Summary
Osteoporosis is a common disease characterised by a systemic impairment of bone mass and microarchitecture that results in fragility fractures. With an ageing population, the medical and socioeconomic impact of osteoporosis in general and postmenopausal osteoporosis in particular, will increase further. A detailed knowledge of bone biology with molecular insights into the communication between bone-forming osteoblasts and bone-resorbing osteoclasts and the orchestrating signalling network has led to the identification of novel therapeutic targets. Based on this, therapeutic strategies have been developed aimed at (I) inhibiting excessive bone resorption and by (II) increasing bone formation. The most promising novel treatments include denosumab, a monoclonal antibody against receptor activator of NF-κB ligand, a key osteoclast cytokine, odanacatib, a specific inhibitor of the osteoclast protease cathepsin K, and antibodies against the proteins sclerostin and dickkopf-1, two endogenous inhibitors of bone formation. This review provides an overview on these novel therapies and explains their underlying physiology.
Introduction
Osteoporosis is an emerging medical and socioeconomic threat characterised by a systemic impairment of bone mass, strength, and microarchitecture which increases the propensity of fragility fractures (figure 1).1 The bone mineral density (BMD) can be assessed with dual X-ray absorptiometry (DXA), and osteoporosis is defined by a T-score −2.5 or more standard deviations below the average of a young adult. About 40% of Caucasian postmenopausal women are affected by osteoporosis, and with an ageing population this number is expected to steadily increase in the near future.2–4 The lifetime fracture risk of a patient with osteoporosis is as high as 40%, and fractures most commonly occur in the spine, the hip, or the wrist (figure 1), but other bones such as the humerus or ribs may also be involved. From a patient’s perspective, a fracture and the subsequent loss of mobility and autonomy often represent a major drop in life quality. In addition, osteoporotic fractures of the hip and spine carry a 12-month excess mortality rate of up to 20%, because they require hospitalisation and subsequently enhance the risk of developing other medical complications, such as pneumonia or thromboembolic disease due to chronic immobilisation.5
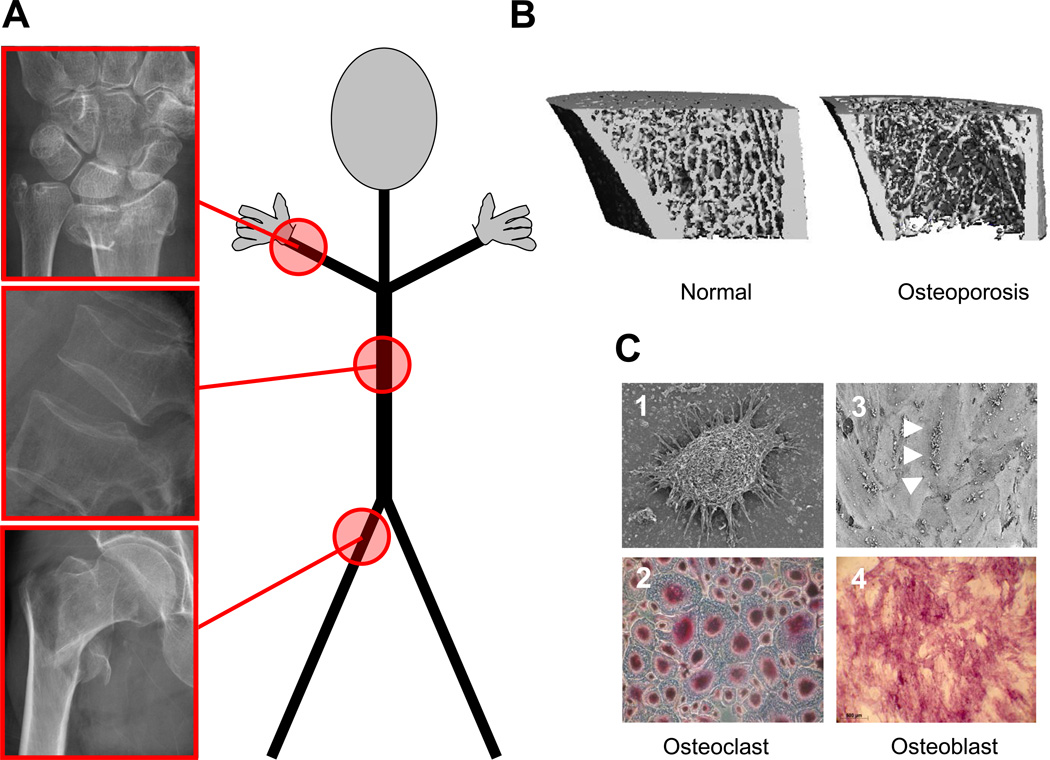
Figure 1. Osteoporosis at a glance.
Osteoporosis is a systemic skeletal disease where bone resorption exceeds bone formation and results in microarchitectural changes. (A) Fragility fractures typically involve the wrist, vertebrae, and the hip. (B) Micro-computed tomography demonstrates marked thinning of bone in a mouse model of osteoporosis. (C) Microscopic view of bone-resorbing osteoclasts and bone-forming osteoblasts; 1- Picture of an Osteoclast, with its distinctive morphology; 2- Tartrate-resistant Acidic Phosphatase (TRAP) staining of multinucleated osteoclasts; 3- Picture of multiple osteoblasts (white arrowheads) on a mineralized matrix; 4- Alizarin red staining, showing the mineralization of osteoblast secreted extracellular matrix.
Early diagnosis of osteoporosis requires a high index of suspicion as elderly patients may concurrently have other comorbidities such as cardiovascular diseases or cancer that receive more attention. Because bone loss occurs insidiously and is initially an asymptomatic process, osteoporosis is frequently only diagnosed after the first clinical fracture has occurred.6,7 Consequently, therapy is often aimed at preventing further fractures. It is therefore important to assess individual osteoporosis risk early enough to prevent the first fracture. National and international guidelines have been implemented to address the question of screening for osteoporosis in an evidence-based and cost-effective manner.8–10 Several risk factors such as age, low body mass index, previous fragility fractures, a family history of fractures, the use of glucocorticoids and active cigarette smoking have to be taken into account.11 The measurement of BMD by DXA is a valid method to diagnose osteoporosis and to predict the risk of fracture.12 New decision-making tools such as the fracture risk assessment tool (FRAX) have integrated clinical risk factors with the DXA-based BMD to predict an individual’s 10-year risk of sustaining a hip fracture as well as the 10-year probability of obtaining a major osteoporotic fracture, defined as clinical spine, forearm, hip or shoulder fracture.6
Osteoporosis therapies fall into two classes, anti-resorptive drugs, which slow down bone resorption or anabolic drugs, which stimulate bone formation. Currently, several approved treatment options exist for the management of osteoporosis that effectively reduce the risk of vertebral, non-vertebral and hip fractures (table 1).13–23 In fact, clear evidence of vertebral fracture risk reduction is a necessary requirement for any novel osteoporotic agent to be registered. Amongst the anti-resorptive drugs, bisphosphonates, with their high affinity for bone and long safety record, constitute the largest class. Bisphosphonates can be administered either orally or intravenously and are most widely used because they can be inexpensive and used across a broad spectrum of osteoporosis types, including postmenopausal, male, and steroid-induced osteoporosis as well as Paget’s disease. Other anti-resorptive drugs such as raloxifene, strontium ranelate, and most recently, denosumab, may represent alternatives for women with postmenopausal osteoporosis. Bone-anabolic agents that build up new bone, rather than preventing its loss, are limited to the full length parathyroid hormone (PTH 1–84) or its N-terminal fragment, teriparatide (PTH 1–34). Both are given subcutaneously, but transdermal application forms of PTH 1–34 are in development.24
Table 1. Established osteoporosis therapies.
Drugs with evidence of reducing the risk of vertebral (and hip) fractures when used with adequate calcium and vitamin D supplementation.
| Drugs | Dose | Interval | Route | Anti-Fracture efficacy against | Side Effects | |
|---|---|---|---|---|---|---|
| Bisphosphonates | Hip fractures Vertebral fractures | Osteonecrosis of the jaw, subtrochanteric femur fractures | ||||
| Alendronate | 70 mg | Weekly | Oral | Black13 | Cummings14 | Esophageal irritation |
| Risedronate | 35 mg/150 mg | Weekly/Monthly | Oral | McClung15 | Harris16 | Esophageal irritation |
| Ibandronate | 150 mg | Monthly | Oral | No data | Chesnut17 | Esophageal irritation |
| 3 mg | Every 3 Months | IV | No data available | No data available | APR | |
| Zoledronic acid | 5 mg | Yearly | IV | Black18 | Black18 | APR, hypocalcaemia, potential renal toxicity |
| Raloxifene | 60 mg | Daily | Oral | No effect | Delmas19 | Thromboembolic disease |
| Strontium ranelate* | 2 g | Daily | Oral | Reginster20 | Meunier21 | Thromboembolic disease; drug rash with eosinophilia systemic syndrome, abdominal discomfort |
| Teriparatide | 20 µg | Daily | SC | No effect | Neer22 | Hypercalcaemia, nausea, diarrhea |
| PTH (1–84)** | 100 µg | Daily | SC | No effect | Greenspan23 | Hypercalcaemia, nausea, diarrhea |
Abbreviations used: IV, intravenous; SC, subcutaneous; APR, Acute-phase reaction
Approved in over 70 countries, but not the US,
approved in Europe, but not the US.
While these drugs are effective, most have some limitations and side effects that impact their long-term administration and adherence (table 1).25 For a more detailed overview of the different therapies for osteoporosis, we recommend recent reviews.26 Here, we summarise the recent progress in bone biology that has defined novel therapeutic targets for drug development and show how this may translate into future osteoporosis therapy.
Recent developments in bone biology
In the past decade, the pathogenesis of osteoporosis has been linked to processes at the tissue, cellular, and molecular levels (figure 1). Signals that act as “master-switches” have been defined that integrate various endocrine, neuroendocrine, inflammatory, and mechanical stimuli. At the cellular level, communication and coupling between the main bone cell types, the bone-forming osteoblasts and the bone-degrading osteoclasts constitute the smallest functional unit (figure 1). Several key molecules conduct the coordinated activities of osteoblasts and osteoclasts during bone remodeling. Detailed knowledge of the molecular and cellular players has created a new concept of bone pathophysiology. With some of these new principles finding their way into clinical practise, we will highlight the most relevant advances in the field.
Osteoclasts and bone resorption
Osteoclasts originate from haematopoietic stem cells and are closely related to monocytes and macrophages (figures 1 and 2). Differentiation from osteoclast precursor cells to fully activated multinucleated osteoclasts depends critically on the presence of receptor activator of NF-κB ligand (RANKL), a member of the TNF family, and the permissive role of macrophage colony-stimulating factor (M-CSF). RANKL is abundantly expressed by bone-forming osteoblasts as well as bone marrow stromal cells, T and B lymphocytes and activates its receptor, RANK expressed on osteoclasts. After RANKL-induced RANK stimulation, several key regulatory transcription factors and enzymes are enhanced to promote the differentiation, proliferation, multinucleation, activation, and survival of osteoclasts. As a result, bone resorption is profoundly induced. Of note, mice with deletion of RANKL or its receptor RANK lack mature osteoclasts.27 Osteoprotegerin (OPG) represents a naturally occurring antagonist of RANKL.28 In women, high RANKL levels in early menopause, the acute phase of estrogen deficiency, subsequently up-regulate bone resorption and cause rapid bone loss.29 In addition to menopause, medical conditions where suppression of sex hormones is induced, e.g. in men with prostate cancer or in women with receptor-positive breast cancer are also associated with an activated RANKL/RANK pathway and enhanced bone resorption. Various hormones30,31 and inflammatory cytokines32 modulate osteoclast biology through the RANKL pathway. In addition, immunological and malignant bone disorders that destroy bone locally are associated with a high RANKL activity, including rheumatoid arthritis,33 periodontal disease,34 myeloma bone disease,35 and osteolytic bone metastasis.36
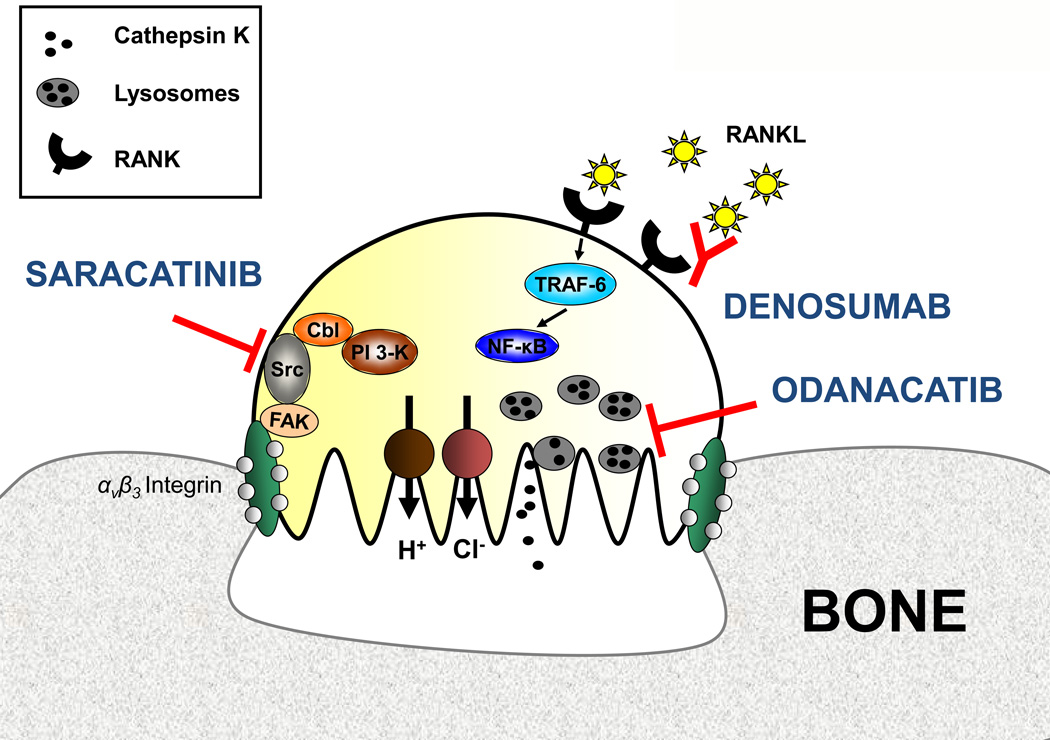
Figure 2. Osteoclast physiology and potential therapeutic targets.
With the help of αvβ3 integrin the osteoclast attaches to the bone surface and forms a sealing zone. Proton pumps and chloride channels produce a highly acidic microenvironment that is essential for the catalytic activity of osteoclastic enzymes such as cathepsin K. Odanacatib inhibits cathepsin K, a lysosomal protease that degrades collagens. The tyrosine Src kinase plays a critical role in osteoclast activity and can be inhibited by saracatinib. RANKL acts as an essential regulator of osteoclast differentiation and activity. The fully human monoclonal antibody denosumab prevents RANKL binding to its receptor RANK. Abbreviations used: FAK, focal adhesion kinase; NF-κB, nuclear factor-κB; PI3K, phosphatidylinositol 3-kinase, RANK, receptor activator of NF-κB; RANKL, RANK ligand; TRAF-6, tumor necrosis factor receptor associated factor-6
The Src kinase is highly expressed in osteoclasts and acts as a mediator of multiple pathways regulating osteoclast activity. Src-deficient mice suffer from osteopetrosis because their osteoclasts lack an intact ruffled border.37,38 Interestingly, the absence of src does not alter the number of osteoclasts38 and is associated with enhanced osteoblastic bone formation rate.39 With their jelly fish-like shape and equipped with a motile cytoskeleton and adhesion molecules such as integrins (figure 2), osteoclasts attach to bone and create a sealing zone on the bone surface which provides a highly enriched acidic microenvironment. Cathepsin K represents a key cystein proteinase of the mature osteoclast that degrades collagen and breaks down bone. Cathepsin K is a critical determinant of resorptive activity by osteoclasts which removes bone of poor quality, where micro-cracks have accumulated and leaves hole-like lacunae. Thus, humans without functioning cathepsin K suffer from pycnodysostosis, a rare disease characterised by osteosclerosis, a dense, but brittle bone phenotype, short stature, and lytic lesions of the distal phalanges as a result of poorly functioning osteoclasts.40 A more severe phenotype, osteopetrosis (“marble bone disease”) has been described in cathepsin K-deficient mice.41
Osteoblasts and bone formation
The osteoblast represents a unique bone-forming cell derived from mesenchymal stem cells (figures 1 and 3). The speed and efficacy of precursor cells differentiating into mature osteoblasts that secrete matrix that can be mineralized and their life span determines the rate of bone formation. These processes are enhanced by vitamin D as well as by intermittent pulses of parathyroid hormone, a treatment scheme employed by daily injections of teriparatide. By contrast, bone formation may be suppressed by exogenous glucocorticoids or be impaired in the elderly. At sites of resorption lacunae, a team of osteoblasts produce an extracellular matrix containing type I collagen and various non-collagenous proteins, including osteocalcin, osteonectin, osteopontin and others. This matrix mineralises under the influence of vitamin D, calcium, and phosphate.
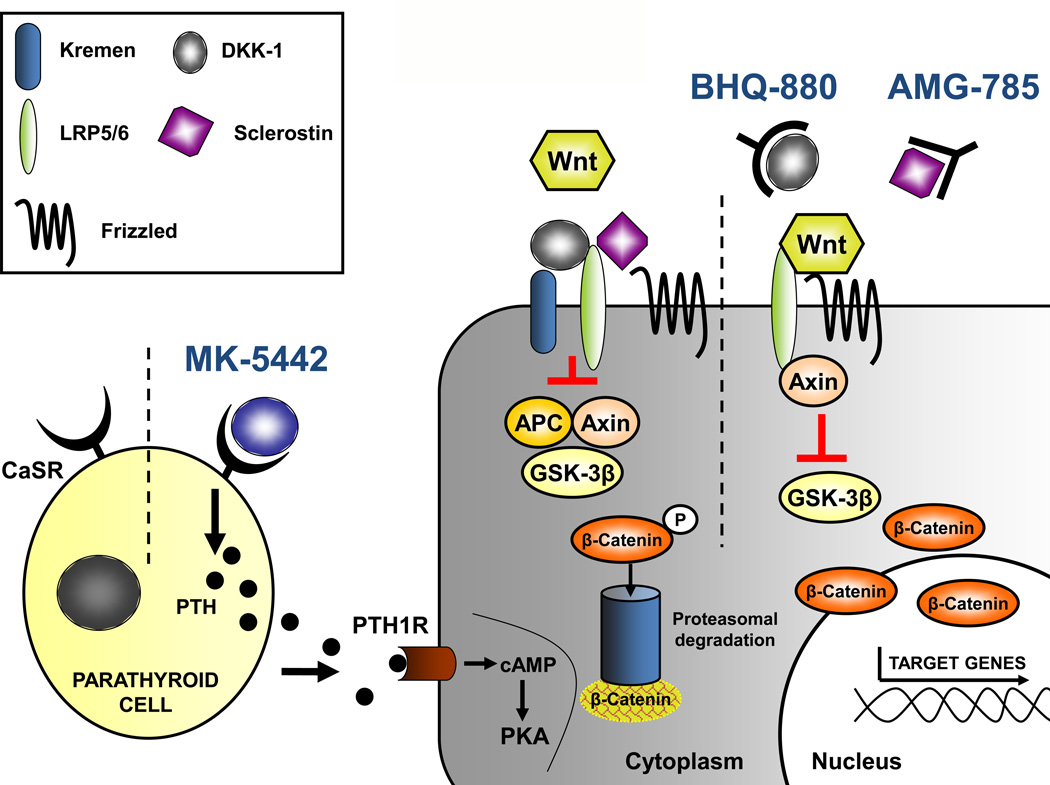
Figure 3. Osteoblast physiology and potential therapeutic targets.
The calcium-sensing receptor is antagonised by MK-5442 and triggers a short burst of PTH secretion. Binding of PTH to its receptor enhances osteoblast functions and bone formation. The presence of the Wnt antagonists Dkk-1 and sclerostin inhibit Wnt signalling. Dkk-1 needs to form a complex with kremen, whereas sclerostin binds LRP5/6 directly. BHQ-880 and AMG-785 are antibodies directed against Dkk-1 and sclerostin, respectively. After neutralising Dkk-1 and sclerostin, Wnt can bind to LRP5/6, which results in the degradation GSK-3β. As a consequence, β-catenin is stabilised, accumulates, and translocates into the nucleus where it regulates the transcription of osteoblastic genes. Abbreviations used: APC, adenomatosis polyposis coli; cAMP, cyclic adenosine monophosphate; CaSR, calcium sensing receptor; Dkk-1, dickkopf-1, GSK, glycogen synthase kinase 3; LRP, low-density lipoprotein receptor-related protein; PKA, protein kinase A; PTH, parathyroid hormone; PTH1R, PTH 1 receptor.
The calcium-sensing receptor (CaSR) on the parathyroid gland controls PTH release to maintain serum calcium levels within a narrow physiological range. While hypocalcaemia stimulates the CaSR and PTH release to elevate serum calcium levels, hypercalcaemia has the opposite effects.42 Accordingly, pharmacologic agents that mimic high calcium levels at the CaSR and suppress PTH secretion are termed calcimimetics (e. g. cinacalcet), whereas drugs that mimic low calcium levels are termed calcilytics (e. g. MK-5442).43 While the physiological role of the CaSR expressed on osteoblasts and osteoclasts is not fully understood, it is thought to mediate some of the effects of the osteoporosis drug strontium ranelate (table 1).44
At the molecular level, activation of the canonical Wnt/β-catenin pathway is the master switch for osteoblastic differentiation.45 This key bone-anabolic pathway is negatively regulated by Wnt inhibitors such as dickkopf-1 (Dkk-1) and sclerostin which bind and block the Wnt receptor LRP-5 (figure 3).46
Osteocytes
Osteocytes account for more than 90% of all bone cells and are found scattered throughout the mineralized matrix. They are terminally differentiated osteoblasts and share morphological similarities with neural cells. Their long dendritic processes form a sensory network, with which they can sense and communicate mechanical stress within the bone. Osteocytes also express a number of factors known to regulate phosphate which suggests a role in matrix mineralisation. In addition osteocytes exclusively produce and secrete sclerostin,47 an inhibitor of the Wnt signaling pathway, thus inhibiting osteoblast differentiation and bone formation.
Novel targets for osteoporosis therapy
Anti-resorptive therapies
Denosumab
The prominent role of RANKL in osteoclastogenesis has made it a prime target for therapy against diseases characterised by excessive bone loss (table 2). Initially, a chimeric OPG-Fc fusion protein was used to antagonise RANKL.48 However, the formation of neutralising antibodies against OPG after administration of the fusion protein and its potential cross-reactivity with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)49 led to a new strategy, the development of denosumab, a fully human monoclonal antibody against RANKL. Denosumab displays a higher specificity and affinity for RANKL with superior pharmacokinetic properties, translating into a longer dosing interval of 6 month.50 A large study program on a wide spectrum of bone diseases, including several types of osteoporosis50–56 and bone metastases is currently ongoing (www.clinicaltrials.gov). With completed phase 3 studies, denosumab is the most advanced of all investigational substances and has recently been approved in Europe and the US for the treatment of osteoporosis.
Table 2. Clinical development of novel osteoporosis therapies.
| Drug | Target | Function | Class | Phase | Route |
|---|---|---|---|---|---|
| Anti-resorptives | |||||
| Denosumab* | RANK ligand | Stem cell factor for osteoclasts | Antibody against RANKL | 3 (completed) | SC |
| Odanacatib | Cathepsin K | Osteoclastic enzyme that degrades collagens | Cathepsin K inhibitor | 3 | PO |
| Saracatinib | c-src kinase | Enzyme involved in osteoclast activation | c-src inhibitor | 2 | PO |
| Anabolic | |||||
| MK-5442 | Calcium-sensing receptor | Triggers PTH release if inhibited | Calcilytic agent | 2 | PO |
| AMG 785 | Sclerostin | Inhibitor of the Wnt/β-catenin pathway | Antibody against sclerostin | 2 | SC |
| BHQ 880 | Dickkopf-1 | Inhibitor of the Wnt/β-catenin pathway | Antibody against dickkopf-1 | 1/2 | SC |
Approved in Europe and the US in May/June, 2010.
Abbreviations: SC, subcutaneous, PO, per os.
In the phase 1 study, a single subcutaneous injection dose-dependently suppressed urinary N-terminal telopeptide of type I collagen (NTX), a biochemical marker of bone resorption, by up to 81% in postmenopausal women for as long as 6 months and was well-tolerated (table 3).50 Subsequently, a phase 2 study with different doses (6–210 mg) and intervals (every 3 to 6 months) was aimed at evaluating the effects on BMD after 12 months in postmenopausal women with low bone mass.51 A dose-dependent suppression of bone turnover was observed, with a decrease of mean levels of serum CTX as early as three days after administration and a maximum mean percentage reduction of 88% among the denosumab groups. Denosumab increased lumbar spine BMD (range: 3.0–6.7%), whereas women receiving placebo experienced a BMD loss of 0.8% in this period. At the total hip, BMD increased by 1.9–3.6% in the denosumab group, but decreased by 0.6% in the placebo group. The optimal denosumab dosing regimen turned out to be 60 mg every 6 months. Extension of this study for another 12 months demonstrated a sustained positive effect of denosumab on BMD at the lumbar spine, total hip, and the distal third of the radius.52 Overall, treatment with denosumab was well-tolerated and not associated with increased serious adverse events when compared to placebo. Of note, discontinuation of denosumab treatment caused a rapid increase of bone turnover markers to values above baseline and even greater than those observed in the placebo group. Subsequently, serum CTX levels returned to near baseline values and after 24 months off treatment were similar to placebo.53 Bone histomorphometry of patients from this study revealed absent osteoclasts in >50% of biopsies in the denosumab group. Tetracycline labelling of trabecular bone was observed in 94% of placebo bones, compared to only 19% of those treated with denosumab, indicating that bone formation and turnover are markedly reduced.54 Long term follow-up is required to determine the clinical effects of suppressed bone turnover during denosumab therapy. The fast reversibility of denosumab on bone remodelling is ambiguous. While prolonged suppression of bone turnover did not occur after denosumab had been stopped, the rapid increase of bone turnover markers, starting 6 months after the last denosumab injection has two practical implication: (I) the need of a reliable recall system to remind the patient of the subsequent injection, and (II) in case of discontinuation, a follow-up strategy with another anti-osteoporosis agent.
Table 3. Study programs involving investigational osteoporosis drugs.
| Trial | n | Primary endpoint | Main results | Ref. |
|---|---|---|---|---|
| DENOSUMAB | ||||
| Phase 1 | ||||
| Single-dose placebo-controlled study in postmenopausal women | 49 | Biochemical markers of bone resorption after 6 months vs. placebo | Urinary NTX decreased by 81% (3 mg/kg denosumab) vs. −10% with placebo (p<0.001) | Bekker50 |
| Phase 2 | ||||
| Efficacy and safety in postmenopausal women with low bone mineral density | 412 | % change from baseline BMD at the lumbar spine after 12 months vs. placebo | Lumbar spine BMD +3.0% to +6.7% vs. −0.6% with placebo (p<0.001) | McClung51 |
| Phase 3 | ||||
| Treatment of postmenopausal osteoporosis (FREEDOM) | 7,868 | Reduction of new vertebral fractures after 36 months vs. placebo | Vertebral fractures decreased by 68% (RR: 0.32, 95%CI 0.26–0.41); hip fractures decreased by 40% (RR: 0.60, 95%CI 0.37–0.97) | Cummings55 |
| Prevention of postmenopausal osteoporosis | 332 | % change from baseline BMD at the lumbar spine after 24 months vs. placebo | Lumbar spine BMD +6.5% vs. −0.6% with placebo (p<0.0001) | Bone57 |
| Comparison with alendronate in postmenopausal Women with low bone mineral density (DECIDE) | 1,189 | % change from baseline BMD at the total hip after 12 months vs. alendronate | Total hip BMD +3.5% vs. +2.6% with alendronate (p<0.0001) | Brown56 |
| Treatment of bone loss in men on androgen-deprivation therapy for non-metastatic prostate cancer (HALT) | 1,468 | % change from baseline BMD at the lumbar spine after 24 months | Lumbar spine BMD +5.6% vs. −1.0 with placebo (p<0.001). After 36 months, vertebral fractures decreased by 62% (RR: 0.38; 95%CI 0.19–0.78) | Smith61 |
| Treatment of bone loss in women on aromatase inhibitors for non-metastatic breast cancer | 252 | % change from baseline BMD at the lumbar spine after 12 months | Lumbar spine BMD +5.5% vs. placebo (p<0.0001) | Ellis60 |
| ODANACATIB | ||||
| Phase 1 | ||||
| Multiple oral doses in healthy adults | 62 | Safety and tolerability | No increase in adverse events; serum CTX decreased by 62% (50 or 100 mg per week) BSAP and osteocalcin remained unaffected | Stoch64 |
| Once-weekly doses in healthy adult females | 78 | |||
| Phase 2 | ||||
| Treatment of postmenopausal osteoporosis | 399 | % change from baseline BMD at the lumbar spine after 24 months | Lumbar spine BMD +5.5% vs. −0.2% with placebo | Bone65 |
| Phase 3 | ||||
| Treatment of postmenopausal osteoporosis | 16,716 | Vertebral, hip, and clinical non-clinical fractures after 36 months | Expected to be completed in July 2012 | NCT00529373 |
| ONO-5334 | ||||
| Postmenopausal women with low BMD | 265 | % change from baseline BMD at the lumbar spine after 12 months | Completed in October 2009, results pending | NCT 00532337 |
| SARACATINIB | ||||
| Phase 1 | ||||
| Multiple oral doses on bone turnover in healthy men | 59 | Effect on bone turnover of multiple daily oral dosing for up to 24 days | Serum CTX −88% (95%CI 84–91%) vs. +17% with placebo (p<0.001); PINP +13 vs. +17% with placebo (p=NS) | Hannon67 |
| MK-5442 (Calcilytic agent) | ||||
| Phase 2 | ||||
| Dose-ranging study in postmenopausal osteoporosis | 384 | % change from baseline BMD at the lumbar spine after 12 months | Expected to be completed in February 2012 | NCT00960934 |
| Postmenopausal osteoporosis previously treated with alendronate | 480 | % change from baseline BMD at the lumbar spine vs. alendronate after 12 months | Expected to be completed in August 2012 | NCT00996801 |
| AMG 785 (Antibody against sclerostin) | ||||
| Phase 1 | ||||
| Healthy men and postmenopausal women | 74 | Safety | No increase in adverse events; after 21 days 3 mg/kg increased PINP, osteocalcin and BSAP by 60 to 100% | NCT01059435 |
| Phase 2 | ||||
| Postmenopausal women with low BMD (vs. alendronate and teriparatide) | 419 | % change from baseline BMD at the lumber spine after 12 months | Expected to be completed in August 2012 | NCT00896532 |
| BHQ 880 (Antibody against dickkopf-1) | ||||
| Phase 1/2 | ||||
| Combination with zoledronic acid in relapsed or refractory myeloma patients | 267 | Time to skeletal-related event; SRE, changes in bone resorption and formation markers after 9 months | Expected to be completed in November 2010 | NCT00741377 |
Abbreviations: BSAP, bone-specific alkaline phosphatase; CTX, C-terminal telopeptide of type I collagen; NTX, N-terminal telopeptide of type I collagen; PINP, serum procollagen propeptide of type I collagen.
In a pivotal randomised placebo-controlled phase 3 study (FREEDOM), denosumab (60 mg every 6 months) was assessed for its fracture reduction in 7,868 women with postmenopausal osteoporosis, of whom 24% had preexisting vertebral fractures.55 After 3 years, denosumab had reduced the risk of new radiographic vertebral fractures by 68%, hip fractures by 40%, and non-vertebral fractures by 20%. Of note, the risk of cardiovascular events, cancer, and infections did not differ between the two groups. However, the incidence of eczema (3.0% vs.1.7%) and cellulitis including erysipelas (0.3% vs. <0.1%) was significantly higher in women receiving denosumab than placebo.55 No unusual pathogens were identified and all infections responded properly to standard antibiotics. Comprehensive assessment of the immune status of patients receiving denosumab for 12 months revealed no relevant changes in white blood cell count, T cell, B cell or NK cell numbers.58 In the follow-up of the FREEDOM cohort, one woman developed osteonecrosis of the jaw after dental extraction. Osteonecrosis of the jaw has been previously described as a rare complication with a frequency of 1:100,000 to 1:10,000 in patients treated for osteoporosis with bisphosphonates.59
Two randomised placebo-controlled phase 3 studies have embarked on the use of denosumab in treatment-related osteoporosis: women receiving aromatase inhibitors for breast cancer and men on androgen-ablation therapy for prostate cancer. In both cases, sex hormone ablation is therapeutically intended as it prolongs disease-free survival, yet it commonly causes rapid bone loss and fragility fractures. In women on aromatase-inhibitor therapy for non-metastatic breast cancer denosumab (60 mg every 6 months for 12 months) increased BMD at the lumbar spine by 5.5% compared to placebo and was similarly effective regardless of the duration of aromatase inhibitor therapy.60 However, this study was not designed to assess fracture reduction. In the HALT study, men under androgen deprivation therapy for prostate cancer were assessed. In this study, denosumab (60 mg every 6 months for 24 months) also increased BMD over 24 months at the lumbar spine by 6.7%, total hip by 4.8%, and distal third of the radius by 5.5% over placebo.61 Importantly, denosumab reduced the incidence of new vertebral fractures by 62% compared to placebo (1.5% vs. 3.9%). Serious side effects were similar between denosumab and placebo, including cancers, infections, and cardiovascular events. Cataracts developed in more men on denosumab (4.7%) compared to placebo (1.2%).
In summary, denosumab represents a novel and effective anti-resorptive therapy for various metabolic bone diseases. While direct comparative studies with fracture endpoints are not available, evidence from completed trials with established surrogates suggests that it may be as effective as the most potent of the amino-bisphosphonates, zoledronic acid. Several important characteristics clearly separate denosumab from bisphosphonates: (I) its reversibility, as it targets RANKL and is not incorporated into the bone mineral, (II) its lack of gastrointestinal side effects and convenient biannual subcutaneous administration that may translate into improved long-term adherence, and (III) its potential use in impaired renal function as it is not eliminated by the kidneys. Of note, while no dose adjustment is required in patients with renal impairment, patients with severe renal impairment (creatinine clearance < 30 mL/min) or receiving dialysis are at greater risk of developing hypocalcemia.
To minimise the risk of osteonecrosis of the jaw, patients who are scheduled to receive denosumab should be seen by a dentist, if additional systemic (glucocorticoid therapy, chemotherapy) or local risk factors (radiation, dental diseases) are present. In addition to recommending improved dental hygiene, invasive dental procedures should be avoided during denosumab therapy.
Odanacatib
Based on the concept that the protease cathepsin K plays an important role in enzymatic bone degradation, the use of cathepsin K inhibitors has emerged as a novel therapeutic approach. A high specificity and affinity for cathepsin K over other cathepsins (B, L and S) that are widely expressed, particularly in the skin, was crucial for this class of compound.62 Odanacatib is currently the only cathepsin K inhibitor under clinical investigation. Other programs of less specific cathepsin K inhibitors were stopped due to cutaneous adverse side effects, including a scleroderma-like skin thickening and rashes.62,63 In phase 1 studies, odanacatib at an oral dose of 50 and 100 mg once a week reduced serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) by 62%.64 Daily administration of odanacatib (10 mg) reduced serum CTX by 81%.64
In a phase 2 study, the effects of weekly oral doses of odanacatib were assessed in 399 women with postmenopausal osteoporosis.65 After 24 months, odanacatib (50 mg) increased the BMD of the lumbar spine and total hip by 5.7% and 4.1% compared to placebo, respectively. Bone resorption markers were dose-dependently suppressed. Of note, odanacatib treatment resulted in a modest and transient reduction of bone formation markers while not suppressing bone formation rate as evident from a subset of 32 women undergoing bone biopsies followed by histomorphometry. Adverse reactions were comparable to placebo and scleroderma-like cutaneous lesions were not observed. Currently, a phase 3 study is being conducted with over 16,000 postmenopausal women to assess the anti-fracture efficacy of odanacatib (table 3). Another cathepsin K inhibitor named ONO-5334 is currently investigated in a phase 2 trial in postmenopausal women with osteopenia or osteoporosis (NCT 00532337).
The underlying bone biology of cathepsin K may give a clue to the distinct clinical findings with odanacatib. Since the levels of secreted cathepsin K determines the potency of osteoclast resorption rather than osteoclast differentiation or apoptosis, its inhibition preserves osteoclast viability, but suppresses their function. This may allow osteoclast-to-osteoblast signaling that maintains bone formation, while suppressing bone resorption.64,65 These uncoupling effects of odanacatib are in contrast to other antiresorptive agents such as bisphosphonates and denosumab which enhance osteoclast apoptosis (figure 4). Denosumab is unique in that it also inhibits osteoclastogenesis and osteoclast activation. These actions at different levels of osteoclast cell biology may explain why bone biopsies obtained from patients treated with denosumab show absent osteoclasts in >50% of samples.54 The importance of giant osteoclasts seen in bisphosphonate-treated patients (Weinstein et al. 2009) and of which one third was apoptotic,66 is only poorly understood.It remains to be seen whether these novel biological effects translate into meaningful clinical endpoints.
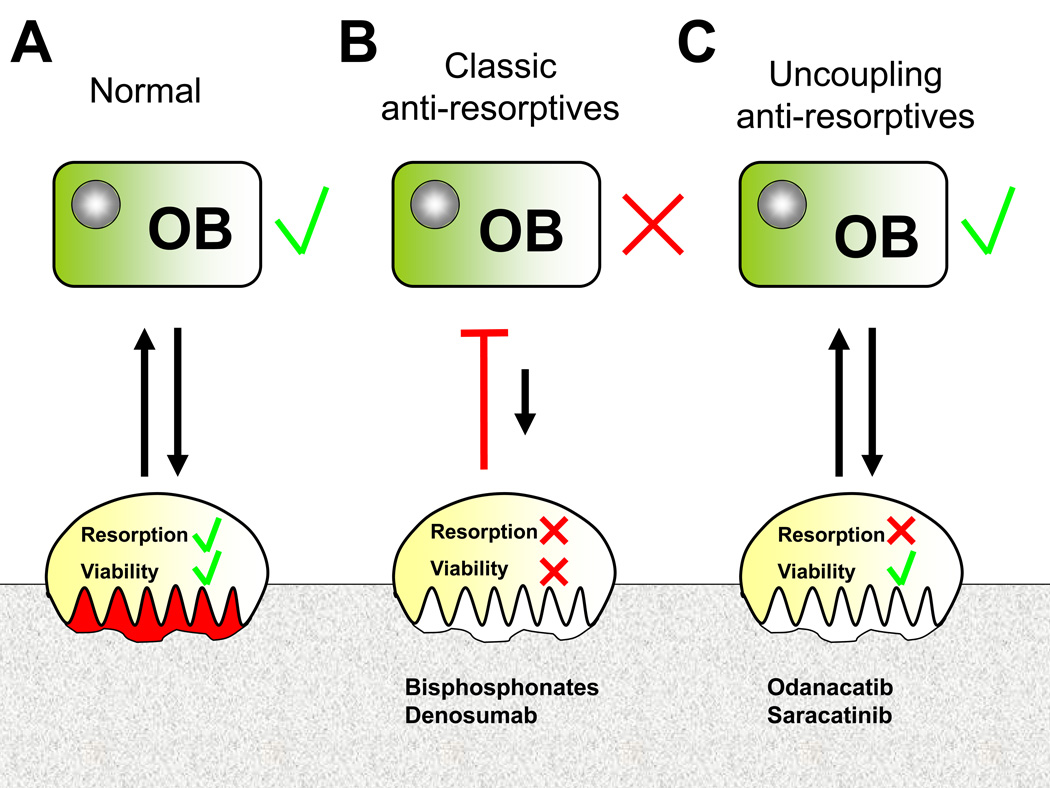
Figure 4. Potential mechanisms of anti-resorptives.
This theoretical concept is based on cellular, preclinical and early clinical data that require clinical validation (A) Physiologically, osteoclastic and osteoblastic functions are coupled with bidirectional communication. (B) Classic anti-resorptives act by reducing osteoclast viability. As a consequence, osteoclastic signalling and subsequently osteoblastic bone formation is suppressed. (C) Uncoupling anti-resorptives inhibit osteoclast activity rather than osteoclast viability, thus allowing physiological communication between osteoclasts and osteoblasts with maintained osteoblastic bone formation. Abbreviations used: OB, osteoblast; OC, osteoclast.
Saracatinib
The effect of impaired osteoclastic functions in src-deficient mice provided the rationale to explore the skeletal effects of a src kinase inhibitor. In a phase 1 trial, the Src kinase inhibitor saracatinib (AZD0530) was evaluated in 59 healthy young men. Saracatinib dose-dependently decreased serum CTX levels and urinary NTX excretion by 88% and 67% (250 mg), respectively after 25 days.67 Bone formation markers were similar to placebo. While there were no significant differences between the saracatinib and placebo groups, papular rash (30% vs. 6%) and loose stools (24% vs. 0%) were more frequent in the treatment compared to the placebo group.67 Saracatinib is currently being explored in phase 2 studies for osteosarcoma (NCT00752206) and bone metastases (NCT00558272), but not osteoporosis.
Others
On the basis of preclinical models, additional osteoclastic targets are currently being evaluated. Eating reduces bone remodeling through release of glucagon-like peptide (GLP)-2, while during nocturnal fasting bone remodeling is enhanced. Treatment with GLP-2 at night time over 4 months increased BMD at the hip by 1.1% from baseline, but not that of the lumbar spine, and did not suppress bone formation markers.68
Other strategies involve inhibition of Atp6v0d2, a subunit of v-ATPase that is required for acidification and the voltage-gated chloride channel ClC-7.69 The chloride channel inhibitor NS3736 prevented bone loss in ovariectomised rats through a marked anti-resorptive effect, without impeding on bone formation markers.69 Another concept that has been evaluated, but is currently no longer pursued are antibodies against αvβ3 integrin that impair the capability of the osteoclast to attach to bone and form the sealing zone.
Anabolic therapies
In contrast to antiresorptive therapies, anabolic agents enhance bone formation instead of preventing further bone loss, and result in a faster increase of bone mass and strength. Currently approved anabolic substances are limited to PTH either as the N-terminal (1–34) fragment, teriparatide, or the full-length PTH (1–84), the latter of which has not been approved in the US.46
Calcilytic agents (MK-5442)
Calcilytics represent a new class of bone-forming agents. They act as antagonists of the CaSR and mimic hypocalcaemia, thus evoking a short pulse of PTH secretion (figure 3). Calcilytics are administered orally and obviate the need for injections as opposed to PTH therapy. A major practical obstacle for calcilytics has been their narrow therapeutic index. Conceptually, a high-amplitude PTH pulse followed by rapid normalisation translates into a bone-anabolic effect. Several programs involving calcilytics have been discontinued because of unfavourable pharmacokinetics70 and lack of efficacy (NCT00471237). These compounds led to sustained PTH secretion and findings that were reminiscent of primary hyperparathyroidism, a catabolic bone disease.
Currently, newer calcilytics with an improved pharmacological profile are being evaluated.71,72 The most advanced compound of this class is MK-5442 that is currently in phase 2 trials for postmenopausal osteoporosis. Results will be available in 2012 (table 3).
Inhibitors of Wnt antagonists
Wnt-dependent nuclear accumulation of β-catenin (figure 3) is a major trigger of osteoblastic differentiation and bone formation.73 The endogenous inhibitors of Wnt signalling, sclerostin and Dkk-1 present potential therapeutic targets to enhance osteoblastic bone formation and are under clinical investigation.74
Sclerostin antibody
Two rare skeletal diseases with a high bone mass, van Buchem disease and sclerosteosis, have been linked to inactivating mutations in the gene encoding for sclerostin.75,76 This highlights the role of sclerostin in the homeostasis of bone mass, and provided the rationale to target sclerostin with monoclonal antibodies to enhance bone formation. In a rat model of postmenopausal osteoporosis due to ovariectomy, treatment with a sclerostin antibody increased bone mass at all skeletal sites and completely prevented bone loss associated with estrogen deficiency.77 Treatment of cynomolgus monkeys with two once-monthly injections of a sclerostin-neutralising antibody yielded an increased bone mass at the femoral neck, radius, and tibia, ranging from 11–29% and enhanced bone strength at the lumbar spine.78 In a phase 1 study, a single subcutaneous injection of a sclerostin antibody (3 mg/kg) was well tolerated and increased bone formation markers by 60 to 100% at day 21.79 Of note, the combination of stimulated bone formation and unchanged bone resorption markers, indicates an uncoupling effect. A phase 2 trial has been initiated to compare the efficacy of sclerostin neutralisation with alendronate and teriparatide (table 3).
Dickkopf-1 antibody (BHQ-880)
Dkk-1 neutralisation is still limited to preclinical trials. Dkk-1 blockade inhibited bone loss in a model of rheumatoid arthritis.80 In a myeloma model, the inhibition of Dkk-1 prevented the formation of osteolytic lesions and increased bone formation rate.81,82 Dkk-1 inhibition is currently being investigated in patients with refractory multiple myeloma (NCT00741377). However, the effects of neutralising Dkk-1 have not yet been investigated in osteoporosis.
Of note, increased Wnt signalling has been associated with human malignancies such as colorectal and hepatocellular cancer.83 More importantly, the Wnt inhibitory factor 1 (WIF), an endogenous inhibitor of Wnt signalling, was found to be absent in 75% of osteosarcomas, leading to enhanced Wnt signalling.84 While patients with van Buchem disease and sclerosteosis carry no increased risk of malignancies,85,86 long-term blockade of Wnt antagonists requires careful monitoring with respect to skeletal and extraskeletal safety.
Summary
With multiple novel anti-osteoporotic compounds in advanced clinical trials, the number of available agents will increase considerably in the coming years. Current anti-resorptive therapies are effective, but some are limited by side effects, concurrent comorbidities, and inadequate long-term compliance. Many of the new drugs combine efficacy with convenient administration that may translate into better adherence. However, conventional anti-resorptives such as aminobisphosphonates and denosumab may profoundly suppress bone turnover with intact coupling (figure 4), and this may possibly be implicated in the pathogenesis of osteonecrosis of the jaw, although this hypothesis has yet to be proven. By contrast, odanacatib and to a certain extent saracatinib represent a distinct class of anti-resorptives that inhibit osteoclast activity rather than impairing their viability. Through these distinct cellular mechanisms, paracrine signalling from osteoclasts to osteoblasts is maintained with suppressed bone resorption and concurrent normal osteoblastic bone formation, consistent with an uncoupling effect (figure 4). Whether these uncoupling compounds have an advantage over conventional anti-resorptives remains to be seen. Apart from this, there is a great need for additional and affordable anabolic therapies in situations of severe osteoporosis, extensive bone loss, and impaired fracture healing. Calcilytics and antagonists of Wnt inhibitors are promising developments.
With a variety of novel drugs that utilise the advanced knowledge of bone cell biology, we have expanded our armamentarium to facilitate the treatment of patients suffering from osteoporosis and other skeletal diseases, thus offering more individualised therapy. Indeed, the development of these multiple novel compounds represents an excellent example of the investment in basic research identifying specific pathways that are being effectively targeted to treat and indeed, reverse osteoporosis.
Burden of osteoporosis – key points.
Characterised by insidious loss of bone mass and strength
Typically associated with vertebral, hip, proximal humerus, wrist (Colles fractures), and suchondral fractures of the femoral head
Associated with chronic pain, loss of autonomy, and increased mortality
DXA measurement is an accurate and valid method for early diagnosis
Current therapies are efficient, but have poor long-term adherence
Search strategy.
We searched MEDLINE and PubMed for articles published between 2000 and 2010. We used the search terms “osteoporosis” in combination with “treatment”, “RANK ligand”, “denosumab”, “cathepsin K”, “odanacatib”, “saracatinib”, “calcium-sensing receptor”, “calcilytic”, “sclerostin”, and “dickkopf-1”. We largely selected original papers and reviews published in the past 5 years, but did not exclude commonly referenced and important older publications. We also searched the ClinicalTrials.gov database for clinical trials. We focussed on randomised controlled trials and meta-analyses, if available. We also analysed the reference sections of identified articles for relevant papers. Recent review articles are cited to provide readers with detailed information. We added selected references as recommended during the peer review process.
Properties of an ideal osteoporosis treatment.
Anti-fracture efficacy at various skeletal sites, including the spine, non-vertebral and the hip
High safety margin
Mode of administration and treatment interval translate into patient’s adherence
Compatibility with drugs prescribed for other medical conditions
Affordable cost
Acknowledgements
We thank Claudia Goettsch, Christine Hamann, Ute Hempel, Martina Rauner, Elena Tsourdi and Cornelia Wolf-Brandstetter for contributing images to Figure 1. Dr. Hofbauer’s research program is supported by the Deutsche Forschungsgemeinschaft, grants Transregio-67 (B2) and HO 1875/8-1.
Footnotes
Contributors
Tilman D. Rachner has contributed to the design, literature search, writing and figure design. Sundeep Khosla has participated in the writing and review of the article. Lorenz C. Hofbauer has contributed to the design, writing, figure design and review of the manuscript.
Conflict of interest
Tilman Rachner has received reimbursement of travel and accommodation expenses from Novartis. Sundeep Khosla has received honoraria for serving on advisory boards for Bone Therapeutics and Pfizer. Lorenz Hofbauer has received honoraria and speakers fees including reimbursement of travel and accommodation expenses from Amgen, Daiichi Sankyo, Merck, Novartis, Nycomed, and Servier.
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 2.Melton LJ, 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992;7:1005–1010. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Ray NF, Chan JK, Thamer M, Melton LJ., 3rd Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 5.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 6.Unnanuntana A, Gladnick BP, Donnelly E, Lane JM. The assessment of fracture risk. J Bone Joint Surg Am. 2010;92:743–753. doi: 10.2106/JBJS.I.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vestergaard P, Rejnmark L, Mosekilde L. Osteoporosis is markedly underdiagnosed: a nationwide study from Denmark. Osteoporos Int. 2005;16:134–141. doi: 10.1007/s00198-004-1680-8. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson SF, Watts NB, Bilezikian JP, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract. 2003;9:544–564. doi: 10.4158/EP.9.6.544. [DOI] [PubMed] [Google Scholar]
- 9.Compston J, Cooper A, Cooper C, et al. Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas. 2009;62:105–108. doi: 10.1016/j.maturitas.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Brown JP, Josse RG. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167:S1–S34. [PMC free article] [PubMed] [Google Scholar]
- 11.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 12.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288:1889–1897. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 13.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 15.McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 16.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 17.Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 18.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 19.Delmas PD, Ensrud KE, Adachi JD, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617. doi: 10.1210/jcem.87.8.8750. [DOI] [PubMed] [Google Scholar]
- 20.Reginster JY, Seeman E, De Vernejoul MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–2822. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- 21.Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 22.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 23.Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- 24.Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid Hormone (1–34)-Coated Microneedle Patch System: Clinical Pharmacokinetics and Pharmacodynamics for Treatment of Osteoporosis. Pharm Res. 2010 doi: 10.1007/s11095-010-0192-9. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122(2 Suppl):S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 27.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 28.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 29.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382–4389. doi: 10.1210/endo.140.10.7034. [DOI] [PubMed] [Google Scholar]
- 31.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 33.Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 34.Teng YT, Nguyen H, Gao X, et al. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:R59–R67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearse RN, Sordillo EM, Yaccoby S, et al. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ. Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res. 2001;61:4432–4436. [PubMed] [Google Scholar]
- 37.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 38.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marzia M, Sims NA, Voit S, et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. 2000;151:311–320. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273:1236–1238. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- 41.Saftig P, Hunziker E, Everts V, et al. Functions of cathepsin K in bone resorption. Lessons from cathepsin K deficient mice. Adv Exp Med Biol. 2000;477:293–303. doi: 10.1007/0-306-46826-3_32. [DOI] [PubMed] [Google Scholar]
- 42.Brown EM. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem. 2007;45:139–167. doi: 10.1007/978-1-4020-6191-2_6. [DOI] [PubMed] [Google Scholar]
- 43.Steddon SJ, Cunningham J. Calcimimetics and calcilytics--fooling the calcium receptor. Lancet. 2005;365:2237–2239. doi: 10.1016/S0140-6736(05)66782-7. [DOI] [PubMed] [Google Scholar]
- 44.Fromigue O, Hay E, Barbara A, et al. Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate. J Cell Mol Med. 2009;13:2189–2199. doi: 10.1111/j.1582-4934.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 46.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 47.Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 48.Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res. 2001;16:348–360. doi: 10.1359/jbmr.2001.16.2.348. [DOI] [PubMed] [Google Scholar]
- 49.Emery JG, McDonnell P, Burke MB, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 50.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 51.McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 52.Lewiecki EM, Miller PD, McClung MR, et al. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res. 2007;22:1832–1841. doi: 10.1359/jbmr.070809. [DOI] [PubMed] [Google Scholar]
- 53.Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Reid I, Miller P, Brown J, Kendler D, Fahrleitner-Pammer A, Valter I, Maasalu K, Bolognese M, Woodson G, Bone H, Ding B, Wagman R, Martin JS, Ominsky M, Dempster D on behalf of the Denosumab Phase 3 Bone Histology Study Group. Effects of denosumab on bone histomorphometry: The FREEDOM and STAND studies. J Bone Miner Res. 2010 doi: 10.1002/jbmr.149. (in press) [DOI] [PubMed] [Google Scholar]
- 55.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 56.Brown JP, Prince RL, Deal C, et al. Comparison of the Effect of Denosumab and Alendronate on Bone Mineral Density and Biochemical Markers of Bone Turnover in Postmenopausal Women With Low Bone Mass: A Randomized, Blinded, Phase 3 Trial. J Bone Miner Res. 2009 doi: 10.1359/jbmr.0809010. IN PRESS. [DOI] [PubMed] [Google Scholar]
- 57.Bone HG, Bolognese MA, Yuen CK, Kendler DL, Wang H, Liu Y, San Martin J. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. Clin Endocrinol Metab. 2008;93:2149–2157. doi: 10.1210/jc.2007-2814. [DOI] [PubMed] [Google Scholar]
- 58.Stolina M, Kostenuik PJ, Dougall WC, Fitzpatrick LA, Zack DJ. RANKL inhibition: from mice to men (and women) Adv Exp Med Biol. 2007;602:143–150. doi: 10.1007/978-0-387-72009-8_18. [DOI] [PubMed] [Google Scholar]
- 59.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 60.Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 61.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gauthier JY, Chauret N, Cromlish W, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008;18:923–928. doi: 10.1016/j.bmcl.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 63.Peroni A, Zini A, Braga V, Colato C, Adami S, Girolomoni G. Drug-induced morphea: report of a case induced by balicatib and review of the literature. J Am Acad Dermatol. 2008;59:125–129. doi: 10.1016/j.jaad.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 64.Stoch SA, Zajic S, Stone J, et al. Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther. 2009;86:175–182. doi: 10.1038/clpt.2009.60. [DOI] [PubMed] [Google Scholar]
- 65.Bone HG, McClung MR, Roux C, et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res. 2010;25:937–947. doi: 10.1359/jbmr.091035. [DOI] [PubMed] [Google Scholar]
- 66.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hannon RA, Clack G, Rimmer M, et al. Effects of the Src kinase inhibitor saracatinib (AZD0530) on bone turnover in healthy men: a randomized, double-blind, placebo-controlled, multiple-ascendingdose phase I trial. J Bone Miner Res. 2010;25:463–471. doi: 10.1359/jbmr.090830. [DOI] [PubMed] [Google Scholar]
- 68.Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, Holst JJ, Christiansen C. Four-month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone. 2009;45:833–842. doi: 10.1016/j.bone.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Schaller S, Henriksen K, Sveigaard C, et al. The chloride channel inhibitor NS3736 [corrected] prevents bone resorption in ovariectomized rats without changing bone formation. J Bone Miner Res. 2004;19:1144–1153. doi: 10.1359/JBMR.040302. [DOI] [PubMed] [Google Scholar]
- 70.Gowen M, Stroup GB, Dodds RA, et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–1604. doi: 10.1172/JCI9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balan G, Bauman J, Bhattacharya S, et al. The discovery of novel calcium sensing receptor negative allosteric modulators. Bioorg Med Chem Lett. 2009;19:3328–3332. doi: 10.1016/j.bmcl.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 72.Kumar S, Matheny CJ, Hoffman SJ, et al. An orally active calcium-sensing receptor antagonist that transiently increases plasma concentrations of PTH and stimulates bone formation. Bone. 2010;46:534–542. doi: 10.1016/j.bone.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 73.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 74.Hoeppner LH, Secreto FJ, Westendorf JJ. Wnt signaling as a therapeutic target for bone diseases. Expert Opin Ther Targets. 2009;13:485–496. doi: 10.1517/14728220902841961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 76.Loots GG, Kneissel M, Keller H, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15:928–935. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 78.Ominsky MS, Vlasseros F, Jolette J, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res. 2010;25:948–959. doi: 10.1002/jbmr.14. [DOI] [PubMed] [Google Scholar]
- 79.Padhi D, Stouch B, Jang G, et al. Anti-sclerostin antibody increases markers of bone formation in healthy postmenopausal women. J Bone Miner Res. 2007;22(Suppl. 1):S37. [Google Scholar]
- 80.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 81.Heath DJ, Chantry AD, Buckle CH, et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;24:425–436. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 82.Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 84.Kansara M, Tsang M, Kodjabachian L, et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119:837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Canalis E. Update in new anabolic therapies for osteoporosis. J Clin Endocrinol Metab. 2010;95:1496–1404. doi: 10.1210/jc.2009-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]