passage 3 (p > 0.05). However, there is a small but significant difference in diameter when H9 cells are compared to H9-MSCs at passage numbers 4-9 (p < 0.05). Although the cell size increases by 12.4% from H9 to H9-MSC (p9), the observed change in the cross-over frequency can be primarily attributed to a significant increase in membrane capacitance of 247% (p < 0.0001).
Figure 6.
Percentage distributions of the DEP cross-over frequencies (fxo) for (a) H1 and differentiated H1-MSC, (b) H9 and H9-MSC, (c) RCM1 and RCM1-trophoblast.
Figure 7.
Membrane capacitance (Cm) values obtained for the human embryonic stem cells and their differentiated progeny.
Figure 8.
The progression of (a) mean membrane capacitance Cm with 95% confidence interval bars, and (b) mean cell diameter with 95% confidence interval error and passage number for the H9 cells cultured on an hyaluronan coated substrate. (**** and *** signify a statistical difference with p < 0.0001 and p < 0.001 between groups).
It is customary to employ the low-frequency (DC) approximation25 to analyse the dielectric and conductive properties of a cell. For a spherical cell of radius r its effective permittivity εeff is given by
| (2) |
where δ is the membrane thickness, εo the permittivity of free space, εm is the mean relative permittivity of the material forming the membrane structure, and ϕm is the membrane-folding factor to take into account cell surface features such as folds, microvilli, ruffles and blebs.26 For a perfectly smooth spherical cell ϕm = 1.
In the DEP literature,7, 14, 26 it is commonly assumed that different values of Cm for different types of cell primarily reflect differences in the ϕm factor, and that the membrane thickness δ and relative permittivity εm in Eq. 2 remain relatively fixed in value (∼5 nm, 2.0–2.2, respectively26, 27). However, recent DEP determination of Cm for fibroblasts and myoblasts, combined with Raman spectroscopic analyses of their membranes, has indicated that the chemical composition (e.g., proportion of saturated to unsaturated lipid content) may have a greater influence on the values for δ and εm than previously thought.28
Our study supports further development of DEP in the context of human embryonic stem cell bioprocessing, in the course of expansion or directed differentiation for industrial or therapeutic applications. The most tractable application would be for purposes of real-time monitoring of the representation of specific cell subpopulations through sampling limited quantities of cells at the time of passaging. This would not require large numbers of cells and could be implemented essentially using methods applied in this study. The application of DEP to the separation of relatively large numbers of cells (>50 000) has been previously demonstrated (e.g., Refs. 13, 28). Scaled separation of tissue derived stem cells and differentiated progeny for further culture by such approaches will require process parallelization.
CONCLUSIONS
The various undifferentiated hESC lines (H1, H9, RCM1, RH1) and the transgenic clonal derivative (T8) studied in this work could not be discriminated in terms of their DEP cross-over frequencies and derived membrane capacitance values (14–20 mF/m2). However, the differentiated progeny (H1-MSC, H9-MSC, RCM1-trophoblast) could be discriminated from their adult precursors (H1, H9, RCM1, respectively) by significant increases in their mean DEP cross-over frequencies and derived membrane capacitance values. The mesenchymal stem cell-like phenotypes (H1-MSC, H9-MSC) derived from differentiation of H1 and H9 exhibited comparable Cm values of 41–49 mF/m2 (p < 0.0001), whereas induction of RCM1 to a trophoblast cell-like phenotype resulted in a smaller increase of mean Cm values from 17.6 to 28.3 mF/m2 (p < 0.0001). Since different hESC lines were derived at different times in different laboratories, and have been cultured in different conditions at different times, yet Cm and fxo are similar, we conclude that a low membrane capacitance is a characteristic property of hESCs. Furthermore, the result that Cm changes upon differentiation suggests that different phenotypes might be discriminated by their Cm values. The transition to a larger membrane capacitance as the H9 cells differentiated to H9-MSCs was progressively evident after each passage of the cell culture up to p9 (Figure 8). All of these findings confirm the existence of distinctive parameters which differ between undifferentiated and differentiating hESCs, and suggests that dielectrophoresis can be applied in the manufacturing process of these cells.
The increase in membrane capacitance during differentiation could arise from an increase in the complexity of the membrane surface (appearance of folds, microvilli, blebs), or a combined thinning and increase of the effective dielectric polarisability of the membrane structure.28 Which of these physico-chemical parameters contributes the most to the observed changes of membrane capacitance remains a focus of our ongoing studies.
ACKNOWLEDGMENTS
The authors would like to thank associates for their technical assistance. Notably Mrs. Judy Fletcher and Ms. Davina Wojtacha for osteogenic differentiation and assessment of calcium deposition; Ms. Courtney Campbell for in situ immunocytochemistry; and Dr. Paz Freile for flow cytometry. This work was supported by EUFP7 funding to P.D.S., as part of a HEALTH-2007-1.4-10 7 program to develop stem cell culture conditions (Project No. 223410), and by funding to R.P. by the Edinburgh Research Partnership in Engineering and Mathematics.
References
- Thomson J. A., Eldor J. I., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., and Jones J. M., Science 282, 1145 (1998). 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Klimanskaya I., Chung Y., Becker S., Lu S. J., and Lanza R., Nature 444, 481 (2006). 10.1038/nature05142 [DOI] [PubMed] [Google Scholar]
- Takahashi K. and Yamanaka S., Cell 126, 663 (2006). 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Gagnon Z. R., Electrophoresis 32, 2466 (2011). 10.1002/elps.201100060 [DOI] [PubMed] [Google Scholar]
- Çetin B. and Li D., Electrophoresis 32, 2410 (2011). 10.1002/elps.201100167 [DOI] [PubMed] [Google Scholar]
- Regtmeier J., Eichhorn R., Viefhues M., Bogunovic L., and Anselmetti D., Electrophoresis 32, 2253 (2011). 10.1002/elps.201100055 [DOI] [PubMed] [Google Scholar]
- Pethig R., Biomicrofluidics 4, 022811 (2010). 10.1063/1.3456626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talary M. S., Mills K. I., Hoy T., Burnett A. K., and Pethig R., Med. Biol. Eng. Comput. 33, 235 (1995). 10.1007/BF02523050 [DOI] [PubMed] [Google Scholar]
- Stephens M., Talary M. S., Pethig R., Burnett A. K., and Mills K. I., Bone Marrow Transplant. 18, 777 (1996), available online at http://www.ncbi.nlm.nih.gov/pubmed/8899194/. [PubMed] [Google Scholar]
- Vykoukal J., Vykoukal D. M., Freyberg S., Alt E. U., and Gascoyne P. R. C., Lab Chip 8, 1386 (2008). 10.1039/b717043b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan L. A., Lu J., Wang L., Marchenko S. A., Jeon N. L., Lee A. P., and Monuki E. S., Stem Cells 26, 656 (2008). 10.1634/stemcells.2007-0810 [DOI] [PubMed] [Google Scholar]
- Labeed F. H., Lu J., Mulhall H. J., Marchenko S. A., Hoettges K. F., Estrada L. C., Lee A. P., Hughes M. P., and Flanagan L. A., PLoS ONE 6(9), e25458 (2011). 10.1371/journal.pone.0025458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Jafferji I., Garza M., Melnikova V. O., Hasegawa D. K., Pethig R., and Davis D. W., Biomicrofluidics 6, 024133 (2012). 10.1063/1.4731647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Becker F. F., and Gascoyne P. R. C., Biochim. Biophys. Acta 1564, 412 (2002). 10.1016/S0005-2736(02)00495-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethig R., Bressler V., Carswell-Crumpton C., Chen Y., Foster-Haje L., García-Ojeda M. E., Lee R. S., Lock G. M., Talary M. S., and Tate K. M., Electrophoresis 23, 2057 (2002). [DOI] [PubMed] [Google Scholar]
- Pethig R. and Talary M. S., IET Nanobiotechnol. 1, 2 (2007). 10.1049/iet-nbt:20060018 [DOI] [PubMed] [Google Scholar]
- Sabuncu A. C., Zhuang J., Kolb J. F., and Beskok A., Biomicrofluidics 6, 034103 (2012). 10.1063/1.4737121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa P. A., Gardner J., Sneddon S., Pells S., Tye B. J., Dand P., Collins D. M., Stewart K., Shaw L., Przyborski S., Cooke M., McLaughlin K. J., Kimber S. J., Lieberman B. A., Wilmut I., and Brison D. R., Stem Cell Res. 2, 188 (2009). 10.1016/j.scr.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Fletcher J. M., Ferrier P. M., Gardner J. O., Harkness L., Dhanjal S., Serhal P., Harper J., Delhanty J., Brownstein D. G., Prasad Y. R., Lebkowski J., Mandalam R., Wilmut I., and De Sousa P. A., Cloning Stem Cells 8, 319 (2006). 10.1089/clo.2006.8.319 [DOI] [PubMed] [Google Scholar]
- Gerrard L., Zhao D., Clark A. J., and Cui W., Stem Cells 23, 124 (2005). 10.1634/stemcells.2004-0102 [DOI] [PubMed] [Google Scholar]
- Harkness L., Mahmood A., Ditzel N., Abdallah B. M., Nygaard J. V., and Kassem M., Bone 48, 231 (2011). 10.1016/j.bone.2010.09.023 [DOI] [PubMed] [Google Scholar]
- Xu R.-H., Chen X., Li D. S., Li R., Addicks G. C., Glennon C., Zwaka T. P., and Thomson J. A., Nat. Biotechnol. 20, 1261 (2002). 10.1038/nbt761 [DOI] [PubMed] [Google Scholar]
- Kapuściński J. and Skoczylas B., Nucleic Acids Res. 5(10), 3775 (1978). 10.1093/nar/5.10.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile V., Thomson A., and McWhir J., Cloning Stem Cells 5, 149–155 (2003). 10.1089/153623003322234759 [DOI] [PubMed] [Google Scholar]
- Schwan H. P., Adv. Biol. Med. Phys. 5, 147 (1957), available online at http://www.ncbi.nlm.nih.gov/pubmed/13520431?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- Wang X. B., Huang Y., Gascoyne P. R., Becker F. F., Holzel R., and Pethig R., Biochim. Biophys. Acta 1193(2), 330 (1994). 10.1016/0005-2736(94)90170-8 [DOI] [PubMed] [Google Scholar]
- Pethig R. and Kell D. B., Phys. Med. Biol. 32, 933 (1987). 10.1088/0031-9155/32/8/001 [DOI] [PubMed] [Google Scholar]
- Muratore M., Srsen V., Waterfall M., Downes A., and Pethig R., Biomicrofluidics 6, 034113 (2012). 10.1063/1.4746252 [DOI] [PMC free article] [PubMed] [Google Scholar]