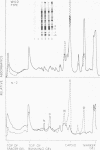
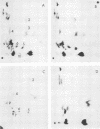
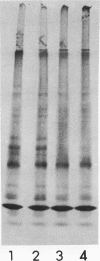
Abstract
Viral messenger RNA was isolated from BHK cells infected with a temperature-sensitive mutant of Sindbis virus and was further purified using an oligo(dT) column. Addition of this mRNA cell-free extracts from rabbit reticulocytes led to formation of discrete authentic viral capsid protein when the reaction was performed at 29 C. However, this same protein-synthesizing system failed to make discrete viral capsid when incubated with the viral RNA at 39 C. Instead, larger-molecular-weight polypeptides that contained the viral capsid peptide sequences were produced. The inability to make a separate viral capside protein in vitro at elevated temperatures by the mRNA from this mutant exactly mimics the phenotype of this ts mutant in viral-infected cells. Three mechanisms are discussed that might account for a temperature-sensitive release of capsid. One of these is based on a model in which there are multiple sites for initiation of translation of polypeptides on a polycistronic viral mRNA.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Burrell C. J., Martin E. M., Cooper P. D. Posttranslational cleavage of virus polypeptides in arbovirus-infected cells. J Gen Virol. 1970 Feb;6(2):319–323. doi: 10.1099/0022-1317-6-2-319. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Formation of Sindbis virus capsid protein in mammalian cell-free extracts programmed with viral messenger RNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1843–1847. doi: 10.1073/pnas.71.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda R., Swanson R., Schlesinger M. J. Effects of different RNAs and components of the cell-free system on in vitro synthesis of Sindbis viral proteins. J Virol. 1974 Sep;14(3):652–663. doi: 10.1128/jvi.14.3.652-663.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Herner A. E., Goldberg I. H. Inhibition by pactamycin of the initiation of protein synthesis. Binding of N-acetylphenylalanyl transfer ribonucleic acid and polyuridylic acid to ribosomes. Biochemistry. 1969 Apr;8(4):1312–1326. doi: 10.1021/bi00832a004. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Primary gene products of an arbovirus. Biochem Biophys Res Commun. 1969 Oct 8;37(2):369–373. doi: 10.1016/0006-291x(69)90744-x. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G., Steitz J. A., Gesteland R. F., Spahr P. F. Gene order in the bacteriophage R17 RNA: 5'-a protein-coat protein-synthetase-3'. Nature. 1970 Apr 18;226(5242):230–237. doi: 10.1038/226230a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Secondary structure of bacteriophage f2 ribonucleic acid and the initiation of in vitro protein biosynthesis. J Mol Biol. 1970 Jun 28;50(3):689–702. doi: 10.1016/0022-2836(70)90093-8. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Lodish H. F. Translation of bacteriophage Q RNA by cytoplasmic extracts of mammalian cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):315–319. doi: 10.1073/pnas.70.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Boyle M. K. Selective inhibition of the synthesis of Sindbis virion proteins by an inhibitor of chymotrypsin. J Virol. 1972 Jan;9(1):187–188. doi: 10.1128/jvi.9.1.187-188.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki M. Nucleocapsid and envelope protein of Semliki forest virus as affected by canavanine. J Gen Virol. 1972 Apr;15(1):59–67. doi: 10.1099/0022-1317-15-1-59. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Virus-specific proteins synthesized in cells infected with RNA+ temperature-sensitive mutants of Sindbis virus. J Virol. 1970 Mar;5(3):329–337. doi: 10.1128/jvi.5.3.329-337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Barnett L., Brenner S., Russell R. L. More mutant tyrosine transfer ribonucleic acids. J Mol Biol. 1970 Nov 28;54(1):1–14. doi: 10.1016/0022-2836(70)90442-0. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: chemical composition, biological activity, and mode of interference. J Virol. 1973 Oct;12(4):862–871. doi: 10.1128/jvi.12.4.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C., Squires C. L. Regulated in vitro synthesis of Escherichia coli tryptophan operon messenger ribonucleic acid and enzymes. J Biol Chem. 1974 Jan 25;249(2):465–475. [PubMed] [Google Scholar]