Abstract
Purpose
Posterior instrumented spinal fusion is indicated for progressive scoliosis that develops in Duchenne muscular dystrophy (DMD) patients. Whilst spinal fusion is known to improve quality of life, there is inconsistency amongst the literature regarding its specific effect on respiratory function. Our objective was to determine the effect of scoliosis correction by posterior spinal fusion on respiratory function in a large cohort of patients with DMD. Patients with DMD undergoing posterior spinal fusion were compared to patients with DMD not undergoing surgical intervention.
Methods
An observational study of 65 patients with DMD associated scoliosis, born between 1961 and 2001: 28 of which underwent correction of scoliosis via posterior spinal fusion (Surgical Group) and 37 of which did not undergo surgical intervention (Non-Surgical Group). Pulmonary function was assessed using traditional spirometry. Comparisons were made between groups at set times, and by way of rates of change over time.
Results
There was no correlation between the level of respiratory dysfunction and the severity of scoliosis (as measured by Cobb angle) for the whole cohort. The Surgical Group had significantly worse respiratory function at a comparable age pre-operatively compared to the Non-Surgical Group, as measured by per cent predicted forced vital capacity (p = 0.02) on spirometry. The rate of decline of forced vital capacity and per cent predicted forced vital capacity was not slowed following surgery compared to the non-operated cases. There was no significant difference in survival between the two groups.
Conclusions
Severity of scoliosis was not a key determinant of respiratory dysfunction. Posterior spinal fusion did not reduce the rate of respiratory function decline. These two points suggest that intrinsic respiratory muscle weakness is the main determinant of decline in respiratory function in DMD.
Keywords: Duchenne muscular dystrophy, Dystrophin, Respiratory function, Spirometry, Scoliosis, Surgical intervention
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive disease that affects 1 in 3,500 male live births [1], making it the commonest form of muscular dystrophy [1, 2]. Dystrophin is a large cell-membrane protein involved with calcium transport in muscle cells. Mutations in the Xp21 region of the X chromosome lead to a deficiency of dystrophin resulting in skeletal, respiratory and cardiac muscle weakness [1, 3].
The natural history of untreated DMD leads to the development of an abnormal gait, calf hypertrophy and difficulty rising from the floor when 2–5 years of age [4]; with wheelchair dependence occurring most commonly by 9–10 years of age [5]; and development of progressive scoliosis in 95 % of patients thereafter [6, 7]. Progressive respiratory muscle weakness decreases respiratory reserve and causes sleep-related hypoventilation [8, 9]. As the disease progresses, hypoventilation starts to occur during daytime. Respiratory failure is still the major cause of death, but heart failure secondary to cardiomyopathy has been implicated in some patients [10, 11].
Medical interventions including influenza vaccination, early antibiotic delivery, glucocorticosteroids, nocturnal non-invasive mechanical ventilation with bi-level positive airway pressure (BiPAP), and the use of angiotensin-converting enzyme inhibitors for the treatment of cardiomyopathy [10, 12] have led to significant improvements in quality of life and life expectancy. The mean age of death for DMD patients in the 1960s was 14 years improving now to around 27 years [5, 13].
Posterior instrumented spinal fusion is the treatment of choice for the progressive scoliosis that develops in DMD patients; it improves sitting balance, comfort, appearance, and quality of life [3, 14–16]. Surgery involves segmental spinal instrumentation with sublaminar wires and more recently pedicle screw fixation with titanium rods often fusing from the second thoracic vertebra to the fifth lumbar vertebra or to the pelvis depending on the degree of pelvic obliquity. The advent of the pedicle screw fixation has led to shorter operation times and reduced blood loss [17]. Some authors advocate prophylactic spinal fusion for low magnitude curves on the assumptions that all curves will progress [18]. Most authors advise spinal stabilization before respiratory and cardiac function have deteriorated to a point that would make general anaesthesia unsafe, and whilst the curve is still mobile [6, 11, 19–21].
There is, however disagreement in the literature as to the specific effect of spinal surgery on respiratory function. Whilst the majority of authors claim that surgery has little impact on the rate of respiratory deterioration as shown by spirometry [3, 15, 21–23], there are some studies that report positive findings [11, 20, 24]. Galasko et al. [20] reported that forced vital capacity (FVC) stabilized for 36 months following spinal fusion, compared with a deterioration of 8 % per year in a non-operated group. It would seem logical that correction of spinal deformity should improve, or at least slow the deterioration of respiratory function; given the mechanical effects of a deformed thorax further adding to deterioration in respiratory function [25].
The purpose of this study is to clarify the effect of posterior spinal fusion on pulmonary function, by comparing surgical to non-surgical patients over a 40-year period.
Materials and methods
Institutional Human Research Ethics Committee approval was obtained for this study. The study cohort included 65 patients with DMD, born between 1961 and 2001 who were treated at a metropolitan children’s hospital muscular dystrophy clinic. The diagnosis of DMD was based on family history, muscle enzyme assay, electrophysiological studies, and muscle biopsy. Patients who walked beyond 13 years old were excluded, as this could indicate a milder, Becker-type dystrophy. Patients with incomplete data (serial pulmonary function tests or serial Cobb measurements) were excluded from the study.
Indications for posterior spinal fusion included documented curve progression, loss of seating balance, pain and/or discomfort. During the 40-year period, 28 patients underwent posterior spinal fusion (Surgical Group, SG) and 37 did not (Non-Surgical Group, NSG). Various non-surgical treatments were offered initially, including bracing (no longer considered to be effective) and glucocorticosteroids. During this time period, the surgical method utilised sublaminar wiring with Luque–Galveston instrumentation, and/or supplemental pedicle screw fixation. From 1984 onwards, the majority of children were supported with positive pressure ventilation; either alone or in addition to surgery. There was no change in rate of surgical intervention throughout the time period.
Data was collected from the case notes, sitting radiographs, and pulmonary function tests for all patients. The severity of the scoliosis was measured using the Cobb method [26]. Pulmonary function tests (PFT) were carried out using the Ohio 842 Spirometer and Jaeger Masterlab System in addition to sleep study data.
Statistical analysis was undertaken using Stata Intercooled version 10.1 for Windows and SPSS version 15.0 for Windows. Cobb angles, FVC, and %FVC data were analysed using the non-parametric Wilcoxon rank sum (Mann–Whitney) test, as data were not normally distributed (as determined by the skewness/kurtosis tests for normality).
Results
The mean age at which the surgery was carried out for the SG was 14.2 years (range 10.8–18.3 years). This same age (14.2 years) was chosen for the NSG for sake of comparison with the SG. Using ‘date of surgery’ as the set point removes the effect of age on respiratory decline.
Data was collected from two time points in each group; For the SG, data was collected pre-operatively and again 1.0 year later. For the NSG, data was collected as close to 14.2 years of age and again 1.8 years later. There were no peri-operative deaths, however three patients developed superficial wound infections, and two had transient blood-loss anaemia. These patients were not excluded.
Cobb angle
For the SG the mean pre-operative Cobb angle was 56.4° [standard deviation (SD) 20.7, range 10–90] (Table 1). The mean post-operative Cobb angle was 21.6° (SD 11.0, range 6–51), representing a mean correction of 34.8° (range 10–84°) or a mean improvement of 61.7 %.
Table 1.
Mean age and Cobb angle data for Surgical Group and Non-Surgical Group at time points 1 and 2
| Surgical Group | Non-Surgical Group | |
|---|---|---|
| Pre-surgery/time 1 | ||
| N | 28 | 26 |
| Mean age (SD) (years) | 14.2 (1.9) | 13.9 (2.2) |
| Mean Cobb angle (°) (SD) | 56.4 (20.7) | 34.9 (26.5) |
| Range Cobb angle (°) | 10–90 | 8–110 |
| Post-surgery/time 2 | ||
| N | 24 | 21 |
| Mean age (SD) (years) | 15.2 (2.5) | 15.7 (1.9) |
| Mean Cobb angle (°) (SD) | 21.6 (11.0) | 51.0 (31.8) |
| Range Cobb angle (°) | 6–51 | 10–128 |
For the NSG, the mean Cobb angle was 34.9° (SD 26.5, range 8–110) prior to the set point. At the second time point, the mean Cobb angle had deteriorated to 51.0° (SD 31.8, range 10–128), representing a mean deterioration of 16.1° or 46.1 %.
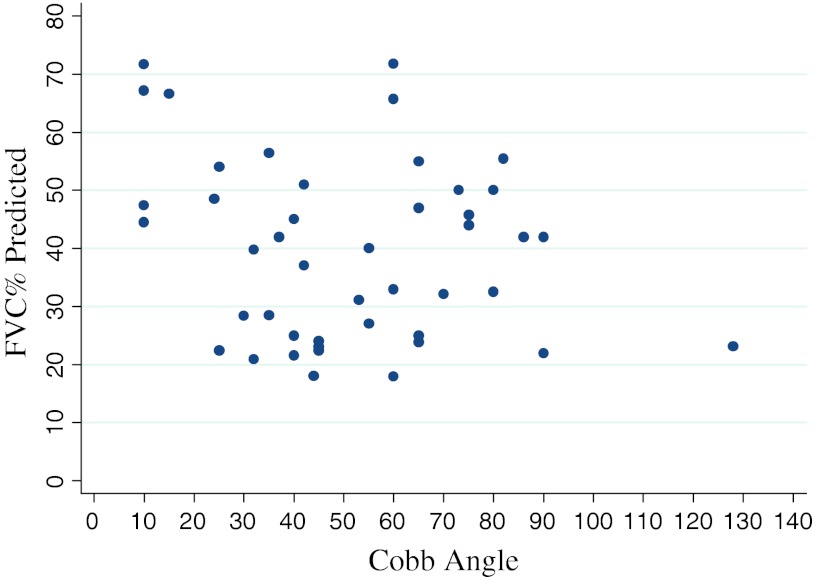
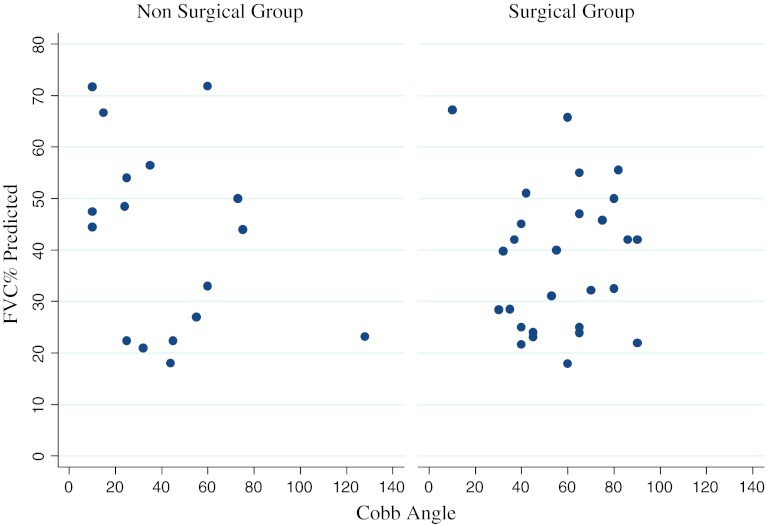
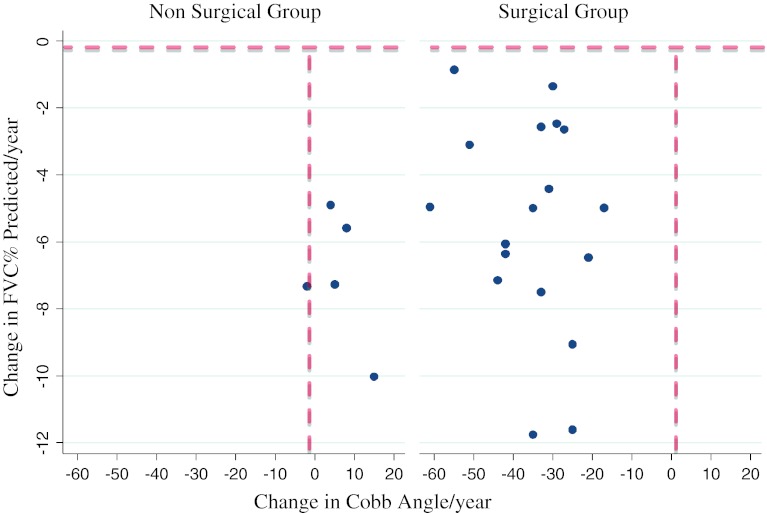
We found no association between curve severity (Cobb angle) and respiratory function (%FVC) at any point in time for the whole group or for the SG or the NSG (p = 0.16) (Figs. 1, 2). Furthermore, there was no association between a change in Cobb angle and a change in %FVC in either group (i.e. an improvement in Cobb angle did not correlate with an improvement in respiratory function or vice versa) (p = 0.14) (Fig. 3).
Fig. 1.
Relationship between Cobb angle and % predicted FVC for the entire cohort (both groups) at 1st time point. No correlation was observed between the severity of scoliosis (Cobb angle) and level of respiratory dysfunction (FVC %) (p = 0.16)
Fig. 2.
Relationship between Cobb angle and % predicted FVC for the Non-Surgical Group (NSG) and the Surgical Group (SG). No correlation was observed between the severity of scoliosis (Cobb angle) and level of respiratory dysfunction (FVC %) in either group (NSG: p = 0.19; SG: p = 0.96)
Fig. 3.
Change in Cobb angle versus Change in % predicted FVC in Non-Surgical Group (NSG) (left) and Surgical Group (SG) (right). Both graphs depict change in Cobb angle, which can be either negative (indicating a improvement in scoliosis) or positive (indicating worsening scoliosis). The vertical dashed lines on both graphs represents no change. Note that all the NSG (left) had INCREASES in the Cobb angle; whereas all the SG (right) had decreases in Cobb angles in the range 20°–60°. The Y-axis shows change in FVC. Note the horizontal dashed line represents zero change and indicates that all patients deteriorated in respiratory function between the two time points. This shows that regardless of whether the Cobb angle deteriorates in the NSG, or improves in the SG, the FVC continues to decline. Along with the previous graph, this indicates that there is no association between Cobb angles and FVCs, even when measured over time with significant improvement in the Cobb angle (NSG: p = 0.37; SG: p = 0.34). Outliers have been excluded
Pulmonary function tests
There was no statistically significant difference in the mean age at which the pulmonary function test data for the two groups were collected (p = 0.30 for time point 1, p = 0.55 for time point 2). However despite the ages at testing being comparable, the SG had a significantly lower %FVC predicted when compared to the NSG (36.2 vs. 54.3 %, respectively, p = 0.02). There was no statistically significant difference in the rate of decline in %FVC per year in the SG when compared to the NSG (SG 5.6 % decline/year vs. NSG 6.9 % decline/year, p = 0.35).
Survival
Of those patients that have died, we found no difference in the mean age of death between the groups (p = 0.11).
Discussion
In this study, posterior spinal fusion in children with Duchene muscular dystrophy was not associated with a reduction in the decline of respiratory function when compared to a similar non-surgical cohort.
Like others who have published in this area [3, 5, 11, 15, 19, 20], our study was not randomised; and conclusions are made after a defined period of observation and measurement for each patient which is relevant in consideration of their progressive disease. Surgery was undertaken for those individuals with documented curve progression, loss of seating balance, pain or discomfort. Results from a previous study suggest that it would be “unethical to randomise any future studies on spinal stabilization in this disease,” given the proven beneficial effects of spinal fusion on quality of life [20].
The pre-operative comparisons of FVC, %FVC, and Cobb angles indicated that the SG had more serious respiratory impairment when compared to their non-surgical counterparts at any age. We speculate that Duchenne’s muscular dystrophy is a heterogeneous disease, with some boys developing symptoms both of a more serious nature, and at a younger age. This is in keeping with the work of others who have published in this area [11].
The lack of correlation between severity of scoliosis (Cobb angle) and respiratory function (FVC, %FVC) was somewhat surprising. We found that across both groups, less severe scoliosis did not partner less severe respiratory dysfunction, and vice versa. Particularly surprising was that this observation held true for both static measurements and dynamic ones, where we compared the reaction of FVC to both scoliosis deterioration and improvement, and found no difference. Logic would tend to suggest that reduced space available for the lung in DMD associated scoliosis would lead to further reduction in pulmonary function over and above that which occurs as a result of respiratory muscle weakness alone, but our data would suggest otherwise. A further explanation is that scoliosis may not cause restrictive pulmonary dysfunction until curves are in the order of 50°–60° [27]. In our study the mean pre-operative Cobb angle for the SG was 56.4° (SD 20.7, range 10–90). We found similar rates of respiratory decline in the members of the SG who did indeed have pre-operative curves in the ranges 80–90°; when compared to patients with less severe scoliosis. This indicates that correction even of the more ‘significant’ curves, still did little to reverse the deterioration in lung function.
Whilst the respiratory failure in DMD has traditionally been attributed to both respiratory muscle weakness and scoliosis; our study questions the contribution that scoliosis adds to the respiratory failure.
With the limitation of non-randomisation in mind, and with these results indicating more serious disease in those patients who underwent surgery, it is important to have an awareness of the heterogeneity of this disease when drawing conclusions. The lack of significant improvement in the SG is perhaps more due to their disease severity, than to the inadequacy of treatment per se. A similar situation presented itself to Miller et al. [15] and Kennedy et al. [3], where, prior to having surgery, the SGs had inferior FVC and %FVC, respectively. Velasco et al. [11] reported considerable variability in disease, despite the mutated dystrophin gene producing a similar phenotype. Given this, and the finding that the rate of FVC decline within individual patients is linear [28], it would be prudent to compare patients with themselves, rather than against others with a different ‘severity’ of DMD.
Analysis of patients with exclusion of intra-disease differences would be ideal. One study attempted this, and reported a reduced rate of FVC decline from 8 %/year preoperatively to 3.9 %/year post-surgery [11]. In contrast, newer techniques in improved ventilatory support such as BiPAP have been shown to improve symptoms, quality of life, and reduce frequency of hospital admissions [29]. Eagle et al. [5] recently reported the median survival of patients treated with posterior spinal fusion and BiPAP to be 30 years of age compared to 22.2 years of age for those treated with BiPAP alone. Furthermore, respiratory function in DMD has traditionally been measured by the FVC1 and the FVC expressed as a percentage of the predicted value based on published normative data (%FVC2). This can predict hypercapnia and survival [30, 31]. The initial respiratory complications of DMD, however, occur during sleep, when respiratory control is at a nadir and muscle atonia leaves only the diaphragm to maintain respirations [32]. Future aims of this study group include the use of sleep studies to more accurately define variations in treatment groups. Boys with DMD-induced diaphragmatic weakness suffer hypoventilation; a pre-cursor to conscious respiratory compromise that is measurable with sleep studies [10].
This study has not demonstrated any significant improvement in respiratory function following correction of scoliosis via posterior spinal fusion for DMD-associated scoliosis. It suggests that respiratory deterioration in itself is not an indication for spinal fusion. Spinal fusion will not improve the intrinsic weakness that affects the respiratory muscles in these patients. This is significant finding given that surgical correction is not without complications; a recent study reported a peri-operative complication rate of 48.1 % following posterior-only pedicle screw instrumentation [33]. Spinal fusion does, however, still have a role, and has clearly been shown to improve sitting balance, pain and discomfort, nursing care, and most importantly quality of life [3, 14–16, 20].
Acknowledgments
AJ Martin (Respiratory Physician, Women’s and Children’s Hospital, Adelaide, South Australia), The Department of Sleep Medicine (Women’s and Children’s Hospital, Adelaide, South Australia).
Conflict of interest
None of the authors or their family members have received financial benefits from any commercial party for this manuscript.
Footnotes
FVC maximum volume of air exhaled from lungs, following maximal inspiration.
%FVC FVC as a percentage of that predicted for a person of the same sex, age, and height.
References
- 1.Hoffman EP, Fischbeck KH. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne’s or Becker’s muscular dystrophy. N Engl J Med. 1988;318(21):1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- 2.Marsh A, Edge G, Lehovsky J. Spinal fusion in patients with Duchenne’s muscular dystrophy and a low forced vital capacity. Eur Spine J. 2003;12(5):507–512. doi: 10.1007/s00586-003-0545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy JD, Staples AJ, Brook PD, et al. Effect of spinal surgery on lung function in Duchenne muscular dystrophy. Thorax. 1995;50(11):1173–1178. doi: 10.1136/thx.50.11.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emery A, Muntoni F. Duchenne muscular dystrophy. Oxford: Oxford University Press; 2003. [Google Scholar]
- 5.Eagle M, Bourke J, Bullock R, et al. Managing Duchenne muscular dystrophy–the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord. 2007;17(6):470–475. doi: 10.1016/j.nmd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Smith AD, Koreska J, Moseley CF. Progression of scoliosis in Duchenne muscular dystrophy. J Bone Joint Surg Am. 1989;71(7):1066–1074. [PubMed] [Google Scholar]
- 7.Oda T, Shimizu N, Yonenobu K, et al. Longitudinal study of spinal deformity in Duchenne muscular dystrophy. J Pediatr Orthop. 1993;13(4):478–488. doi: 10.1097/01241398-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Khan Y, Heckmatt JZ. Obstructive apnoeas in Duchenne muscular dystrophy. Thorax. 1994;49(2):157–161. doi: 10.1136/thx.49.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PE, Calverley PM, Edwards RH. Hypoxemia during sleep in Duchenne muscular dystrophy. Am Rev Respir Dis. 1988;137(4):884–888. doi: 10.1164/ajrccm/137.4.884. [DOI] [PubMed] [Google Scholar]
- 10.Manzur AY, Kinali M, Muntoni F. Update on the management of Duchenne muscular dystrophy. Arch Dis Child. 2008;93(11):986–990. doi: 10.1136/adc.2007.118141. [DOI] [PubMed] [Google Scholar]
- 11.Velasco MV, Colin AA, Zurakowski D, et al. Posterior spinal fusion for scoliosis in Duchenne muscular dystrophy diminishes the rate of respiratory decline. Spine (Phila Pa 1976) 2007;32(4):459–465. doi: 10.1097/01.brs.0000255062.94744.52. [DOI] [PubMed] [Google Scholar]
- 12.Simonds AK, Muntoni F, Heather S, Fielding S. Impact of nasal ventilation on survival in hypercapnic Duchenne muscular dystrophy. Thorax. 1998;53(11):949–952. doi: 10.1136/thx.53.11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12(10):926–929. doi: 10.1016/S0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 14.Granata C, Merlini L, Cervellati S, et al. Long-term results of spine surgery in Duchenne muscular dystrophy. Neuromuscul Disord. 1996;6(1):61–68. doi: 10.1016/0960-8966(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 15.Miller RG, Chalmers AC, Dao H, et al. The effect of spine fusion on respiratory function in Duchenne muscular dystrophy. Neurology. 1991;41(1):38–40. doi: 10.1212/WNL.41.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Sussman MD. Advantage of early spinal stabilization and fusion in patients with Duchenne muscular dystrophy. J Pediatr Orthop. 1984;4(5):532–537. [PubMed] [Google Scholar]
- 17.Arun R, Srinivas S, Mehdian SM. Scoliosis in Duchenne’s muscular dystrophy: a changing trend in surgical management: a historical surgical outcome study comparing sublaminar, hybrid and pedicle screw instrumentation systems. Eur Spine J. 2010;19(3):376–383. doi: 10.1007/s00586-009-1163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rideau Y, Glorion B, Delaubier A, et al. The treatment of scoliosis in Duchenne muscular dystrophy. Muscle Nerve. 1984;7(4):281–286. doi: 10.1002/mus.880070405. [DOI] [PubMed] [Google Scholar]
- 19.Brook PD, Kennedy JD, Stern LM, et al. Spinal fusion in Duchenne’s muscular dystrophy. J Pediatr Orthop. 1996;16(3):324–331. doi: 10.1097/01241398-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Galasko CS, Delaney C, Morris P. Spinal stabilization in Duchenne muscular dystrophy. J Bone Joint Surg Br. 1992;74(2):210–214. doi: 10.1302/0301-620X.74B2.1544954. [DOI] [PubMed] [Google Scholar]
- 21.Miller F, Moseley CF, Koreska J. Spinal fusion in Duchenne muscular dystrophy. Dev Med Child Neurol. 1992;34(9):775–786. doi: 10.1111/j.1469-8749.1992.tb11516.x. [DOI] [PubMed] [Google Scholar]
- 22.Gayet LE. Surgical treatment of scoliosis due to Duchenne muscular dystrophy. Chirurgie. 1999;124(4):423–431. doi: 10.1016/S0001-4001(00)80016-1. [DOI] [PubMed] [Google Scholar]
- 23.Kinali M, Messina S, Mercuri E, et al. Management of scoliosis in Duchenne muscular dystrophy: a large 10-year retrospective study. Dev Med Child Neurol. 2006;48(6):513–518. doi: 10.1017/S0012162206001083. [DOI] [PubMed] [Google Scholar]
- 24.Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39(4):475–481. doi: 10.1212/WNL.39.4.475. [DOI] [PubMed] [Google Scholar]
- 25.Kurz LT, Mubarak SJ, Schultz P, et al. Correlation of scoliosis and pulmonary function in Duchenne muscular dystrophy. J Pediatr Orthop. 1983;3(3):347–353. doi: 10.1097/01241398-198307000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Cobb J. Outline for the study of scoliosis. Instr Course Lect. 1948;5:261–275. [Google Scholar]
- 27.Tsiligiannis T, Grivas T. Pulmonary function in children with idiopathic scoliosis. Scoliosis. 2012;7(1):7. doi: 10.1186/1748-7161-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller F, Mosely CF, Koreska J, Levison H. Pulmonary function and scoliosis in Duchenne dystrophy. J Pediatr Orthop. 1988;8(2):133–137. [PubMed] [Google Scholar]
- 29.Young HK, Lowe A, Fitzgerald DA, et al. Outcome of non-invasive ventilation in children with neuromuscular disease. Neurology. 2007;68(3):198–201. doi: 10.1212/01.wnl.0000251299.54608.13. [DOI] [PubMed] [Google Scholar]
- 30.Toussaint M, Steens M, Soudon P. Lung function accurately predicts hypercapnia in patients with Duchenne muscular dystrophy. Chest. 2007;131(2):368–375. doi: 10.1378/chest.06-1265. [DOI] [PubMed] [Google Scholar]
- 31.Bourke SC, Gibson GJ. Sleep and breathing in neuromuscular disease. Eur Respir J. 2002;19(6):1194–1201. doi: 10.1183/09031936.02.01302001a. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy JD, Martin AJ. Chronic respiratory failure and neuromuscular disease. Pediatr Clin North Am. 2009;56(1):261–273. doi: 10.1016/j.pcl.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Modi HN, Suh SW, Hong JY, et al. Treatment and complications in flaccid neuromuscular scoliosis (Duchenne muscular dystrophy and spinal muscular atrophy) with posterior-only pedicle screw instrumentation. Eur Spine J. 2010;19(3):384–393. doi: 10.1007/s00586-009-1198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]