Abstract
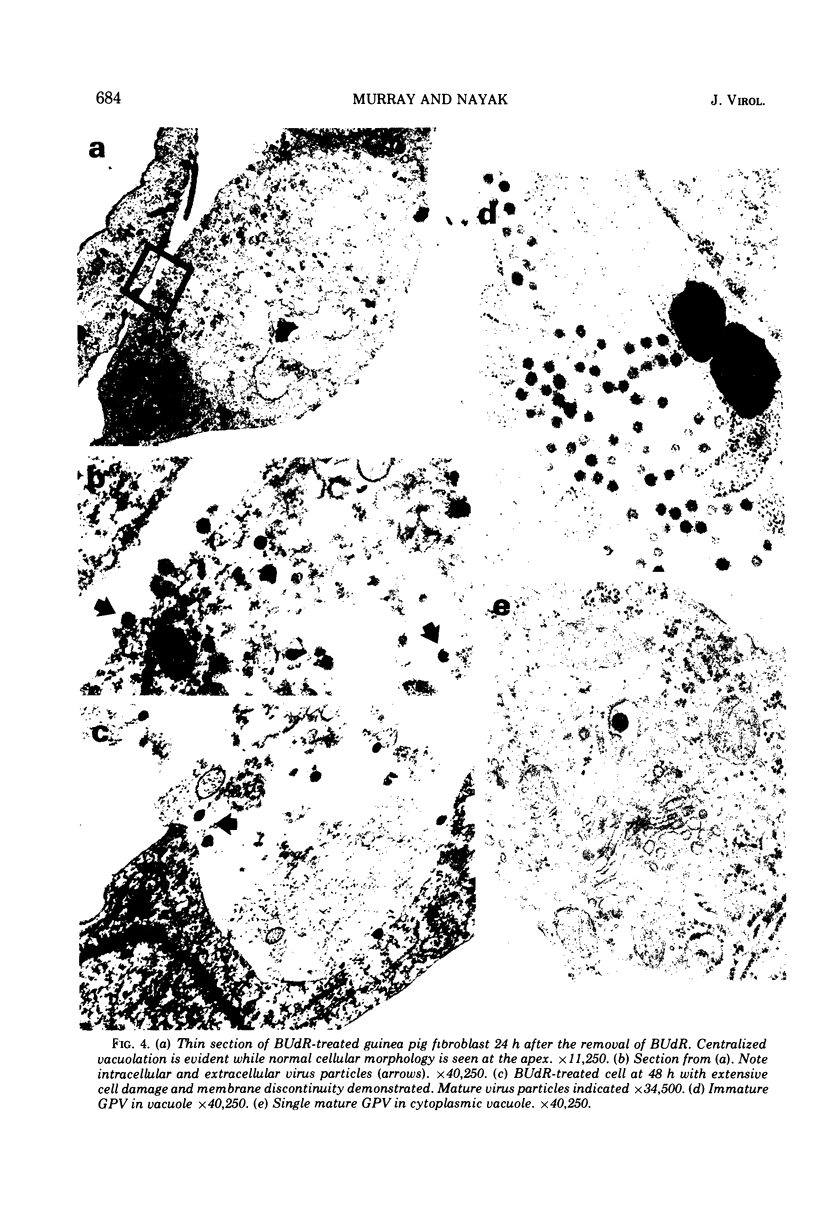
An endogenous virus (GPV) was induced after 5-bromodeoxyuridine treatment of cultured guinea pig cells. Compared to Gross murine leukemia virus (G-MuLV) GPV has a reproducibly heterogenous density of about 1.16 to 1.18 g/ml. The virion-associated RNA is slightly larger than that in G-MuLV. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of dissociated GPV resolved five major structural proteins: I (molecular weight 70,000), II (molecular weight 36,000), III (molecular weight 24,000), IV (molecular weight 18,000), and V (molecular weight 16,000) which are similar to but distinct from G-MuLV proteins. Proteins I and II were demonstrated to be glycoproteins by incorporation of [3H]glucosamine. GPV and G-MuLV did not have any appreciable genetic homology or any common group-specific antigens when analyzed by immunodiffusion, radioimmunoassay, and indirect immunofluorescence. Morphogenesis of GPV also differed from that of a typical type C oncornavirus and proceeded via two pathways: (i) a majority of virus particles were formed in cytoplasmic vacuoles and were released after cellular disruption; and (ii) a minor population of particles were assembled in the cytoplasmic matrix and then migrated to the plasma membrane where they budded into the extracellular space. To date, GPV has been unable to initiate or maintain a productive replication in any cell line tested.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. K., Jeppesen T. Virus-like particles in guinea pig oogonia and oocytes. J Natl Cancer Inst. 1972 Nov;49(5):1403–1410. [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S., Liszczak T., Korol W., Jensen E. M. Type-C particles in human tissues. I. Electron microscopic study of embryonic tissues in vivo and in vitro. Int J Cancer. 1970 Jul 15;6(1):40–45. doi: 10.1002/ijc.2910060107. [DOI] [PubMed] [Google Scholar]
- Dalton A. J. RNA tumor viruses. Terminology and ultrastructural aspects of virion morphology and replication. J Natl Cancer Inst. 1972 Aug;49(2):323–327. [PubMed] [Google Scholar]
- Feldman D. G., Gross L. Electron microscopic study of the guinea pig leukemia virus. Cancer Res. 1970 Nov;30(11):2702–2711. [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Rongey R. W., Estes J. D., Huebner R. J. Spontaneous tumors of aging wild house mice. Incidence, pathology, and C-type virus expression. J Natl Cancer Inst. 1973 Mar;50(3):719–734. doi: 10.1093/jnci/50.3.719. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Sylvester S. S. Cell line derived from Balb-3T3 that is transformed by murine leukaemia virus: a focus assay for leukaemia virus. Nat New Biol. 1972 Oct 11;239(93):164–166. doi: 10.1038/newbio239164a0. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D. Activation of guinea pig C-type virus in cultured spleen cells by 5-bromo-2'-deoxyuridine. J Natl Cancer Inst. 1972 Aug;49(2):567–570. [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Kramarsky B., Sarkar N. H., Moore D. H. Ultrastructural comparison of a virus from a Rhesus-monkey mammary carcinoma with four oncogenic RNA viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1603–1607. doi: 10.1073/pnas.68.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. M., Todaro G. J. Spontaneous and induced production of endogenous type-C RNA virus from a clonal line of spontaneously transformed BALB-3T3. Int J Cancer. 1973 May;11(3):616–627. doi: 10.1002/ijc.2910110313. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Todaro G. J. Endogenous type C virus from a cat cell clone with properties distinct from previously described feline type C virus. Virology. 1973 May;53(1):142–151. doi: 10.1016/0042-6822(73)90473-x. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Moroni C. Structural proteins of Rauscher leukemia virus and Harvey sarcoma virus. Virology. 1972 Jan;47(1):1–7. doi: 10.1016/0042-6822(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Nayak D. P. Endogenous guinea pig virus: equability of virus-specific DNA in normal, leukemic, and virus-producing cells. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1164–1168. doi: 10.1073/pnas.71.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P. Isolation and characterization of a herpesvirus from leukemic guinea pigs. J Virol. 1971 Oct;8(4):579–588. doi: 10.1128/jvi.8.4.579-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Murray P. R. Induction of type C viruses in cultured guinea pig cells. J Virol. 1973 Jul;12(1):177–187. doi: 10.1128/jvi.12.1.177-187.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Edynak E., Sarkar N. H. Serological and structural properties of Mason-Pfizer monkey virus isolated from the mammary tumor of a Rhesus monkey. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1608–1612. doi: 10.1073/pnas.68.7.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. L., Spahn G. J., Rabstein L. S., Kelloff G. J., Huebner R. J. Murine C-type RNA virus from spontaneous neoplasms: in vitro host range and oncogenic potential. Science. 1973 Aug 17;181(4100):665–667. doi: 10.1126/science.181.4100.665. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Duh F. G., Cho H. Y., Wuu K. D., Vernon M. L. Brief communication: Activation by 5-bromo-2'-deoxyuridine of particles resembling guinea-pig leukemia virus from guinea-pig nonproducer cells. J Natl Cancer Inst. 1973 Oct;51(4):1327–1331. doi: 10.1093/jnci/51.4.1327. [DOI] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Pelster B., Brandis H. Cytochalasin B-induced activation in the synthesis of L-cell virus particles. J Gen Virol. 1973 Nov;21(2):435–440. doi: 10.1099/0022-1317-21-2-435. [DOI] [PubMed] [Google Scholar]
- Stewart S. E., Kasnic G., Jr, Draycott C., Ben T. Activation of viruses in human tumors by 5-iododeoxyuridine and dimethyl sulfoxide. Science. 1972 Jan 14;175(4018):198–199. doi: 10.1126/science.175.4018.198. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Tamura A., Tsujimura D. Properties of the intracytoplasmic A particles purified from mouse tumors. Virology. 1972 Jul;49(1):61–78. doi: 10.1016/s0042-6822(72)80007-2. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]