Abstract
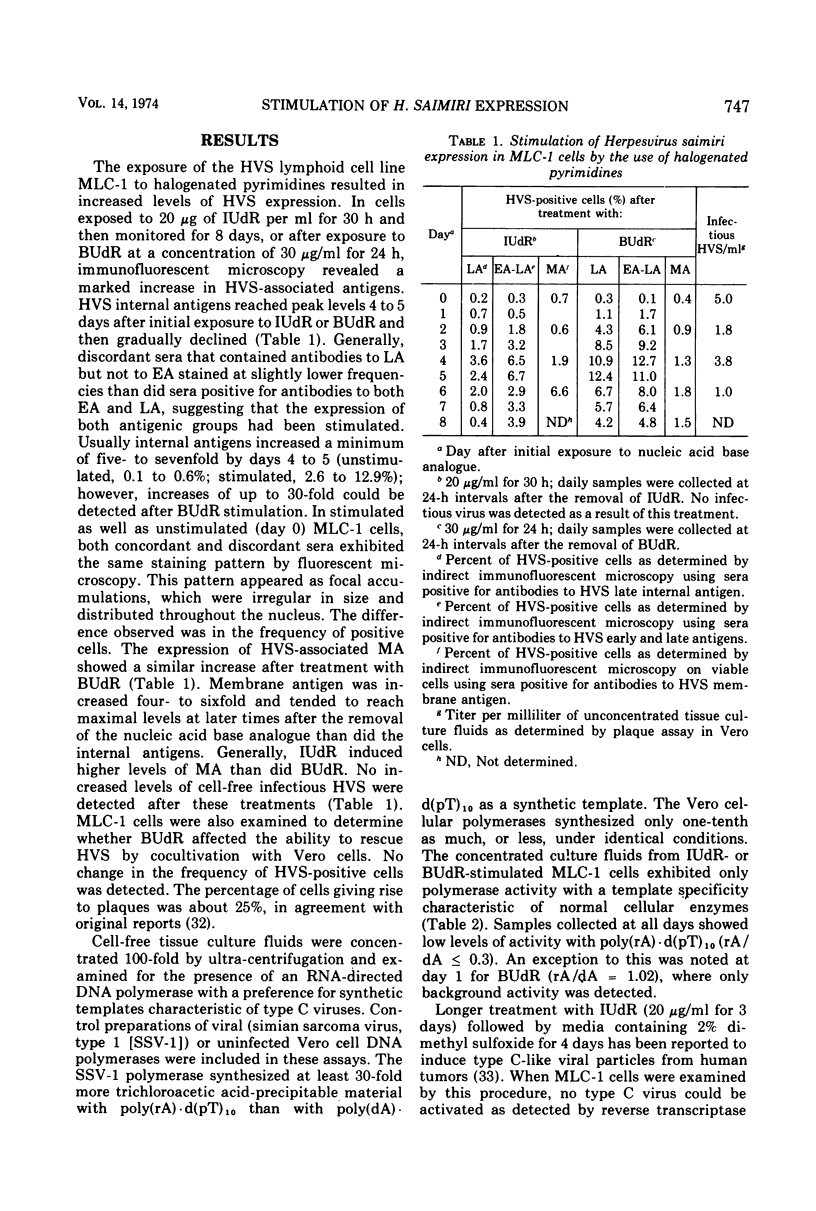
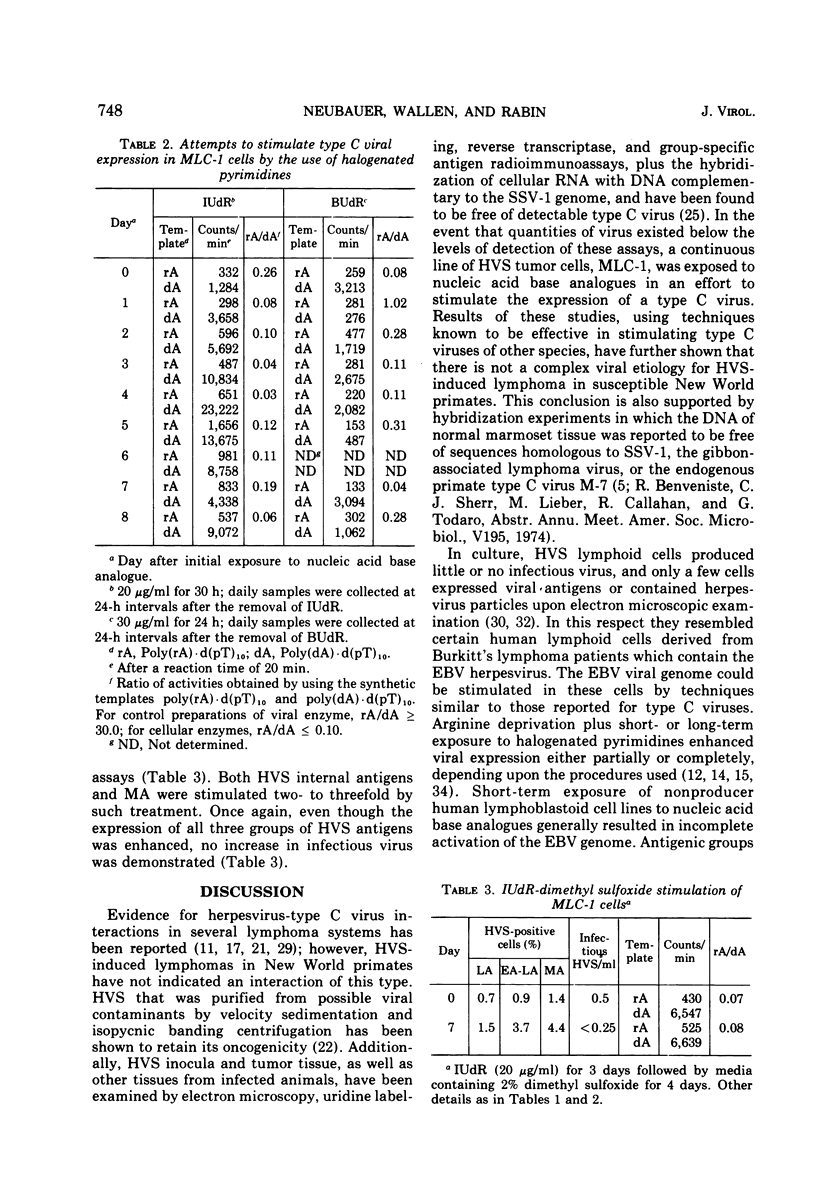
Nucleic acid base analogues were used to examine a Herpesvirus saimiri (HVS)-infected marmoset lymphoid cell line (MLC-1) for possible association with type C viruses. Synthetic templates poly(rA)·d(pT)10 and poly(dA)·d(pT)10 were used to detect RNA-directed DNA polymerase activity in 100-fold concentrated tissue culture fluids. HVS was monitored by immunofluorescence for early, late, and membrane antigens. MLC-1 cells were exposed to 30 μg of 5-bromo-2′-deoxyuridine (BUdR) per ml for 24 h and examined daily. Similar experiments used 5-iodo-2′-deoxyuridine (IUdR) (20 μg/ml) for 30 h or IUdR (20 μg/ml) for 3 days followed by 2% dimethyl sulfoxide for 4 days. Results of these experiments failed to show any type C virus-like polymerase; however, HVS expression was greatly stimulated. BUdR and IUdR enhanced expression of HVS-associated antigens five- to sevenfold, with maximal stimulation being observed 3 to 4 days after removal of the analogue. IUdR-dimethyl sulfoxide treatment was generally less effective. Although more cells showed HVS antigens, the treatments did not increase cell-free infectious virus. The data suggest that HVS-infected lymphoid cells can be stimulated to express virus in a manner similar to that of the Epstein-Barr virus in Burkitt's lymphoma cells. No evidence of type C virus was found in stimulated cultures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Ablashi D. V., Loeb W. F., Valerio M. G., Adamson R. H., Armstrong G. R., Bennett D. G., Heine U. Malignant lymphoma with lymphocytic leukemia induced in owl monkeys by Herpesvirus saimiri. J Natl Cancer Inst. 1971 Oct;47(4):837–855. [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnek B. W., Ubertini T., Adldinger H. K. Viral antigen, virus particles, and infectivity of tissues from chickens with Marek's disease. J Natl Cancer Inst. 1970 Aug;45(2):341–351. [PubMed] [Google Scholar]
- Daniel M. D., Rabin H., Barahona H. H., Meléndez L. V. Herpesvirus saimiri. 3. Plaque formation under multi agar, methyl cellulose and starch overlays. Proc Soc Exp Biol Med. 1971 Apr;136(4):1192–1196. doi: 10.3181/00379727-136-35456. [DOI] [PubMed] [Google Scholar]
- Deinhardt F. Oncogenic herpesviruses in species other than owl monkeys. A review. J Med Primatol. 1974;3(1):79–88. doi: 10.1159/000459967. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Herpesvirus saimiri: experimental infection of squirrel monkeys (Saimir sciureus). J Natl Cancer Inst. 1973 Jul;51(1):165–170. doi: 10.1093/jnci/51.1.165. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Isolation of Herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus). J Natl Cancer Inst. 1972 May;48(5):1499–1505. [PubMed] [Google Scholar]
- Frankel J. W., Groupé V. Interactions between Marek's disease herpesvirus and avian leucosis virus in tissue culture. Nat New Biol. 1971 Nov 24;234(47):125–126. doi: 10.1038/newbio234125a0. [DOI] [PubMed] [Google Scholar]
- Gerber P., Lucas S. Epstein-Barr virus-associated antigens activated in human cells by 5-bromodeoxyuridine. Proc Soc Exp Biol Med. 1972 Nov;141(2):431–438. doi: 10.3181/00379727-141-36791. [DOI] [PubMed] [Google Scholar]
- Gross P. A., Fong C. K., Hsiung C. D. Characterization of guinea pig C-type virus. Proc Soc Exp Biol Med. 1973 Jun;143(2):367–370. doi: 10.3181/00379727-143-37322. [DOI] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Martos L. M., Walker J. L. Synthesis of Epstein-Barr virus after activation of the viral genome in a "virus-negative" human lymphoblastoid cell (Raji) made resistant to 5-bromodeoxyuridine (thymidine kinase-virus antigen-immunofluorescence-herpesvirus fingerprints). Proc Natl Acad Sci U S A. 1972 Jan;69(1):78–82. doi: 10.1073/pnas.69.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G. Effect of arginine-deficient media on the herpes-type virus associated with cultured Burkitt tumor cells. J Virol. 1968 Mar;2(3):182–191. doi: 10.1128/jvi.2.3.182-191.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G. Evidence for a relation of Epstein-Barr virus to Burkitt's lymphoma and nasopharyngeal carcinoma. Bibl Haematol. 1970;(36):706–713. doi: 10.1159/000391777. [DOI] [PubMed] [Google Scholar]
- Hirumi H., Frankel J. W., Prickett C. O., Maramorosch K. Coexistence of particles resembling herpesvirus and type-C virus in feather follicle epithelium of chickens. J Natl Cancer Inst. 1974 Jan;52(1):303–306. doi: 10.1093/jnci/52.1.303. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Fong C. K., Gross P. A. Oncogenic potential of guinea pig herpes- and C-type viruses. Cancer Res. 1973 Jun;33(6):1436–1442. [PubMed] [Google Scholar]
- Klein G., Pearson G., Rabson A., Ablashi D. V., Falk L., Wolfe L., Dienhardt F., Rabin H. Antibody reactions to herpesvirus saimiri (HVS)-induced early and late antigens (EA and LA) in HVS-infected squirrel, marmoset and owl monkeys. Int J Cancer. 1973 Jul 15;12(1):270–289. doi: 10.1002/ijc.2910120128. [DOI] [PubMed] [Google Scholar]
- Kufe D., Magrath I. T., Ziegler J. L., Spiegelman S. Burkitt's tumors contain particles encapsulating RNA-instructed DNA polymerase and high molecular weight virus-related RNA. Proc Natl Acad Sci U S A. 1973 Mar;70(3):737–741. doi: 10.1073/pnas.70.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs R., Fleckenstein B. Malignant lymphoma induced by partially purified Herpesvirus saimiri and recovery of infectious virus from tumorous lymph nodes. Med Microbiol Immunol. 1972;158(2):135–146. doi: 10.1007/BF02120479. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Daniel M. D., Hunt R. D., Garcia F. G. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab Anim Care. 1968 Jun;18(3):374–381. [PubMed] [Google Scholar]
- Neubauer R. H., Wallen W. C., Parks W. P., Rabin H., Cicmanec J. L. Attempts to demonstrate type-C virus in normal and neoplastic tissues of nonhuman primate origin. Lab Anim Sci. 1974 Feb;24(1):235–240. [PubMed] [Google Scholar]
- Neubauer R. H., Wallen W. C., Rabin H., Pearson G. R., Ablashi D. V. Virological investigations of Herpesvirus saimiri-infected owl monkeys. J Med Primatol. 1974;3(1):27–40. doi: 10.1159/000459962. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Orr T., Rabin H., Cicmanec J., Ablashi D., Armstrong G. Antibody patterns to Herpesvirus saimiri-induced antigens in owl monkeys. J Natl Cancer Inst. 1973 Dec;51(6):1939–1943. doi: 10.1093/jnci/51.6.1939. [DOI] [PubMed] [Google Scholar]
- Pearson G., Ablashi D., Orr T., Rabin H., Armstrong G. Intracellular and membrane immunofluorescence investigations on cells infected with Herpesvirus saimiri. J Natl Cancer Inst. 1972 Nov;49(5):1417–1424. [PubMed] [Google Scholar]
- Peters W. P., Kufe D., Schlom J., Frankel J. W., Prickett C. O., Groupé V., Spiegelman S. Biological and biochemical evidence for an interaction between Marek's disease herpesvirus and avian leukosis virus in vivo. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3175–3178. doi: 10.1073/pnas.70.11.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin H., Pearson G., Chopra H. C., Orr T., Ablashi D. V., Armstrong G. R. Characteristics of Herpesvirus saimiri-induced lymphoma cells in tissue culture. In Vitro. 1973 Sep-Oct;9(2):65–72. doi: 10.1007/BF02616002. [DOI] [PubMed] [Google Scholar]
- Rabin H., Pearson G., Klein G., Ablashi D., Wallen W., Cicmanec J. Herpesvirus saimiri antigens and virus recovery from cultured cells and antibody levels and virus isolations from squirrel monkeys. Am J Phys Anthropol. 1973 Mar;38(2):491–496. doi: 10.1002/ajpa.1330380253. [DOI] [PubMed] [Google Scholar]
- Rabson A. S., O'Conor G. T., Lorenz D. E., Kirschstein R. L., Legallais F. Y., Tralka T. S. Lymphoid cell-culture line derived from lymph node of marmoset infected wtih Herpesvirus saimiri--preliminary report. J Natl Cancer Inst. 1971 May;46(5):1099–1109. [PubMed] [Google Scholar]
- Stewart S. E., Kasnic G., Jr, Draycott C., Ben T. Activation of viruses in human tumors by 5-iododeoxyuridine and dimethyl sulfoxide. Science. 1972 Jan 14;175(4018):198–199. doi: 10.1126/science.175.4018.198. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Mizuno F., Osato T. Induction of Epstein-Barr virus-related membrane antigens by 5-iododeoxyuridine in non-producer human lymphoblastoid cells. Nat New Biol. 1973 Nov 21;246(151):70–72. doi: 10.1038/newbio246070a0. [DOI] [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H. In vitro immunological characteristics of lymphoid cells derived from owl monkeys infected with Herpesvirus saimiri. J Med Primatol. 1974;3(1):41–53. doi: 10.1159/000459963. [DOI] [PubMed] [Google Scholar]
- Wolfe L. G., Falk L. A., Deinhardt F. Oncogenicity of herpesvirus saimiri in marmoset monkeys. J Natl Cancer Inst. 1971 Nov;47(5):1145–1162. [PubMed] [Google Scholar]
- Yang S. S., Ablashi D., Armstrong G., Ting R. C. RNA-dependent DNA polymerase associated with a simian lymphoid cell line derived from a Herpesvirus saimiri-induced lymphoma. Int J Cancer. 1974 Jan 15;13(1):82–90. doi: 10.1002/ijc.2910130110. [DOI] [PubMed] [Google Scholar]



