Abstract
Cell migration is an important physiological process, which is involved in cancer metastasis. Therefore, the investigation of cell migration may lead to the development of novel therapeutic approaches. In this study, we have successfully developed a microsystem for culture of two cell types (non-malignant and carcinoma) and for analysis of cell migration dependence on distance between them. Finally, we studied quantitatively the influence of photodynamic therapy (PDT) procedures on the viability of pairs of non-malignant (MRC5 or Balb/3T3) and carcinoma (A549) cells coculture. The proposed geometry of the microsystem allowed for separate introduction of two cell lines and analysis of cells migration dependence on distance between the cells. We found that a length of connecting microchannel has an influence on cell migration and viability of non-malignant cells after PDT procedure. Summarizing, the developed microsystem can constitute a new tool for carrying out experiments, which offers a few functions: cell migration analysis, carcinoma and non-malignant cells coculture, and evaluation of PDT procedure in the various steps of cell migration.
INTRODUCTION
Cell culture techniques that mimic in vivo conditions are very important in biological and biochemical research.1 Microsystems have now become accepted tools used for fundamental biological studies as they enable one to perform highly controlled in vitro experiments. A number of devices for the cell cultivation, lysis, single-cell analysis, and cell based toxicity tests are reported.2, 3, 4, 5, 6 In microsystems, cells can be easily manipulated and cellular environment can be precisely controlled.7, 8 There is a wide range of materials from which microsystems can be fabricated depending on their application: polymers, glass, silicone, paper, as well combinations of these materials (hybrid systems).9, 10 The most important parameters, which must be considered when the appropriate material is being chosen are: biocompatibility, surface chemistry, optical and electrical properties, cost, easiness of method for fabrication and integration. Microfluidic devices have many advantages, i.e., miniaturisation of cells assays and precise control of cellular environment. Microscale systems dedicated for cell and tissue engineering enable control of temporal and spatial resolution, which is important in cells studies. These systems create the ability to control a cellular microenvironment including the supply and transfer of media, buffers, and waste products, which mimic the human circulatory system better.11
In the native environment, cells strongly interact with the extracellular matrix and adjacent cells. Directed cell migration is an integrated process essential for development, growth, and life of cells. Moreover, cell-cell and cell-microenvironment interaction are crucial for various biological functions. The understanding of cells' interaction and migration mechanism is essential for elaboration of new anticancer therapies and drugs.12 Cell migrations or communications with other cell types are especially important when one wishes to examine cells culture in vitro, which reacts in the same way as components of tissue and organs.13, 14, 15 Moreover, development of new anticancer methods requires the investigation of communications between non-malignant and carcinoma cells. Cell movement during cell migration can be driven by chemical gradients (i.e., chemotaxis)16, 17, 18, 19, 20 or physical parameters, i.e., mechanical stimulation, magnetic fields, and electric fields (i.e., electrotaxis).21, 22, 23, 24 Microsystems are valuable tools for chemotaxis and electrotaxis studies due to the possibility of precise configuration of chemical gradients and direct current electric fields generation (dcEF). Various gradient-generating microscale devices have been applied to chemotaxis research.25, 26 Moreover, microfluidics-based migration research for different cell types towards electric fields was performed.27, 28, 29 There is also an example of a device for studies of cell migration in co-existing chemical gradients and electric fields.30 In spite of the fact that there are many examples of microdevices for observation and monitoring of cell migration in 2D and 3D cultures31 and for evaluation of photodynamic therapy (PDT) efficiency,32, 33 there is no example of a device applied for cell migration studies and also for PDT efficiency investigation. Migration of the cells can also have an influence on the efficiency of PDT procedure. Therefore, it is important to test the interaction between non-malignant and carcinoma cells cultured in coculture and to assess/evaluate the effectiveness after PDT procedure. It can mimic in vivo conditions during the PDT treatment. PDT procedure requires administration of a non-toxic photosensitizing substance (pre-formed photosensitizer or precursor of photosensitizer), which accumulates primarily in the carcinoma cells. After that, the cells are irradiated with light of wavelength that is absorbed by the photosensitizer. The excited photosensitizer generates the reactive oxygen species (ROS), which are toxic to the cells.34, 35, 36 5-aminolevulinic acid (ALA) is an often-investigated precursor of a photosensitizer. When the exogenous ALA is administered, it penetrates into all cells, where it is metabolized into an active sensitizer PpIX. However, a higher activity of enzymes in the tumour than in the non-malignant cells leads to a higher PpIX accumulation in these cells. Finally, PpIX is present in a lower concentration in the non-malignant than in carcinoma cells.37, 38 The comparison of the toxic effect after PDT between non-malignant and carcinoma cells is important, because the selectivity of the method is essential for effective anticancer therapy. These tests were performed using classical methods (96-well plates)39 and using the microfluidic system.33 The previously designed microfluidic system was used for the examination of PDT procedure on the non-malignant and carcinoma cells cultured in the separated and the “mixed” culture. However, the influence of migration was not controlled.
Integration in the microfluidic system of functions such as: migration analysis, coculture formation, and PDT procedure performance enabled evaluation of PDT procedure in conditions that mimic cellular environment better than a classic cell monoculture. The aim of the research was to check whether the presence of another cell type would enhance/weaken the viability of a cell line and to observe if cells' migration would appear. Our device enables the evaluation of PDT therapy effectiveness influenced by the presence of two types of cells (non-malignant and carcinoma cells). In our system, both types of cells grow in separated microchambers connected by microchannels. The special architecture of the microsystem enabled examination of how intercellular signals contribute to cellular communities. It was found that medium exchanging and death signals, which can be released by either cell type40 (in the connecting microchannel region), influence the efficiency of the PDT procedure.
MATERIALS AND METHODS
Biological material
The A549 (human lung carcinoma cell line), MRC5 (human fetal lung fibroblast cells), Balb/3T3 (mouse embryonic fibroblast cell line) were used as the model cells for experiments. The cells were obtained from European Type Culture Collection (EATCC). Cells were cultured in MEME supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% streptomycin and penicillin, and 2 mM l-glutamine. The cells were cultured at 37 °C in a humidified atmosphere including 5% CO2.
Microfluidic device preparation
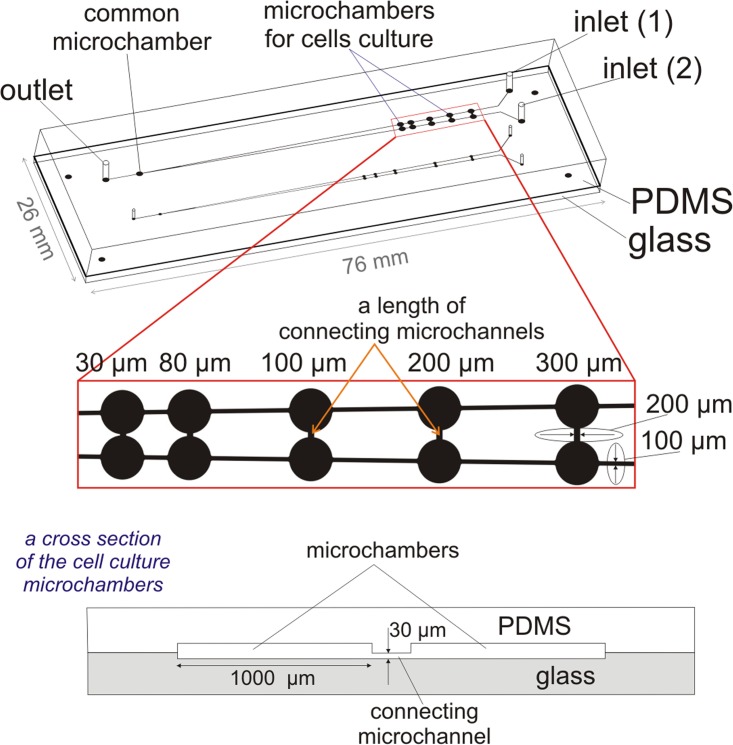
The microfluidic system for carcinoma and non-malignant cells migration analysis consisted of two layers: polydimethylsiloxane (PDMS) (Sylgard 184, Dow Corning) and a glass plate (76 mm × 26 mm × 1 mm, Helmand). The geometry of the microsystem was designed using AutoCAD and the design was printed on a transparency mask by a high-resolution printer (3600 DPI). The geometry of the microsystem contains: a microchannels' network (a width—100 μm, a height—50 μm) and five pairs of microchambers connected with additional microchannel. Moreover, at the end of the microchannels' network, a common microchamber was placed (Fig. 1). The arrangement of the microchannels' network on the plate creates a V-shaped structure. Furthermore, each pair of microchambers is connected with an additional microchannel (a length of 300 μm, 200 μm, 100 μm, 80 μm, 30 μm, respectively). They were designed for migration analysis of cells cultured at different distances. A width and a height of all connecting microchannels were the same, it equals 200 μm and 30 μm, respectively. In turn, the common microchamber was designed to obtain an automatically mixed culture of two cell lines side by side culturing in the same microchamber. In Fig. 1, on the enlarged part of the microsystem dimensions of the microstructure are also shown. Moreover, a cross section scheme of the pair of microchambers is presented. The geometry of the microsystem consists also of two independent networks of microchannels with different sizes of microchambers (with a diameter of 1 mm and 500 μm). It was designed for monitoring of the growth and migration of two cultures in different conditions (different value of surface area to volume ratio—SAV) simultaneously.
Figure 1.
The geometry of the multi-function microsystem for cells migration analysis and evaluation of PDT procedure. On the enlarged part of the microsystem, the important dimensions of the microstructure are also shown. Dimensions of the microchannels (V-shaped structure) obtained in the PDMS equal: a height of 50 μm, a diameter of 100 μm. In the glass were obtained: microchambers (a diameter of 1000 μm, a height of 30 μm) and connecting microchannels (a width—200 μm, a height—30 μm). A length of connecting microchannels equals—300 μm, 200 μm, 100 μm, 80 μm, 30 μm, respectively. The cross section scheme of the pair of microchambers is also presented.
The fabrication process of the microsystem includes two parts: the development of microstructures (a) in PDMS and (b) in the glass plate. The pairs of microchambers were fabricated in the hydrophilic glass plate, because it assured a site for good adhesion and proliferation of adherent cells. The chambers etched in the glass (a diameter of 1 mm, a depth of 30 μm) provided good cells adhesion conditions and minimised the hydrodynamic stress caused by the medium flow over the cell culture. Moreover, the connecting microchannels were designed in the glass. Thus, no reservoir edge in the glass existed; therefore, it did not impede migration. In turn, the microchannels (with a V-shaped structure) were designed to provide cells and medium dosage into the cell microchambers. Microstructures in PDMS were obtained using photolithography and replica moulding techniques, which were used in the preceding work.33, 41 Finally, 1.3 mm diameter holes for tubings were drilled in the PDMS plate. The microchambers with five connecting microchannels were fabricated in the hydrophilic glass plate using photolithography and the wet etching method.33, 41 Finally, the PDMS plate with microchannels network and access holes was bonded with the glass plate using surface plasma activation (Plasma Preen System, Inc., II 973).
Cell culture and cells migration analysis
The fabricated microdevice was sterilized under ultraviolet light and by flushing with 70% volume ethyl alcohol for 20 min. After that, the medium was introduced in the microfluidic system. Syringe pumps (NE 1000 New Era Pump Systems Inc.) were used for introduction of all fluids and cells. After culture medium introduction, the microsystem was placed in incubator (5% CO2, 37 °C) for 2 h. The proper condition for adherent cell culture was, thus, ensured. Next, the cell suspensions of 1 × 106 A549 cells/ml, 1 × 106 MRC5 cells/ml, and 1 × 106 Balb/3T3 cells/ml were prepared. The A549 with MRC5 and A549 with Balb/3T3 cells (in two independent tests) were introduced in the microchambers through two inlets of one microstructure with a flow rate of 20 μl/min. The sealed microfluidic device was placed in the incubator at 37 °C and 5% CO2. The medium in the microchambers was replaced every 24 h (with a flow rate of 1.2 μl/min for 20 min) to maintain suitable conditions for cell culture. The temperature of the microsystem was controlled by use of a heated microscope table. The A549, MRC5, and Balb/3T3 cells were cultured over 48 h. Cell migration between two microchambers was observed, using an inverted fluorescent microscope (Olympus IX-71) connected with a CCD camera. Moreover, in the independent tests, the cell trackers were used to mark non-malignant and carcinoma cells. For this purpose, CellTracker™ Red CMTPX and CellTracker Green CMFDA (Invitrogen) for MRC-5 (or Balb/3T3) and A549 cells staining were used, respectively. Solution of CellTrackers was prepared according to the producer's instructions. The above tests were performed in order to show that two types of cells are not mixed during the cells seeding. Details of this part of the research were described in the supplementary material.42
PDT procedure in multi-function microsystem
ALA (Sigma-Aldrich) was used as a precursor for the photosensitizer—PPIX. The samples of 0.75 mM ALA were prepared in the culture medium without FBS. PDT procedure in microscale was elaborated in the preceding work.33 Briefly, 48 h after cells seeding (A549-Balb/3T3 and A549-MRC5) in the fabricated microsystem (when migration was observed), the cells were rinsed with PBS at a flow rate 1.2 μl/min for 10 min. Next, the cells were incubated with 0.75 mM ALA solution and then the microsystem was placed for 4 h in the incubator. This time of incubation is necessary for selective penetration of exogenous ALA into the carcinoma cells.43 After that, the cells were washed with PBS with a flow rate of 1.2 μl/min for 10 min. This PDT procedure step was performed under a microscope. At the end, the microdevice was exposed to the light generated by a LED (a distance 10 mm, time 60 s, λ = 625 nm, energy dose 30 J/cm2, a bandwidth of a LED 25 nm). After irradiation, a fresh medium containing 10% (v/v) FBS was introduced into the microsystem and the microchip was placed in the incubator for 2 h. The PDT procedure in details was presented in our previous work, so here only short description of this method was added.33
Viability tests and data analysis
To evaluate the toxic effect after PDT in the microsystem, calceine AM (CAM) and propidium iodide (PI) were used. 1 μl of 2 mM calceine AM (Sigma Aldrich) and 50 μl of 1 mg ml−1 propidium iodide (Sigma Aldrich) in 0.5 ml of culture medium were used. The fluorescent dyes were introduced in the microstructure at a flow rate of 1.2 μl/min for 20 min. Calceine AM penetrates into the living cell through the cell membrane and gives a green fluorescence, while propidium iodide combines with the nucleic acids of necrotic cells and gives a red fluorescence. For each microchamber, three images were recorded at 10× magnification using an inverted fluorescent microscope. The number of dead cells was determined by counting the number of green (corresponding to live cells) and red (dead cells) objects with image processing software (cellSens Dimension, OLYMPUS). Experimental data are expressed as mean ± standard deviation (SD) from at least three independent experiments.
RESULTS
Fabrication result of the microsystem
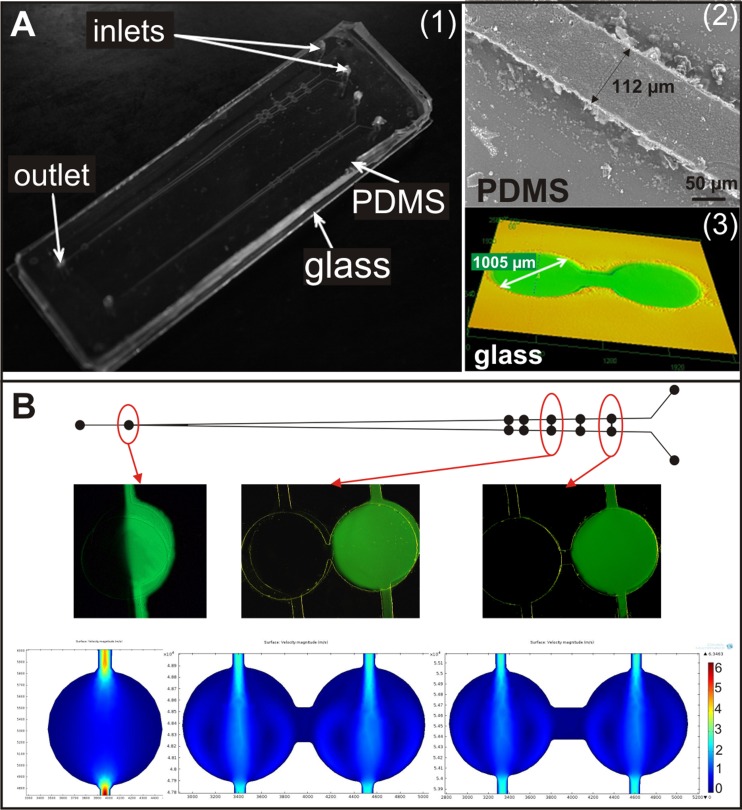
Photolithography, replica moulding, and wet etching technologies were used to successfully fabricate the microsystem. The microstructures obtained in the PDMS and glass plates were checked using an electron microscope (Hitachi TM-1000) and a confocal laser microscope (LEXT OLS3100 Olympus) (Fig. 2a). It was found that the microstructures dimensions correspond to the photomask design.
Figure 2.
(a) A photographs of (1) the designed and fabricated microsystem for carcinoma and non-malignant cells migration analysis, (2) the microchannel fabricated in the PDMS plate (Hitachi TM1000), and (3) the microchambers developed in the glass (LEXT OLS3100 Olympus). (b) Tests of solution flow—pairs of microchambers during the introduction of fluorescein solution and distilled water. At the end simulation of flow rate of two introduced substances through microchambers (comsol multiphysics) are presented.
The aim of our work was to fabricate the microsystem allowing for automatic and separate introduction of non-malignant and carcinoma cells into the microchambers. Moreover, the fabricated microsystem should provide analysis of migration of cells between cultured cells. The designed microsystem fulfilled these assumptions. Prior to cell loading, the flow through the microdevice of two different solutions was analysed. It was studied in order to verify the geometry of the microsystem, which must be appropriate for separate introduction of non-malignant and carcinoma cells. The aqueous solution of fluorescein (Fluka) was prepared with the concentration 3 × 10−4 M. The fluorescein solution and distilled water were introduced into the microdevice (filled with water) through the inlets, with a flow rate of 20 μl/min each. Fluorescence intensity was measured for each microchamber during a flow rate. Image analysis was performed using an inverted fluorescent microscope (Olympus IX71) with an integrated CCD camera. Figure 2b shows the microchambers with the two introduced solutions showing both introduced solutions flow separately through the channels (without mixing). Based on images analysis, these flows of introduced solutions were confirmed. In the common microchamber, both fluorescein solution and water were placed. Fluid flow in the designed structure was also simulated by computer modeling using MEMS Module of comsol multiphysics software. The results of computer modeling of fluid flow in the microchannels show that the solution can be introduced in the microsystem without mixing in connecting microchannels. The experiment confirmed proper microchannel geometry of the designed microchip. Therefore, it was expected that during the introduction of non-malignant and carcinoma cells to the microsystem, they shall not be mixed and shall be placed in separate microchambers for culture.
Cell culture in the microsystem
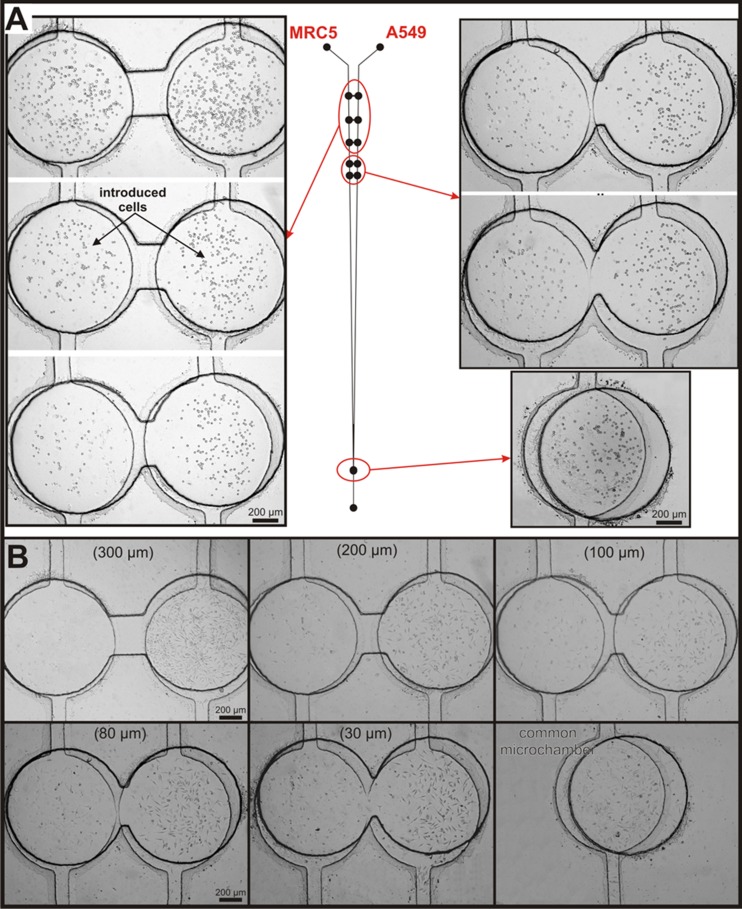
In this paper, A549, MRC5, and Balb/3T3 cell lines cultured in the microfluidic system for cell migration are presented. The migration between cells is dependent on the type of the cells. Therefore, two pairs of non-malignant and carcinoma cells were used: A549 with MRC5 and A549 with Balb/3T3. The carcinoma (A549) and non-malignant (MRC-5 or Balb/3T3) cells were seeded (through inlets) in the appropriate microchambers without affecting each other. Cells were introduced in the microsystem previously filled with culture medium. Due to this, all of the connecting microchannels remained unfilled with cells. The microchambers with A549 and MRC5 cells after introduction are shown in Fig. 3a. The suspension density of both cell lines and a flow rate used for cell docking in microchambers enabled introduction of the cells to each culture microchamber in a sufficient amount for future culture. Moreover, the seeded cell density allowed maintenance of cellular interactions (between the same cells) to ensure proper cell growth (Fig. 3b). In the last microchamber, both of the tested cell lines were cultured together. The final effect was monitored—viability of the cells—after PDT procedure. The same culture conditions of both cells and interchange of signal(s) released by either cell type influence on growth of the cells were tested. Microchambers with cells stained with CellTrackers are shown in the supplementary material.42
Figure 3.
The MRC5 and A549 cells cultured in the microchambers with connecting microchannels for migration analysis (a) after cells introduction and (b) 24 h after cells seeding (microchannels length: 300, 200, 100, 80, and 30 μm, respectively). The last microchamber is common for both cell lines, a coculture was formed.
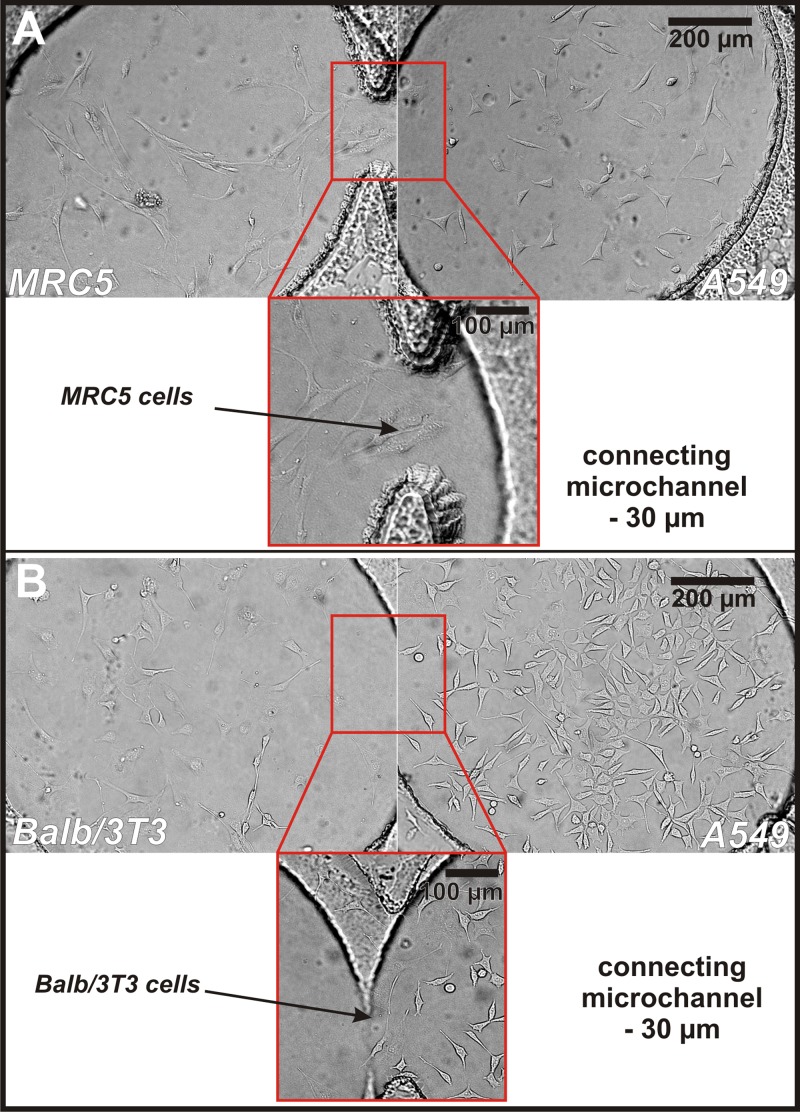
Cells' adhesion and proliferation were observed within 48 h after seeding. It was investigated to ensure that the geometry of the microsystem and the applied procedures did not cause hydrodynamic stress for the cells cultured in the microchambers. After the introduction of fresh medium, the cells in the microchamber were adhered to the glass. A flow rate of the introduced medium did not influence the morphology and growth of cells. 24 h after cells' seeding cell migration was observed. At first, cells of the same line moved together, then migration to the other cell type was observed (cells appeared in the connecting microchannels). Two different cells cultured separately can migrate in the special fabricated connecting channels. After 24 h, mainly Balb/3T3 cells migrated along connecting microchannels (A549-Balb/3T3 cell pair). Individual A549 cells were adhered in connecting microchannels. In the case of the second cell pair (A549-MRC5 cell pair), only isolated MRC5 cells migrated in the connecting microchannels. It has been found that after 24 h culture, cells migrated only in the shortest distance between microchambers. Fig. 4 shows the microchambers (a length of connecting microchannels—30 μm) with two different cell pairs (A) A549 - MRC5 and (B) A549 - Balb/3T3. Both of the cell pairs are very well adhered to growth surface. One can also observe that the non-malignant cells (MRC5 and Balb/3T3) migrated along the connecting microchannel.
Figure 4.
(a) Culture of MRC5 (left microchambers) and A549 (right microchamber) cells 24 h after seeding. (b) Culture of Balb/3T3 (left microchambers) and A549 (right microchamber) cells 24 h after seeding (the connecting microchannel with a length of 30 μm). Pictures show that all of the cultured cells are very well adhered to the growth surface. It indicates that the culture of two types of cells and medium exchanging along connecting microchannel not influence the viability and growth of these cells. Moreover, it was observed that 24 h after cells seeding, non-malignant cells grow in the shortest connecting microchannels. Type of the cells was distinguished based on their morphology. However, in the independent tests, cell trackers were used to mark two types of cells (see supplementary material42).
These experiments confirmed that appropriately designed microchannel geometry enables simultaneous migration analysis of non-malignant and carcinoma cells whereas in cells' microchambers arranged at different distances this was not evident. It is an advantage, distinguishing it from macroscale, where usually definition of the distance between two cell types cultured together is not possible. The above studies confirmed that the microsystem can be used to analyse the migration of non-malignant and carcinoma cells cultured separately and connected by a microchannel. It was also investigated that the dimension of microchambers (500 or 1000 μm) did not have influence on the cells' growth and migration. The geometry of the microsystem also enables the exchange of medium from non-malignant and carcinoma cells. It can influence the cell growth. 48 h after cells seeding, the viability of cells was analysed. Both cell lines were still proliferating, growing, and maintaining their basic life functions. The cells were still attached to the hydrophilic glass substrate. Therefore, the proposed cultures were used to test PDT procedure.
Effectiveness of PDT procedure
In clinical application, PDT is effective when it is selective only towards the tumour cells. Obviously, concentrations of a photosensitiser, duration of ALA exposure to the cells, and also the exposure time and dose of light have a significant influence on the viability of the cells. PDT procedure must be optimised so that the non-malignant cells remain alive. Therefore, it is important to perform tests related to the evaluation of the toxicity of PDT procedure in the same conditions on non-malignant and carcinoma cells. An alternative tool is proposed, which enables simultaneous testing of PDT procedure using different cell lines, during the different steps of migration. Two pairs of the cells, selected for the investigation, constituted a good model, because they represented non-malignant and carcinoma cells. First, the cultured pair of A549-Balb/3T3 cells was selected as a good adherent cell model used in our previous tests.33 In turn, A549-MRC5 cells were derived from the same organ. It mimics growth and communications between carcinoma and non-malignant cells present in a living organism. First, the toxic effect after PDT procedures (with ALA) on the separated Balb/3T3, MRC-5 and A549 cell culture was investigated. It was proved that the usage of 0.75 mM ALA for PDT caused death of 95% of carcinoma A549 cells, whereas only 5% of MRC-5 and 10% of Balb/3T3 non-malignant cells were killed. These tests confirmed that the used PDT parameters had a toxic effect mainly on the carcinoma cells. Non-malignant cells were still alive during exposure of the same PDT parameters.
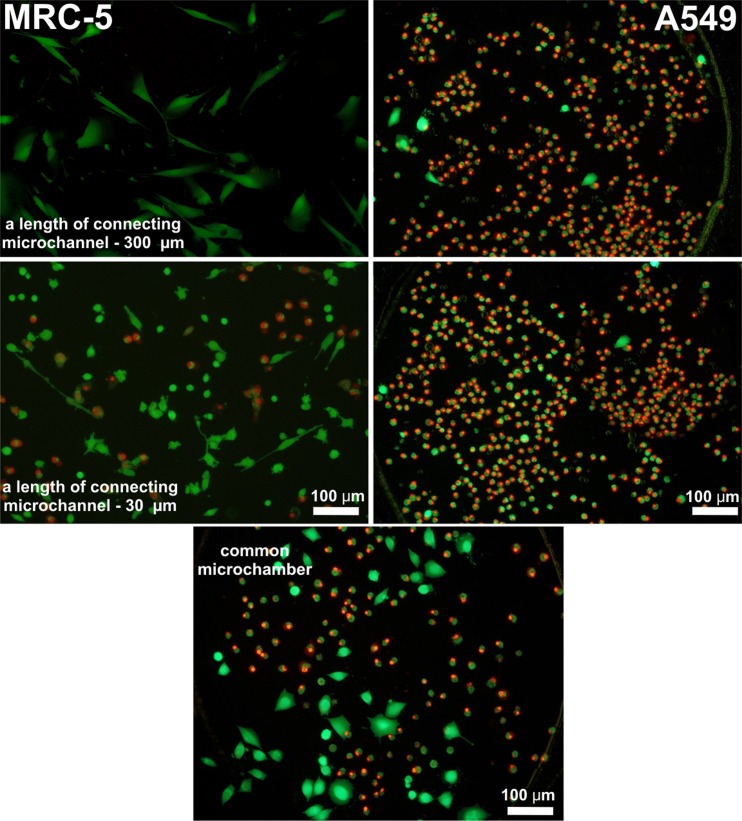
Next, influence of the cells migration and medium exchanging in connecting microchannels on the viability of the A549-MRC5 and A549-Balb/3T3 cells after PDT procedure was studied. It was observed that the exchange of nutrient between both types of cells has an effect on the viability of non-malignant cells. After the viability test (with calceine–AM and propidium iodide), non-malignant cells (Balb/3T3 or MRC-5) were still alive in the microchamber connected by long microchannels, whereas carcinoma cells (A549) were dead. In Fig. 5, the viability test with calceine AM and propidium iodide on MRC-5 and A549 cells after PDT procedures is shown. MRC5 cells were alive in pairs of microchambers connected with microchannels with a length of 80–300 μm. It was observed that the number of dead non-malignant cells in the shortest connecting microchannel (30 μm) and the common microchamber was the highest. Cell numbers in the common microchamber (distinction between the non-malignant and carcinoma cells) were determined based on the morphology of non-malignant and carcinoma cells. Moreover, during the introduction of cells into the microsystem, they were tracked with CellTracker™ Red CMTPX (MRC-5 or Balb/3T3 cells) and CellTracker Green CMFDA (A549 cells). The tracking enabled confirmation of proper seeding of cells in the suitable microchambers and also calculation of the number of each type of cells in the common microchamber.
Figure 5.
The MRC-5 and A549 cells cultured in the microchambers after PDT procedure and viability test. Viability is examined after LIVE/DEAD cell staining with PI and CAM. For each ALA concentration, three images of the interested area were taken at 10× magnification using a fluorescent microscope. Cell viability was determined by counting the number of green objects (corresponding to live cells) and red (dead cells) with an image processing software (cellSens Dimension, Olympus).
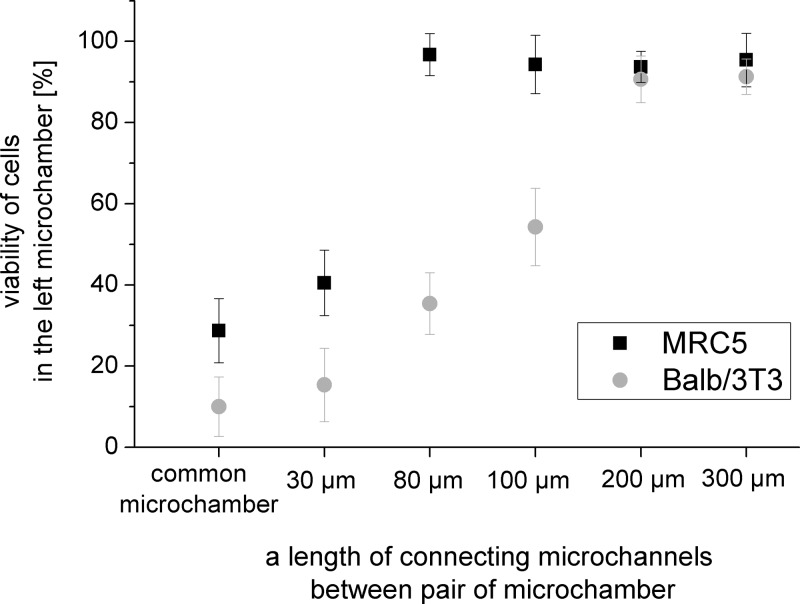
The same tendency was observed for both cells pairs: A549-Balb/3T3 and A549-MRC5 (Fig. 6). The number of live cells increased with the length of the connecting microchannels. Non-malignant cells (in both tested cells pairs) were alive in microchambers with connecting microchannels of lengths of 200 and 300 μm. The number of live cells equals 95.4 ± 6.5, 91.3 ± 4.3 (for MRC5 cells), and 93.7 ± 3.8, 90.7 ± 5.7 (for Balb/3T3 cells) in sequence for microchannels with lengths of 200 and 300 μm. In the common microchamber for both tested cell pairs, the highest toxic effect was observed. Viability of cells equals 28.7 ± 7.9 for MRC5 cells and 10.0 ± 7.3 for Balb/3T3 cells. In this microchamber, a coculture was created during cell seeding. Communication between these cells was the strongest, because they grew directly next to each other.
Figure 6.
The number of dead non-malignant cells (MRC-5 and Balb/3T3) in each of the microchambers.
Probably, reactive oxygen species, which were produced by the carcinoma A549 cells, have a toxic effect also on non-malignant cells cultured in the microchambers placed in common microchamber. However, due to limited lifetime of ROS, it is rather impossible that ROS would diffuse through the connecting microchannel. For example, the distance diffused by the reactive intermediate, mainly 1O2, causing the photodegradation was estimated to be on the order of 0.01-0.02 μm, which corresponds to an intermediate lifetime of 0.01–0.04 ms in the cells44, 45, 46, 47 The higher death rate for short connection channels is due to the release of death signals from the dying A549 cells (i.e., TNFα factor).40, 48 Moreover, it was observed that cell viability is dependent on the type of cell pair. For A549-Balb/3T3 pair, the number of dead non-malignant cells was higher than for A549-MRC5 cells. The highest difference of the number of dead cells was observed for microchambers connected to the microchannels with lengths of 80 μm and 100 μm. Here, the viability of MRC5 cells (A549-MRC5 pair) was, respectively, three times and twice the viability of Balb/3T3 cells (A549-Balb/3T3).
In the preceding work,49 it was proved that the PDT procedure performed on the “mixed” culture caused the number of dead cells to be the highest in the culture with the higher density of the carcinoma cells. Here, there is the same cell density but different distances between non-malignant and carcinoma cells. It was proved that the PDT procedure has no toxic effect on the non-malignant cells in the separate cultures, however, a toxic effect was observed in cultures where medium and factors exchange are possible. Carcinoma (A549) cells produce death signals, which can have toxic effect also on the non-malignant cells, cultured in the same environment, connected by a short microchannel. Examination showed that the number of dead cells after PDT is dependent on the type of cell culture. Besides advantages resulting from the miniaturisation, the usage of the microsystem enabled comparison and testing of PDT procedure on the non-malignant and carcinoma cells cultured in the same conditions, placed in the microchip in a controlled manner. Special geometry of the microsystem enabled evaluation of difference in the cells viability after PDT procedure resulting from the distances between non-malignant and carcinoma cells. In this stage of the research, it was investigated whether the presence of carcinoma cells in the culture has an influence on the viability of non-malignant cells. It was indicated that their viability was dependent on the distance between both types of cells. In future work, it is intended to determine the type and concentration of death factors, which can be produced by carcinoma cells.
CONCLUSIONS
We developed the new multi-function microsystem for simple cell lines introduction and cultivation of two adherent cell types. Special connecting microchannel geometry allows for precise cell lines deposition in microchambers. Transparent material chosen for system fabrication enables cell coculture observation. In the proposed device, continuous media exchange can be realized. The microchannel geometry allows for cells interactions assisted by common medium and close distance between two types of cells. In our opinion, a cell coculture microsystem based on non-malignant and carcinoma cell lines is a useful device for anticancer drug studies and therapies as well as for fundamental studies of cells interactions (observation of proliferation and growth changes). The proposed multi-function microfluidic system for cell culture is a convenient tool for cell-based applications such as cell migration studies, toxicological test as well as new drug and evaluation of how the presence of another cell type has influence on cells viability after PDT procedure. Finally, the microsystem can have numerous applications in the study of a new photosentitizers and parameters of PDT procedure on the cells (during the migration of various cells under precisely controlled conditions and coculture formation).
ACKNOWLEDGMENTS
This work was realized with a frame of project Iuventus Plus, which is financed by the Ministry of Science and Higher Education, Contract No. 0205/IP2/2011/71. This work was realized with a frame of Project 1450/B/H03/2011/40, which was financed by the National Centre of Research.
References
- Marimuthu M. and Kim S., Anal. Biochem. 413, 81 (2011). 10.1016/j.ab.2011.02.027 [DOI] [PubMed] [Google Scholar]
- El-Ali J., Sorger P., and Jensen K., Nature 442, 403 (2006). 10.1038/nature05063 [DOI] [PubMed] [Google Scholar]
- Walker G., Ozers M., and Beebe D., Biomed. Microdevices 4, 161 (2002). 10.1023/A:1016088128057 [DOI] [Google Scholar]
- Liu T., Lin B., and Qin J., Lab Chip 10, 1671 (2010). 10.1039/c000022a [DOI] [PubMed] [Google Scholar]
- Wang M., Tu E., Raymond D., Yang J., Zhang H., Hagen N., Dees B., Mercer E., Forster A., Kariv I., Marchand P., and Butler W., Nat. Biotechnol. 23, 83 (2005). 10.1038/nbt1050 [DOI] [PubMed] [Google Scholar]
- Pihl J., Sinclair J., Karlsson M., and Orwar O., Mater. Today 8, 46 (2005). 10.1016/S1369-7021(05)71224-4 [DOI] [Google Scholar]
- Ziolkowska K., Jedrych E., Kwapiszewski R., Lopacinska J., Skolimowski M., and Chudy M., Sens. Actuators B 145, 533 (2010). 10.1016/j.snb.2009.11.010 [DOI] [Google Scholar]
- Griffith L. and Swartz M., Nat. Rev. Mol. Cell Biol. 7, 211 (2006). 10.1038/nrm1858 [DOI] [PubMed] [Google Scholar]
- Guber A. E., Heckele M., Herrmann D., Muslija A., Saile V., Eichhorn L., Gietzelt T., Hoffmann W., Hauser P. C., Tanyanyiwa J., Gerlach A., Gottschlich N., and Knebel G., Chem. Eng. J. 101, 447 (2004). 10.1016/j.cej.2004.01.016 [DOI] [Google Scholar]
- Greve F., Seemann L., Hierlemann A., and Lichtenberg J., J. Micromech. Microeng. 17, 1721 (2007). 10.1088/0960-1317/17/8/040 [DOI] [Google Scholar]
- Meer A., Poot A., Duits M., Feijen J., and Vermes I., J. Biomed. Biotechnol. 2009, 823148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz R. and Webb D., Curr. Biol. 13, R756 (2003). 10.1016/j.cub.2003.09.014 [DOI] [PubMed] [Google Scholar]
- Wei C. W., Cheng J. Y., and Young T. H., Biomed. Microdevices 8, 65 (2006). 10.1007/s10544-006-6384-8 [DOI] [PubMed] [Google Scholar]
- Bhatia S. N., Yarmush M. L., and Toner M., J. Biomed. Mater. Res. 34, 189 (1997). [DOI] [PubMed] [Google Scholar]
- Tan W. and Desai T. A., J. Biomed. Mater. Res. Part A 72, 146 (2005). 10.1002/jbm.a.30182 [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., and Simmons R. L., J. Immunol. 115, 1650 (1975). [PubMed] [Google Scholar]
- Boyden S., J. Exp. Med. 115, 453 (1962). 10.1084/jem.115.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof A., Quillan M., Dan Y., and Poo M., J. Neurosci. 12, 1253 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S., J. Cell Biol. 75, 606 (1977). 10.1083/jcb.75.2.606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman E. F., Campbell J. J., and Butcher E. C., J. Cell Biol. 139, 1349 (1997). 10.1083/jcb.139.5.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S. and Beningo K. A., PLoS ONE 6, e17277 (2011). 10.1371/journal.pone.0017277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Gu Y., Pu J., Reid B., Zhao Z., and Zhao M., Nat. Protoc. 2, 1479 (2007). 10.1038/nprot.2007.205 [DOI] [PubMed] [Google Scholar]
- Li J., Nandagopal S., Wu D., Romanuik S. F., Paul K., Thomson D. J., and Lin F., Lab Chip 11, 1298 (2011). 10.1039/c0lc00371a [DOI] [PubMed] [Google Scholar]
- Lin F., Baldessari F., Gyenge C., Sato T., Chambers R., Santiago J., and Butcher E., J. Immunol. 181, 2465 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. and Lin F., Trends Cell Biol. 21, 489 (2011). 10.1016/j.tcb.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Kim S., Kim H. J., and Jeon N. L., Integr. Biol. 2, 584 (2010). 10.1039/c0ib00055h [DOI] [PubMed] [Google Scholar]
- Rezai P., Siddiqui A., Selvaganapathy P., and Gupta B., Lab Chip 10, 220 (2010). 10.1039/b917486a [DOI] [PubMed] [Google Scholar]
- Minc N. and Chang F., Curr. Biol. 20, 710 (2010). 10.1016/j.cub.2010.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Kao Y. C., Chi P. Y., Huang C. W., Lin J. Y., Chou C. F., Cheng J. Y., and Lee C. H., Lab Chip 11, 695 (2011). 10.1039/c0lc00155d [DOI] [PubMed] [Google Scholar]
- Li J., Zhu L., Zhang M., and Lin F., Biomicrofluidics 6, 024121 (2012). 10.1063/1.4718721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Sudo R., Mack P. J., Wan Ch -R., Vickerman V., and Kamm R., Lab Chip 9, 269 (2009). 10.1039/b807585a [DOI] [PubMed] [Google Scholar]
- Lou X., Kim G., Lee Y. K., Kopelman R., and Yoon E., in Proceedings of 15th International Conference on mTAS (2011), p. 2058.
- Jedrych E., Pawlicka Z., Chudy M., Dybko A., and Brzozka Z., Anal. Chim. Acta 683(2), 149 (2011). 10.1016/j.aca.2010.10.005 [DOI] [PubMed] [Google Scholar]
- Friesen S. A., Hjortland G. O., Madsen S. J., Hirschberg H., Engebraten O., Nesland J. M., and Peng Q., Int. J. Oncol. 21, 577 (2002). [PubMed] [Google Scholar]
- Rossi V. M., White B. M., Newton M. J., Jacques S. L., and Baugher P. J., Proc. SPIE 7886, 1 (2011). 10.1117/12.876337 [DOI] [Google Scholar]
- Alam M. F., Atif M., AlSalhi M. S., Siddique M., Kishwar S., Qadir M. I., and Willander M., Laser Phys. 21, 972 (2011). 10.1134/S1054660X11090076 [DOI] [Google Scholar]
- Collaud S., Juzeniene A., Moan J., and Lange N., Curr. Med. Chem. Anticancer Agents 4, 301 (2004). 10.2174/1568011043352984 [DOI] [PubMed] [Google Scholar]
- Ohgari Y., Nakayasu Y., Kitajima S., Sawamoto M., Mori H., Shimokawa O., Matsui H., and Taketani S., Biochem. Pharmacol. 71, 42 (2005). 10.1016/j.bcp.2005.10.019 [DOI] [PubMed] [Google Scholar]
- Kastle M., Grimm S., Nagel R., Breusing N., and Grune T., Free Radic. Biol. Med. 50, 305 (2011). 10.1016/j.freeradbiomed.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Fiers W., Beyaert R., Declercq W., and Vandenabeele P., Oncogene 18, 7719 (1999). 10.1038/sj.onc.1203249 [DOI] [PubMed] [Google Scholar]
- Jedrych E., Flis S., Jastrzebski Z., Chudy M., Dybko A., and Brzozka Z., Sens. Actuators B 160, 1544 (2011). 10.1016/j.snb.2011.08.074 [DOI] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4771966 for comparison of cells' introduction and growth in the microsystem after trackered with Long Term CellTrackers.
- Betz Ch., Lai J. P., Xiang W., Janda P., Henrich P., Stepp H., Baumgartner R., and Leunig A., Photochem. Photobiol. Sci. 1, 315 (2002). 10.1039/b109817a [DOI] [PubMed] [Google Scholar]
- Lu Ch., Song G., and Lin J. M., TrAC, Trends Anal. Chem. 25, 985 (2006). 10.1016/j.trac.2006.07.007 [DOI] [Google Scholar]
- Peng Q., Nesland J. M., Madslien K., Danielsen H. E., and Moan J., Proc. SPIE 2628, 102 (1996). 10.1117/12.229977 [DOI] [Google Scholar]
- Moan J. and Berg K., Photochem. Photobiol. 53, 549 (1991). 10.1111/j.1751-1097.1991.tb03669.x [DOI] [PubMed] [Google Scholar]
- Bronshtein I., Aulova S., Juzeniene A., Iani V., Ma L.-W., Smith K. M., Malik Z., Moan J., and Ehrenberg B., Photochem. Photobiol. 82, 1319 (2006). 10.1562/2006-04-02-RA-865 [DOI] [PubMed] [Google Scholar]
- Korobowicz A., Pol. Merkuriusz Lek. 21(124), 358 (2006). [PubMed] [Google Scholar]
- Jedrych E., Chudy M., Dybko A., and Brzozka Z., Biomicrofluidics 5, 041101 (2011). 10.1063/1.3658842 [DOI] [PMC free article] [PubMed] [Google Scholar]