Abstract
Purpose
This study aimed to explore the feasibility of ultra-short-course chemotherapy in the treatment of spinal tuberculosis.
Methods
One hundred and eighty-five patients with confirmed spinal tuberculosis and surgical indication were included. The chemotherapy regimen was 2SHRZ/XHRZ. According to the duration of the chemotherapy, the patients were divided into two groups, the ultra-short-course chemotherapy group with an average duration of 4.5 months, and the standard chemotherapy group with an average duration of 9 months. The same surgery was performed for patients in the two groups.
Results
The duration of the follow-up ranged from 61 to 87 months, with an average of 69.1 months. Erythrocyte sedimentation rate and C-reactive protein, kyphosis and nerve function, recovery of work, and activities of daily living were not significantly different between the two groups before or after treatment; however, the aforementioned indices were significantly different before and after treatment within groups. There was no significant difference in postoperative bone graft healing between the two groups. The drug side effects were significantly different between the two groups.
Conclusions
With thorough focus debridement, bone grafting, and internal fixation, the efficacy of ultra-short chemotherapy was similar to that of standard chemotherapy for the treatment of spinal tuberculosis. The ultra-short-course chemotherapy can shorten the course of treatment and reduce drug side effects.
Keywords: Spine, Tuberculosis, Ultra-short-course chemotherapy, Efficacy, Thorough debridement
Introduction
Spinal tuberculosis is a common infectious disease, and its incidence has increased in both developed and developing countries in recent years [1, 2]. Chemotherapy combined with surgical treatment is the main method of treatment for spinal tuberculosis patients with surgical indications. With the emergence of the new theory and new techniques of spinal surgery, the surgical treatment of spinal tuberculosis has developed rapidly; however, drug treatment is still the 9-month standard chemotherapy regimen that has been used in the past several decades. The traditional standard chemotherapy regimen has achieved satisfactory effectiveness in the treatment of spinal tuberculosis [3], but its greatest shortcoming is its excessively long duration. Clinical studies have proven the effectiveness of 6- to 9-month short-course chemotherapy for the treatment of spinal tuberculosis. On that basis, can the duration of chemotherapy be further shortened for the treatment of spinal tuberculosis? Chemotherapy regimens of less than 6 months are referred to as ultra-short-course chemotherapy in the treatment of pulmonary tuberculosis [4–8], whose success in the treatment of pulmonary tuberculosis has been reported [9]; however, its application in the treatment of bone and joint tuberculosis has not been reported.
In 2007, the authors reported satisfactory preliminary results of 4- to 6-month ultra-short-course chemotherapy regimen for the treatment of spinal tuberculosis [10]. In order to investigate the exact effects of ultra-short-course chemotherapy, we conducted this 5-year follow-up study to compare the effectiveness of ultra-short-course chemotherapy and standard chemotherapy with thorough surgical debridement in patients with spinal tuberculosis.
Materials and methods
Study design
To compare the effectiveness of ultra-short-course chemotherapy and standard chemotherapy in combination with thorough debridement in patients with spinal tuberculosis through a prospective cohort study, patients with spinal tuberculosis treated at our hospital from January 1998 to January 2006 were included in this study, according to the different chemotherapy regimens divided into ultra-short-course chemotherapy group and the standard chemotherapy group. The effectiveness of chemotherapy was observed of two groups for at least 5 years.
Inclusion criteria
Spinal tuberculosis was diagnosed according to symptoms, physical signs, imaging examination results, and etiological and histopathological results. Patients were evaluated to be appropriate candidates for surgical focus debridement for tuberculosis and no contraindications to surgery. The indications for surgery were as follows: cases with spinal cord or cauda equina nerve or root compression causing neurological dysfunction; cases where the stability of the spine had been destroyed; cases with serious or progressive kyphosis deformities; cases accompanied by large abscess formation, bone sequestration, cavitation or sinus tract formation. The patients met the study criteria signed informed consent.
Grouping
One hundred and eighty-five patients met the inclusion criteria and were randomly divided into two treatment groups according to the different chemotherapy regimens (Table 1). Randomized patients extract the envelope which had a random number with different chemotherapy regimens.
Table 1.
Preoperative data in the ultra-short-course chemotherapy group and in the standard chemotherapy group
| The ultra-short-course chemotherapy group | The standard chemotherapy group | 95 % CI | P value | |
|---|---|---|---|---|
| Mean age (x ± s) | 37.96 ± 16.30 | 41.53 ± 15.76 | −8.227 to 1.087 | 0.132 |
| Number of males/females (n = 185) | 54/42 (96) | 50/39 (89) | – | – |
| Lesion location (n = 185) | 96 | 89 | – | – |
| Cervical vertebrae | 4/96 (4.2) | 3/89 (3.4) | – | – |
| Cervical and thoracic vertebrae | 2/96 (2.1) | 1/89 (1.1) | – | – |
| Thoracic vertebrae | 20/96 (20.8) | 18/89 (20.2) | – | – |
| Thoracolumbar segment | 17/96 (17.7) | 25/89 (28.1) | – | – |
| Lumbar vertebrae | 40/96 (41.7) | 32/89 (36.0) | – | – |
| Lumbosacral segment | 13/96 (13.5) | 10/89 (11.2) | – | – |
| Vertebral involvement (n = 185) | 96 | 89 | – | – |
| 1 or 2 vertebrae | 64/96 (66.7) | 66/89 (74.2) | – | – |
| ≥3 vertebrae | 32/96 (33.3) | 23/89 (25.8) | – | – |
| The kyphosis Cobb angle (n = 185) | 20.53 ± 8.64° | 19.35 ± 8.65° | −1.327 to 3.693 | 0.354 |
| Abscess (n = 160) | 83/96 (86.5) | 77/89 (86.5) | – | – |
| Nerve compression (n = 54) | 30/96 (31.3) | 24/89 (27.0) | – | – |
| Combined with other parts TB | 6 | 7 | – | – |
| ESR (mm/H) | 53.18 ± 18.12 | 54.66 ± 18.72 | −6.831 to 3.859 | 0.548 |
| CRP (mg/L) | 27.11 ± 11.93 | 28.15 ± 13.44 | −4.728 to 2.633 | 0.575 |
| Duration of follow-up (months) | 74.15 ± 7.44 | 74.08 ± 7.38 | −2.084 to 2.219 | 0.951 |
The values without a unit are shown as number of cases/number of total cases (%)
There were 96 patients in the ultra-short-course chemotherapy group (ultra-short group), of whom 54 were males and 42 females; The mean age was 37.96 ± 16.30 years; The lesions affected 1–2 segments in 64 patients, and three or more segments in 32 patients, and the average number of affected vertebrae was 1.51 segments. The most commonly affected vertebrae were the lumbar vertebrae (41.7 %).
There were 89 patients in the standard chemotherapy group (standard group), of whom 50 were males and 39 females. The mean age was 41.53 ± 15.76 years. The lesions affected 1–2 segments in 66 patients, and three or more segments in 23 patients, and the average number of affected vertebrae was 1.37. The lumbar vertebrae were the most commonly affected vertebrae (36.0 %).
Methods
Preoperative chemotherapy
Preoperative chemotherapy was the same in the two groups. The duration of preoperative chemotherapy ranged from 2 to 4 weeks, with an average of 3.1 weeks. The chemotherapy was stopped when systemic toxic manifestations of tuberculosis were alleviated and the general condition of the patients improved. Surgery was performed when the patients could tolerate surgery.
Surgical method
Anterior radical focus debridement, placement of an intervertebral bone graft between the affected vertebrae and anterior or posterior internal fixation were adopted in the two groups. Radical focus debridement not only thoroughly removes the sequestrum, abscesses, granulation tissue, caseous necrotic substance, sinus, and necrotic intervertebral disc in the affected vertebrae, but also resects the dead space or cavity, hardened wall, and pathologic bony bridge [10].
One hundred and forty-two cases underwent posterior fixation, combined with anterior debridement and strut graft. All cases underwent intervertebral focal surgery. All cases were operated in the forced prone position, and a posterior median incision was made centered on the affected vertebral body. In cases where the residual height of the body was one-third to two-thirds of normal, the normal screws were used via a posterior approach at the affected body; in cases where the residual height of the body was less than one-third of normal, a 25- to 35-mm short screw was used and a connecting rod was added to increase stability. All incisions were closed after fixation. The cases were then changed to the lateral position, with the deteriorated body super. After that, focal debridement and graft implantation were undertaken via thoracic, thoracolumbar, or peritoneal approaches for different affected segments; All cases underwent implantation of autologous iliac bone. Posterior fixation, including CD, GSS, M8, and SINO, was selected.
Forty-three cases underwent one-stage anterior fixation, combined with anterior debridement and strut graft. This strategy was used for cases where the residual height of the body was two-third of normal; the normal screws can be used at the affected body. All cases were operated in the lateral position, with the deteriorated body super. After that, focal debridement, graft implantation, anterior deformity correction, and internal fixation were performed, and were undertaken via thoracic, thoracolumbar, or peritoneal approaches for different affected segments; all cases underwent implantation of autologous iliac bone. Anterior fixation, including Z-Plate and Ventrofix, was selected.
Chemotherapy regimen
The duration of chemotherapy was 4–6 months with an average of 4.5 months in the ultra-short group. The chemotherapy regimen was 2SHRZ/2–4HRZ. Streptomycin (S) 20 mg/kg/d, with a maximum dose of 1.0 g, was given by intramuscular injection, once per day. The following agents were taken orally, once daily: isoniazid (H) 5 mg/kg/d, with a maximum dose of 300 mg; rifampicin (R) 10 mg/kg/d, with a maximum dose of 0.6 g; and pyrazinamide (Z) 25 mg/kg/d, with a maximum dose of 2.0 g. The intensification phase was 2 months. The consolidation phase was 2–4 months, during which time the doses were the same as those during the intensification phase. The duration of chemotherapy was 9 months in the standard group, and the average duration was 12 months. The chemotherapy regimen was 2SHRZ/7HRZ. The daily dose of standard group was the same as that in ultra-short-course chemotherapy group.
Observation methods and outcomes
A unified clinical data collection form was used to collect data, and a spinal tuberculosis database was established. Patients were supervised by a specific person and were examined regularly, and the results were recorded. Patients were examined after admission, before surgery, 2 weeks after surgery, every month within 6 months after surgery, and every year within 10 years after surgery. The following outcomes were recorded. (1) Clinical manifestations: Toxic symptoms of TB, local pain, recovery of ability to work and to perform activities of daily living. (2) Recovery of neurological function was assessed using the ASIA scales. (3) Bone graft healing and correction of deformity were observed using imaging including X-ray, CT scan or CT reconstruction, and MRI scan or contrast-enhanced MRI. The time of examination was before surgery, 2 weeks after surgery, 5 months after surgery, and every year within 10 years after surgery. The Cobb angle was measured in the lateral X-ray image for the assessment of correction of deformity, and bone graft healing was assessed according to Moon’s standard [11]. (4) Laboratory tests employed were ESR, CRP, and tests to assess drug side effects, including liver and kidney function. (5) B-mode ultrasound was used to assess cold abscess. The aforementioned time of examination was adjusted if concerns arose during the course of treatment.
The DOTS recommended by the World Health Organization (WHO) was used, and the short-course chemotherapy regimen was changed into the ultra-short-course chemotherapy regimens. The direct observation was changed as follows: (1) A specific person was responsible for supervision. (2) Regarding health education, patients were taught the methods and the possible outcomes of the disease. (3) A follow-up card was created, and was given to each patient, and each patient received medicine and had follow-up visits according to the instructions on that card. (4) Telephone and/or the Internet was used for communication.
Statistical analysis
SPSS11.5. was used for data analysis. The quantitative data of the two groups were shown as 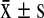 and were compared using pairwise t test. The categorical data of the two groups were compared using x2 test. α = 0.005 used for the test standards.
and were compared using pairwise t test. The categorical data of the two groups were compared using x2 test. α = 0.005 used for the test standards.
Results
One hundred and eighty-five patients met the inclusion criteria and were followed. There were 96 patients in the ultra-short group and 89 patients in the standard group. The duration of follow-up ranged from 61 to 84 months in the ultra-short group, with an average duration of 67.5 months. The duration of follow-up ranged from 64 to 87 months in the standard group, with an average duration of 70.3 months. There was no significant difference in the preoperative age, sex, lesion location, number of affected vertebrae, number of patients with abscess, the kyphosis Cobb angle, ESR and CRP test, neurological function score, or occurrence of combined extraspinal tuberculosis between the two groups (Table 1).
Clinical symptoms
Disappearance of toxic symptoms of TB and local pain and recovery of ability to work and to perform activities of daily living were seen in 90/96 (93.8 %) in the ultra-short group, and 85/89 (95.5 %) in the standard group.
Correction of deformity and fusion of bone graft
The mean preoperative Cobb angle was 20.53 ± 8.64° in the ultra-short group, and the Cobb angle was 5.65 ± 4.60° after surgery. The deformity correction rate was 72.5 %. The Cobb angle was 7.45 ± 4.87° at the last follow-up in that group. The loss correction Rate was 8.8 %. The mean preoperative Cobb angle was 19.35 ± 8.65° in the standard group, and the Cobb angle was 6.15 ± 4.92° after surgery. The deformity correction rate was 68.2 %. The Cobb angle was 7.62 ± 5.17° at the last follow-up in that group. Loss correction Rate was 7.6 % at the last follow-up in that group. There was no significant difference in the Cobb angle between the two groups before treatment (P > 0.05) or after treatment (P > 0.05). For each group, the Cobb angle was significantly different before and after treatment (P < 0.05). The average healing time was 5.3 months (range 3.0–5.5). At the last follow-up, 100 % of grafts had achieved fusion in the ultra-short group, and 98.9 % of grafts had achieved fusion in the standard group. Bone was not healed in one patient with severe acute renal failure, who was not compliant with the recommended chemotherapy. There was no significant difference in bone healing between the two groups (P > 0.05).
Neurological function ASIA scores
In both groups, neurological function ASIA scores were lower than normal before surgery, but they were not significantly different between the two groups (P > 0.05). The neurological function ASIA score increased in the two groups after surgery. There were no significant differences between the two groups (P > 0.05). Within each group, the neurological function ASIA score were significantly different before and after treatment (P < 0.05) (Table 2).
Table 2.
Neurological conditions in the two groups (ASIA scores)
| Group | Motor function scores | P value | Sensory function scores | P value | ||
|---|---|---|---|---|---|---|
| Before treatment | Last follow-up | Before treatment | Last follow-up | |||
| Ultra-short-course chemotherapy group | 76.18 ± 11.54 | 98.21 ± 4.72 | 0.000 | 181.59 ± 24.53 | 220.90 ± 8.67 | 0.000 |
| Standard chemotherapy group | 76.21 ± 15.5 | 96.69 ± 6.18 | 0.000 | 180.13 ± 32.19 | 218.09 ± 11.54 | 0.000 |
| P value | 0.985 | 0.063 | 0.731 | 0.065 | ||
The normal total scores of motor function: 200. The normal total scores of sensory function: 224
ESR and CRP
In both groups, ESR and CRP were higher than normal before treatment, but they were not significantly different between the two groups (P > 0.05). ESR and CRP of 88 cases in the ultra-short-course chemotherapy group and 83 cases in the standard chemotherapy group returned to normal after treatment. There were no significant differences between the two groups (P > 0.05). Within each group, ESR and CRP were significantly different before and after treatment (P < 0.05) (Table 3).
Table 3.
Changes in ESR and CRP in the two groups
| Groups | ESR (mm/H) | CRP (mg/L) | ||
|---|---|---|---|---|
| Before treatment | At the end of treatment | Before treatment | At the end of treatment | |
| Ultra-short-course chemotherapy group | 53.18 ± 18.12 | 7.74 ± 3.58 | 27.11 ± 11.93 | 1.00 ± 0.52 |
| Standard chemotherapy group | 54.66 ± 18.72 | 8.19 ± 3.98 | 28.15 ± 13.44 | 0.90 ± 0.55 |
| P value | 0.548 | 0.418 | 0.575 | 0.231 |
Normal range of ESR: male 0–15 mm/h; female 0–20 mm/h. Normal range of CRP: 0–2.87 mg/L
Unhealed lesions at the end of chemotherapy
At the end of the chemotherapy, good therapeutic results were achieved in 171 of 185 patients in the two groups. ESR and CRP did not return to normal in only 14 patients: 8 cases (8.3 %) in the ultra-short-course chemotherapy group, and 6 cases (6.7 %) in the standard chemotherapy group. The causes were explored (Table 4). Of the 14 patients, 13 were cured after surgery and by adjusting the chemotherapy regimen (Table 4). One patient discontinued chemotherapy because of acute renal dysfunction and had protracted disease.
Table 4.
Unhealed lesions at the end of chemotherapy in the two groups
| Reasons for poor efficacy | Ultra-short-course chemotherapy group (cases) | Standard chemotherapy group (cases) | x2 | P |
|---|---|---|---|---|
| Multidrug-resistant TB | 3 | 2 | – | – |
| Other parts of TB | 3 | 1 | – | – |
| Severe liver and renal dysfunction | 0 | 2 | – | – |
| Diabetes | 1 | 1 | – | – |
| Rheumatic disease | 1 | 0 | – | – |
| Total | 8 | 6 | 5.101 | 0.021 |
Side effects
Side effects included allergic skin reactions and gastrointestinal reactions, blurred vision, hearing impairment, and abnormal liver and kidney function. Adverse events occurred in 8 (8.3 %) patients in the ultra-short-course chemotherapy group, and in 18 (20.2 %) patients in the standard chemotherapy group. The incidence of adverse events in the standard group was significantly higher than that in the ultra-short group (P < 0.05). All patients experiencing adverse events were treated with conservative treatment, and all of them recovered (Table 5).
Table 5.
Side effects of chemotherapy in the two groups
| Side effects | Ultra-short-course chemotherapy group (cases) | Standard chemotherapy group (cases) | x2 | P |
|---|---|---|---|---|
| Allergic reactions (rash, fever) | 1 | 2 | – | – |
| Nausea and vomiting | 3 | 4 | – | – |
| Vision dysfunction | 0 | 3 | – | – |
| Auditory dysfunction | 1 | 3 | – | – |
| Liver and kidney dysfunction | 3 | 6 | – | – |
| Total | 8 | 18 | 5.407 | 0.020 |
Discussion
The 9- to 18-month standard chemotherapy regimen has its shortcomings for the treatment of spinal tuberculosis. The major disadvantage is the long duration of drug treatment, which many patients cannot tolerate. Second, the long drug administration makes supervision laborious and time-consuming, and long-term combination therapy may readily lead to side effects, increasing the incidence of tissue and organ damage [12, 13]. In our patients, the overall incidence of side effects was 20.2 % in the standard chemotherapy group and 8.3 % in the ultra-short group. The incidence in the standard chemotherapy group was significantly higher than that in the ultra-short-course chemotherapy group. Anti-TB-drug-induced side effects not only bring additional pain to patients, but also affect compliance with chemotherapy, which may even lead to failure of chemotherapy. Faced with a series of problems caused by long-term chemotherapy, further shortening of the duration of chemotherapy may be necessary for the treatment of spinal tuberculosis in clinical practice.
Ultra-short-course chemotherapy is feasible in theory and practice for the treatment of spinal tuberculosis. In the 1970s, the short-course chemotherapy regimen of the MRC (Medical Research Council) proved that the duration of chemotherapy for pulmonary tuberculosis can be shortened to 6–9 months [14], and short-course chemotherapy was also successful in the treatment of spinal tuberculosis [15–18]. Ultra-short-course chemotherapy has been explored and applied in the treatment of pulmonary tuberculosis. The recognized ultra-short-course chemotherapy regimen was 4.0–5.5 months [8]. When the 2SHRZ/4HR-based ultra-short-course chemotherapy regimen with an average duration of 4.7 months was adopted, the rate of conversion to negative results in smear-positive pulmonary tuberculosis was 98.3 %, and the bacteriological relapse rate was only 1.9 % during the 2-year follow-up [19]. The 5-month ultra-short-course chemotherapy regimen for the initial treatment of smear-positive pulmonary tuberculosis reached a conversion rate of 97.0 %, and a 3-year recurrence rate of 2.2 % 9. Therefore, we inferred that ultra-short-course chemotherapy should be feasible in the treatment of spinal tuberculosis. First, all of the chemotherapy regimens for pulmonary tuberculosis are appropriate for extrapulmonary tuberculosis. The effects of short-course chemotherapy in extrapulmonary tuberculosis are similar to those in pulmonary tuberculosis [20–22]; spinal tuberculosis is a common type of extrapulmonary tuberculosis; therefore, the ultra short-course chemotherapy should have the same effects. Second, the density of Mycobacterium tuberculosis organisms in lesions of spinal tuberculosis is less than the density in lesions of pulmonary tuberculosis [23]. The chemotherapy with an average duration of 4.5 months should be effective in the treatment of spinal tuberculosis. Third, the effectiveness of surgery plus chemotherapy in the treatment of spinal tuberculosis should be superior to that of simple chemotherapy in the treatment of pulmonary tuberculosis. Spinal tuberculosis is treated with surgery, which removes the tuberculous lesions to the maximal extent. Surgery limits the pathological complexity of lesions, enabling more rapid progress in the direction of recovery, thus greatly shortening the course of treatment. Fourth, thorough focal debridement removes the mechanical barrier to the anti-TB drug, thus allowing it to enter the lesions more effectively and to kill residual Mycobacterium tuberculosis [24].
In 2007, we reported a 3-year follow-up study on the effects of the ultra-short-course chemotherapy in the treatment of spinal tuberculosis 10, and the preliminary effectiveness was satisfactory. This 5-year follow-up study showed more clearly that there was no significant difference in correction of deformity, improvement of function, healing of the bone graft, or recovery of ESR and CRP between the ultra-short-course chemotherapy and the standard chemotherapy. Compared with the results reported in the literature, the ultra-short-course chemotherapy achieved satisfactory results in correcting or maintaining the kyphosis angle [25–28].
Our data showed that in the ultra-short-course chemotherapy group, the number of cases with abnormal ESR and CRP caused by incomplete removal of the focus, and persistent extraspinal TB was higher than that in the standard chemotherapy group at the end of chemotherapy, which might be caused by the insufficient duration of the chemotherapy. This study shows that on the basis of thorough debridement, the efficacy of ultra-short-course chemotherapy in the treatment of spinal tuberculosis is similar to that with standard chemotherapy, and significantly shortens the course of treatment. The new chemotherapy regimens can be implemented easily and be cost-efficient, especially in the developing countries and poor areas to alleviate the shortage of healthcare funding.
In this group of patients, ultra-short-course chemotherapy is appropriate when the following conditions are met: (1) the focus can be removed completely. Incomplete debridement of the focus is the main factor limiting the effectiveness of the ultra-short-course chemotherapy. (2) Patients do not have active extraspinal tuberculosis. Extraspinal tuberculosis requires extended chemotherapy. (3) Patients have no serious liver and kidney dysfunction. Patients with serious liver and kidney dysfunction show poor compliance with chemotherapy. (4) Patients have no comorbid conditions. The effectiveness of the ultra-short-course chemotherapy is poor in patients with severe comorbidities (such as diabetes and active rheumatic diseases). (5) The ultra-short-course chemotherapy is suitable for patients receiving either initial or repeated chemotherapy.
Application of the DOTS strategy is the key to ensuring the success of chemotherapy. DOTS strategy is summarized by the WHO and is an effective method to improve the cure rate of chemotherapy. It is the most cost-effective strategy [29]. DOTS strategy has achieved a high cure rate in the treatment of pulmonary tuberculosis, but its efficacy in the treatment of spinal tuberculosis has not been reported. We modified the DOTS strategy, applied it in the ultra-short-course chemotherapy for the treatment of spinal tuberculosis, and obtained excellent results; however, we must emphasize that some surgeons believe that surgery is more important than chemotherapy, and this misconception should be corrected. Understanding this concept is important for the implementation of the DOTS strategy.
Conclusions
This study demonstrated that in conjunction with thorough focal debridement, bone grafting, and internal fixation, the effectiveness of ultra-short chemotherapy was similar to that of standard chemotherapy in the treatment of spinal tuberculosis. Ultra-short-course chemotherapy can shorten the course of treatment and reduce drug side effects; however, ultra-short chemotherapy is not suitable for patients with incomplete focus debridement, concurrent active extraspinal tuberculosis, severe liver and kidney dysfunction, or serious medical comorbidities.
As a prospective cohort study, the data used for this study were collected routinely; all patients had signed the informed consent form before treatment. This study did not require special approval by the Ethics Committee.
Conflict of interest
None.
References
- 1.Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br. 2010;92(7):905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 2.Tuli SM. Tuberculosis of the spine: a historical review. Clin Orthop Relat Res. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- 3.Won Park Dae, Sohn JW, Kim E-H, et al. Outcome and management of spinal tuberculosis according to the severity of disease. Spine. 2007;32(4):E130–E135. doi: 10.1097/01.brs.0000255216.54085.21. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal IM, Zhang M, Williams KN, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4(12):e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies GR, Nuermberger EL. Pharmacokinetics and pharmacodynamics in the development of anti-tuberculosis drugs. Tuberculosis (Edinb) 2008;88(S1):S65–S74. doi: 10.1016/S1472-9792(08)70037-4. [DOI] [PubMed] [Google Scholar]
- 6.Nuermberger EL, Spigelman MK, Yew WW. Current development and future prospects in chemotherapy of tuberculosis. Respirology. 2010;15(5):764–778. doi: 10.1111/j.1440-1843.2010.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young DB, Perkins MD, Duncan K. Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;118(4):1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreis B, Pretet S, Birenbaum J, et al. Two three-month treatment regimens for pulmonary tuberculosis. Bull Int Union Tuberc. 1976;51(1):71–75. [PubMed] [Google Scholar]
- 9.National Collaborative Group of short-course chemotherapy for pulmonary tuberculosis Five-month short-course chemotherapy and six-month whole-course intermittent regimen for the treatment of smear-positive pulmonary tuberculosis: a controlled clinical study. Chin J Tuberc Respir Dis. 1998;21:388–391. [Google Scholar]
- 10.Wang Z, Ge Z, Jin W, et al. Treatment of spinal tuberculosis with ultrashort-course chemotherapy in conjunction with partial excision of pathologic vertebrae. Spine J. 2007;7(6):671–681. doi: 10.1016/j.spinee.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Moon MS, Woo YK, Lee KS, et al. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20:1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Xia A, Zhan S. Comprehensive analysis of the incidence of anti-tuberculosis drug-induced adverse reactions in China. Chin J Tuberc Respir Dis. 2007;30:419–423. [PubMed] [Google Scholar]
- 13.Jin W, Wang Z, Ma X, et al. Clinical analysis of side effects of anti-tuberculosis drugs in 156 patients with spinal tuberculosis. Trans Third Military Med Univ. 2009;31:1932–1936. [Google Scholar]
- 14.East African/British Medical Reasearch Council (1972) Controlled clinical trial of short-course (6-month) regimens of chemotherapy for treatmen of pulmonary tuberculosis. Lanced, 1:1079–1085 [PubMed]
- 15.Cattamanchi A, Dantes RB, Metcalfe JZ, et al. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin Infect Dis. 2009;48(2):179–185. doi: 10.1086/595689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swaminathan S, Deivanayagam CN, Rajasekaran S, et al. Long term follow up of HIV-infected patients with tuberculosis treated with 6-month intermittent short course chemotherapy. Natl Med J India. 2008;21(1):3–8. [PubMed] [Google Scholar]
- 17.Guo LX, Ma YZ, Chen X, et al. Clinical study of short-course chemotherapy combined with radical operation in retreating spinal tuberculosis. Zhongguo Gu Shang. 2010;23(7):491–494. [PubMed] [Google Scholar]
- 18.Parthasarathy R, Sriram K, Santha T, et al. Short-course chemotherapy for tuberculosis of the spine A comparison between ambulant treatmentand radical surgery-ten-year report. J BoneJoint Surge (Br). 1999;81:464–471. doi: 10.1302/0301-620X.81B3.9043. [DOI] [PubMed] [Google Scholar]
- 19.Xiao C, Fu Y, Li Z, et al. Initial studies on short-course chemotherapy with irregular chemotherapeutic terms. Chin J Tuberc Respir. 1997;20:258–260. [Google Scholar]
- 20.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 21.Arora S, Sabat D, Maini L, et al. The results of nonoperative treatment of craniovertebral junction tuberculosis: a review of twenty-six cases. J Bone Joint Surg Am. 2011;93(6):540–547. doi: 10.2106/JBJS.J.00634. [DOI] [PubMed] [Google Scholar]
- 22.Mwachaka PM, Ranketi SS, et al. Spinal tuberculosis among human immunodeficiency virus-negative patients in a Kenyan tertiary hospital: a 5-year synopsis. Spine J. 2011;11(4):265–269. doi: 10.1016/j.spinee.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman EB, Crosier JH, Cremin BJ. Imaging in children with spinal tuberculosis: a comparison of radiography, computed tomography and magnetic resonance imaging. J Bone Joint Surg [Br] 1993;75-B:233–239. doi: 10.1302/0301-620X.75B2.8444943. [DOI] [PubMed] [Google Scholar]
- 24.Ge Z, Wang Z, Wei M. Measurement of the concentration of three antituberculosis drugs in the focus of spinal tuberculosis. Eur Spine J. 2008;17(11):1482–1487. doi: 10.1007/s00586-008-0778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erturer E, Tezer M, Aydogan M, et al. The results of simultaneous posterior-anterior-posterior surgery in multilevel tuberculosis spondylitis associated with severe kyphosis. Eur Spine J. 2010;19(12):2209–2215. doi: 10.1007/s00586-010-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon MS, Kim SS, Lee BJ, et al. Surgical management of severe rigid tuberculous kyphosis of dorsolumbar spine. Int Orthop. 2011;35(1):75–81. doi: 10.1007/s00264-010-0999-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erturer E, Tezer M, Aydogan M, et al. The results of simultaneous posterior-anterior-posterior surgery in multilevel tuberculosis spondylitis associated with severe kyphosis. Eur Spine J. 2010;19(12):2209–2215. doi: 10.1007/s00586-010-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Zhang Y, Zhang X, et al. Posterior-only multilevel modified vertebral column resection for extremely severe Pott’s kyphotic deformity. Eur Spine J. 2009;18(10):1436–1441. doi: 10.1007/s00586-009-1067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steffen R, Menzies D, Oxlade O, et al. Patients’ costs and cost-effectiveness of tuberculosis treatment in DOTS and non-DOTS facilities in Rio de Janeiro, Brazil. PLoS ONE. 2010;5(11):e14014. doi: 10.1371/journal.pone.0014014. [DOI] [PMC free article] [PubMed] [Google Scholar]


