Abstract
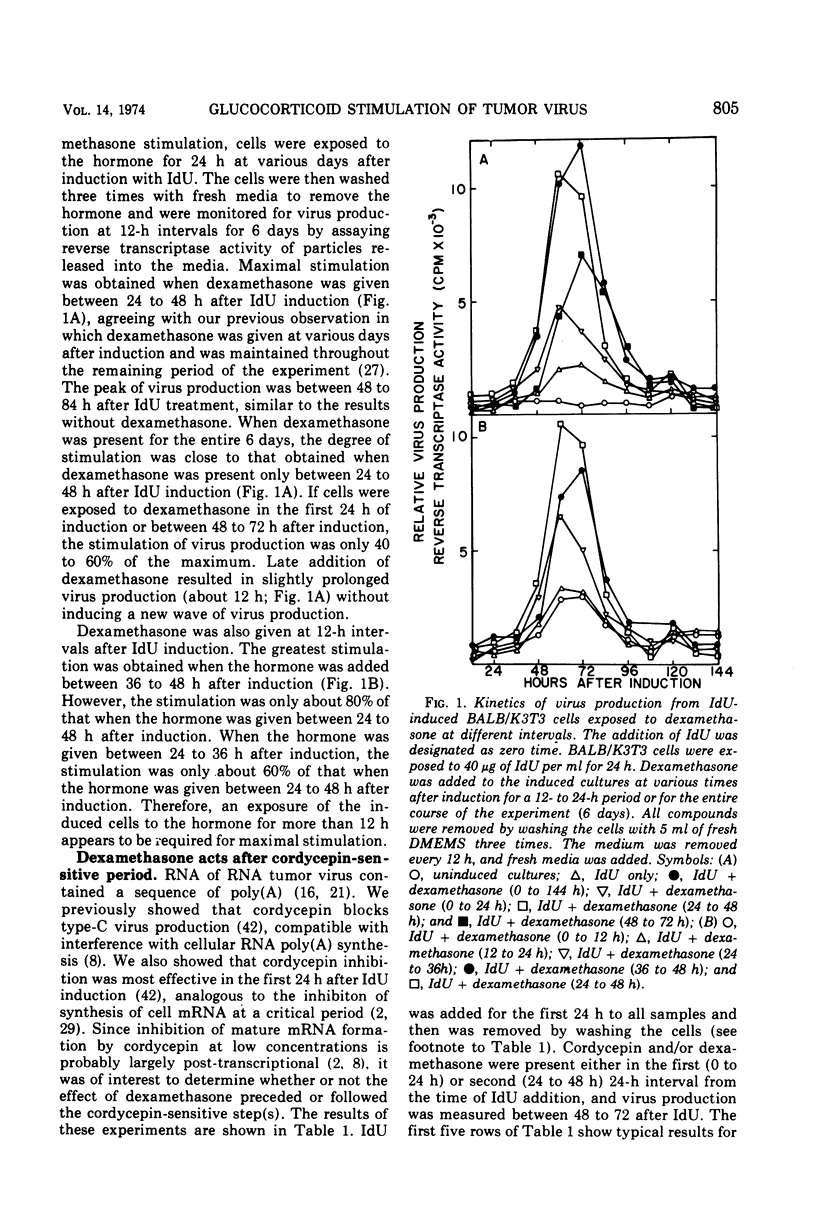
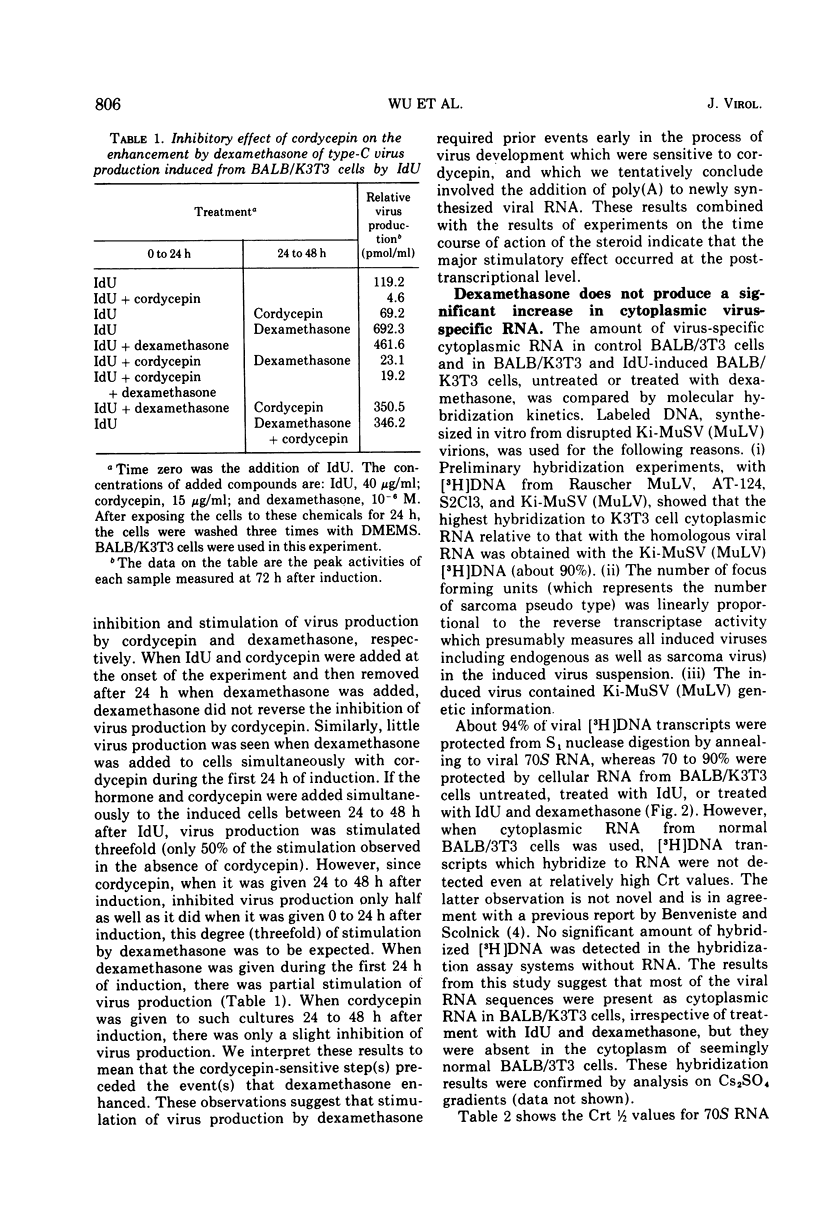
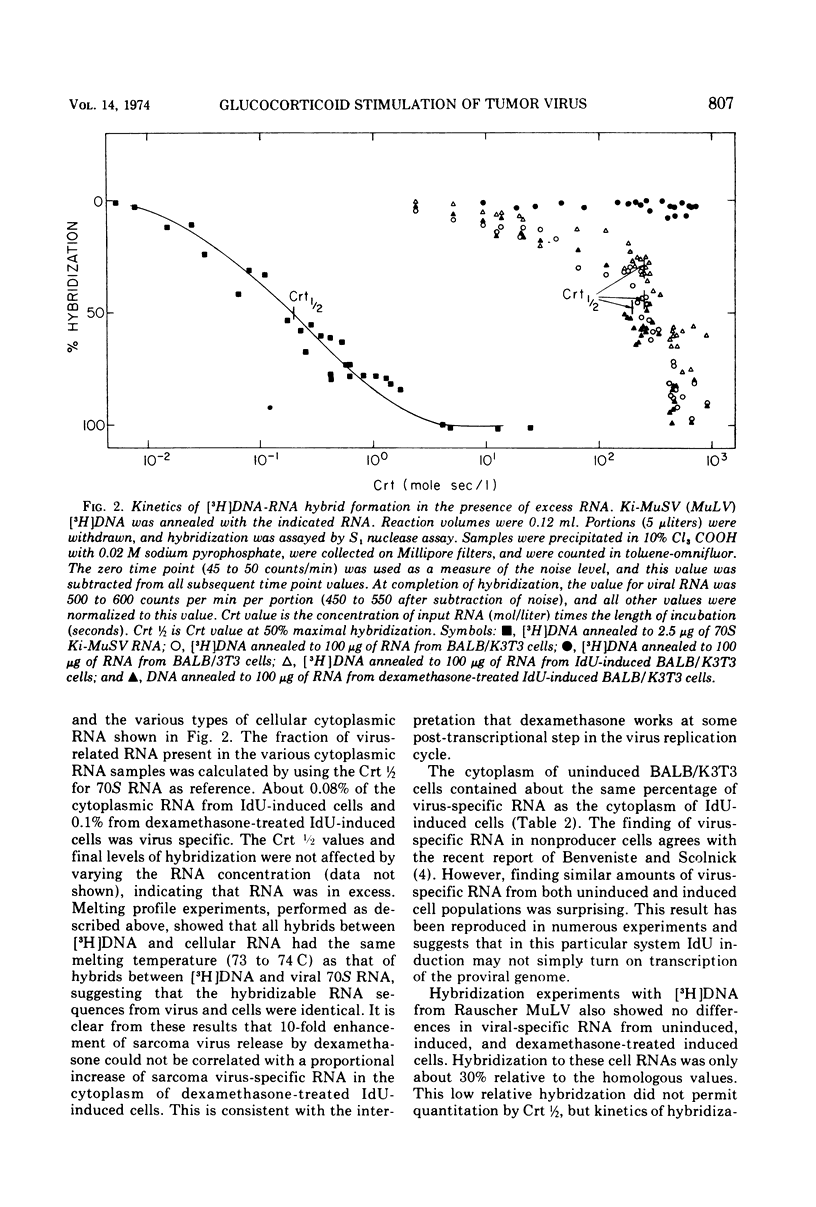
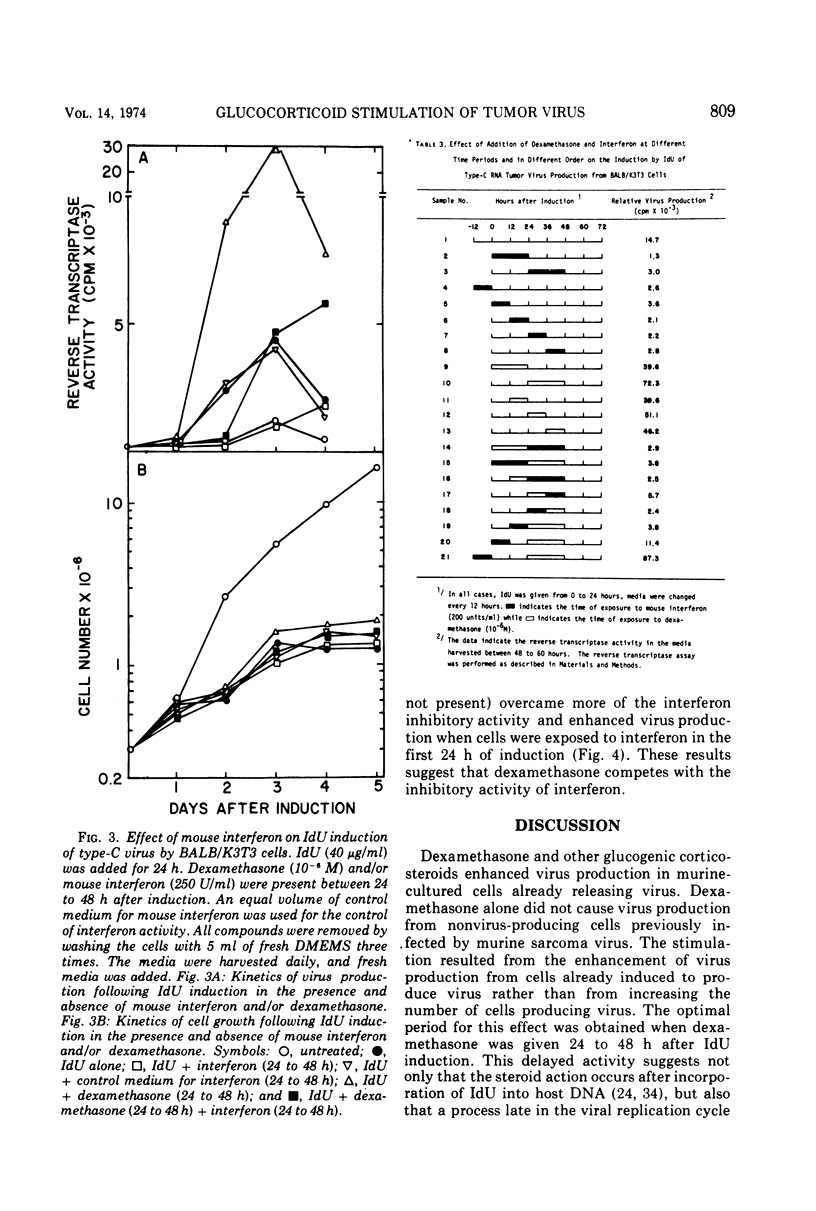
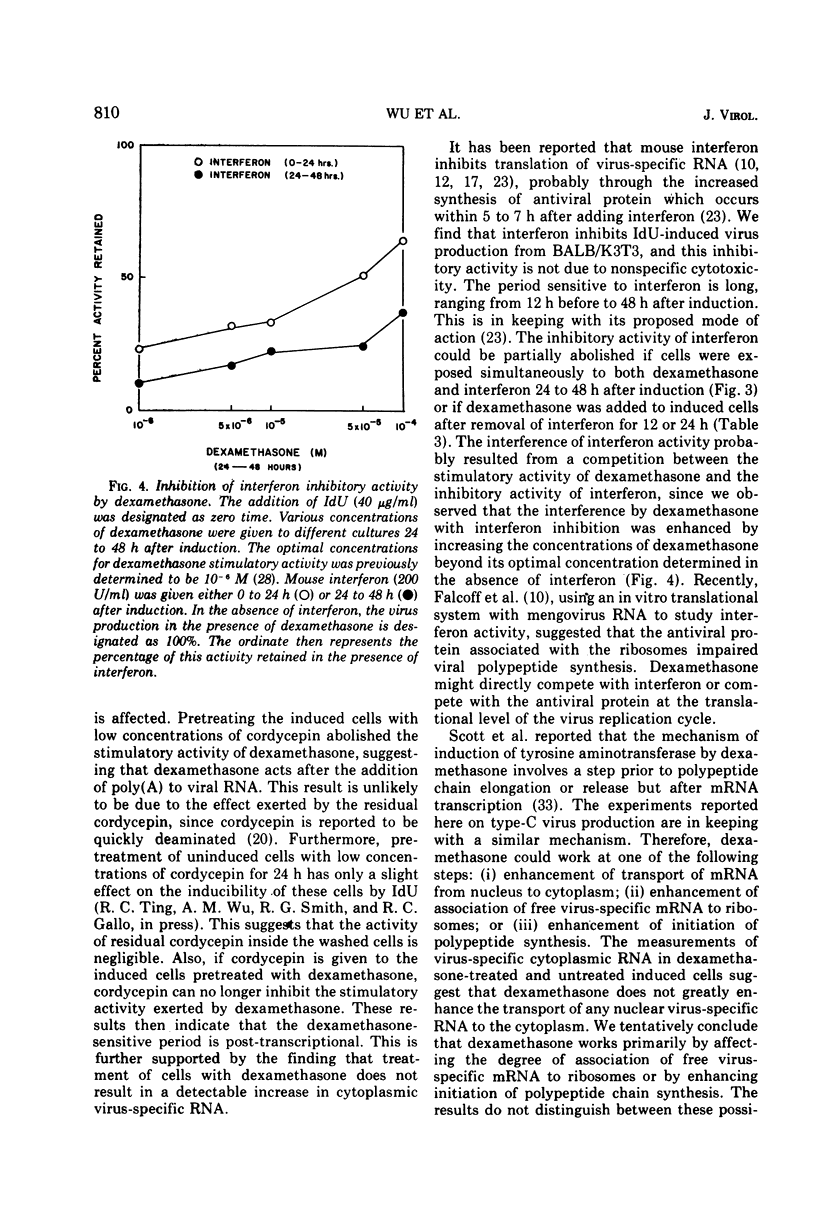
We have previously shown that dexamethasone stimulates production of type-C virus from seemingly normal murine fibroblasts (BALB/3T3) and from transformed (Kirsten sarcoma-leukemia virus) nonproducing cells (BALB/K3T3) induced by 5-iododeoxyuridine. In this report, we further examine the mechanism of this effect by using BALB/K3T3 cells. Several observations suggest that this effect is post-transcriptional. The optimal stimulation by dexamethasone is obtained when dexamethasone is given 24 to 48 h after 5-iododeoxyuridine induction. Although this effect is late, time course experiments suggest that dexamethasone does not act to promote release of preformed virions. The stimulation by dexamethasone is blocked when cells are treated with cordycepin (3′-deoxyadenosine) during the first 24 h of induction, but not when cordycepin is added later. Conversely, interferon, which inhibits virus production, interferes with dexamethasone when it is added late or after removal of the steroid. The results of molecular hybridization experiments show that there is no detectable increase in Kirsten sarcoma-leukemia virus-specific RNA in dexamethasone-treated cells (with or without 5-iododeoxyuridine). The results of the time course studies, and the cordycepin, interferon, and hybridization experiments, suggest that the effect of dexamethasone on type-C virus production in this system is post-transcriptional.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya J., Xuma M., Reitz M., Sarin P. S., Gallo R. C. Utilization of mammalian 70S RNA by a purified reverse transcriptase from human myelocytic leukemic cells. Biochem Biophys Res Commun. 1973 Sep 5;54(1):324–334. doi: 10.1016/0006-291x(73)90926-1. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Buckler C. E., Baron S. Effect of cycloheximide on the antiviral action of interferon. Proc Soc Exp Biol Med. 1969 Feb;130(2):519–523. doi: 10.3181/00379727-130-33595. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Canaani E. Complementarity between Rous sarcoma virus (RSV) RNA and the in vitro-synthesized DNA of the virus-associated DNA polymerase. Virology. 1970 Nov;42(3):783–788. doi: 10.1016/0042-6822(70)90325-9. [DOI] [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Lebleu B., Revel M. Correlation between the antiviral effect of interferon treatment and the inhibition of in vitro mRNA translation in noninfected L cells. J Virol. 1973 Sep;12(3):421–430. doi: 10.1128/jvi.12.3.421-430.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Metz D. H., Esteban R. M., Tovell D. R., Ball L. A., Kerr I. M. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J Virol. 1972 Dec;10(6):1184–1198. doi: 10.1128/jvi.10.6.1184-1198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Aaronson S. A., Martin M. A. Heterogeneity of murine leukemia virus in vitro DNA; detection of viral DNA in mammalian cells. Science. 1971 Jun 25;172(3990):1353–1355. doi: 10.1126/science.172.3990.1353. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Inhibition of protein synthesis directed by added viral and cellular messenger RNAs in extracts of interferon-treated Ehrlich ascites tumor cells. Location and dominance of the inhibitor(s). Biochem Biophys Res Commun. 1973 Sep 18;54(2):777–783. doi: 10.1016/0006-291x(73)91491-5. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLENOW H. FORMATION OF THE MONO-, DI- AND TRIPHOSPHATE OF CORDYCEPIN IN EHRLICH ASCITES-TUMOR CELLS IN VITRO. Biochim Biophys Acta. 1963 Nov 22;76:347–353. [PubMed] [Google Scholar]
- Kenney F. T., Lee K. L., Stiles C. D., Fritz J. E. Further evidence against post-transcriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1973 Dec 19;246(155):208–210. doi: 10.1038/newbio246208a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Morhenn V., Rabinowitz Z., Tomkins G. M. Effects of adrenal glucocorticoids on polyoma virus replication. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1088–1089. doi: 10.1073/pnas.70.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie H. K., Gazdar A. F., Buckler C. E., Baron S. High interferon producing line of transformed murine cells. J Gen Virol. 1972 Oct;17(1):107–109. doi: 10.1099/0022-1317-17-1-107. [DOI] [PubMed] [Google Scholar]
- Paran M., Gallo R. C., Richardson L. S., Wu A. M. Adrenal corticosteroids enhance production of type-C virus induced by 5-iodo-2'-deoxyuridine from cultured mouse fibroblasts. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2391–2395. doi: 10.1073/pnas.70.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Abrell J. W., Trainor C. D., Gallo R. C. Precipitation of nucleic acids with cetyltrimethylammonium bromide: a method for preparing viral and cellular DNA polymerase products for cesium sulfate density gradient analysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):30–38. doi: 10.1016/0006-291x(72)90005-8. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Shiu G., Baron S., Huebner R. J. Inhibitory effect of interferon on murine sarcoma and leukaemia virus infection in vitro. Nature. 1969 Aug 23;223(5208):845–846. doi: 10.1038/223845a0. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Sarin P. S., Reitz M. S., Gallo R. C. Reverse transcriptase activity of human acute leukaemic cells: purification of the enzyme, response to AMV 70S RNA, and characterization of the DNA product. Nat New Biol. 1972 Nov 15;240(98):67–72. doi: 10.1038/newbio240067a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Shields R., Tomkins G. M. Mechanism of hormonal induction of tyrosine aminotransferase studied by measurement of the concentration of growing enzyme molecules. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2937–2941. doi: 10.1073/pnas.69.10.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich N., Lowy D. R., Hartley J. W., Rowe W. P. Studies of the mechanism of induction of infectious murine leukemia virus from AKR mouse embryo cell lines by 5-iododeoxyuridine and 5-bromodeoxyuridine. Virology. 1973 Jan;51(1):163–173. doi: 10.1016/0042-6822(73)90376-0. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J. Spontaneous release of type C viruses from clonal lines of spontaneously transformed Blab-3T3 cells. Nat New Biol. 1972 Nov 29;240(100):157–160. doi: 10.1038/newbio240157a0. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Gallo R. C. RNA-directed DNA polymerase and virus-induced leukemia in mice. Proc Natl Acad Sci U S A. 1973 May;70(5):1298–1302. doi: 10.1073/pnas.70.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Paran M., Gallo R. C. Cordycepin inhibits induction of murine leukovirus production by 5-iodo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3820–3824. doi: 10.1073/pnas.69.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]



