Abstract
Background
The prognostic significance of p16 promoter hypermethylation in patients with non-small cell lung cancer (NSCLC) is still controversial. This analysis presents pooled estimates of the association to better elucidate whether p16 methylation has a prognostic role in NSCLC.
Methods
Relevant studies were identified by searching PubMed, Embase and Web of Science databases until June 2012. The association of p16 methylation with both overall survival (OS) and disease-free survival (DFS) was preformed. Studies were pooled and summary hazard ratios (HR) were calculated. Subgroup analyses, sensitivity analysis and publication bias were also conducted.
Results
A total of 18 studies containing 2432 patients met the inclusion criteria and had sufficient survival data for quantitative aggregation. The results showed that p16 methylation was an indicator of poor prognosis in NSCLC. The HR was 1.36 (95% CI: 1.08–1.73, I2 = 56.7%) and 1.68 (95% CI: 1.12–2.52, I2 = 38.7%) for OS and DFS, respectively. Subgroup analyses were carried out. The HRs of fresh and paraffin tissue were 1.50 (95% CI: 1.11–2.01) and 1.10 (95% CI: 0.77–1.57). The pooled HR was 1.40 (95% CI: 1.02–1.92) for methylation-specific PCR (MSP) and 1.26 (95% CI: 0.87–1.82) for quantitative MSP (Q-MSP). The combined HR of the 16 studies reporting NSCLC as a whole indicated that patients with p16 hypermethylation had poor prognosis. No significant association was found when adenocarcinoma subtype pooled. When seven studies on DFS were aggregated, the HR was 1.68 (95% CI: 1.12–2.52) without significant heterogeneity. Moreover, no obvious publication bias was detected on both OS and DFS.
Conclusion
The meta-analysis findings support the hypothesis that p16 methylation is associated with OS and DFS in NSCLC patients. Large well-designed prospective studies are now needed to confirm the clinical utility of p16 methylation as an independent prognostic marker.
Introduction
With more than 1.6 million cases diagnosed annually, lung cancer is the leading cause of cancer-related deaths in men and the second leading cause of cancer deaths in women worldwide [1]. Given the clinical burden of lung cancer, with non–small cell lung cancer (NSCLC) accounting for approximately 85% of all cases, it has become a major health problem, worldwide. Despite major advances in cancer treatment in the past two decades, the prognosis of patients with lung cancer has improved only minimally, the overall 5-year survival rate for NSCLC (all stages combined) is roughly 15% (http://seer.cancer.gov/statfacts/html/lungb.html).
Although TNM stage is the most significant clinical parameter to be considered on cancer prognosis, the variability of survival within staging groups requires additional parameters affecting outcome, independent of tumor stage. Since multiple gene and gene-related alterations contribute to NSCLC development and progression. Therefore, determination of the biological behavior and identification of prognostic biomarkers is important for the early detection of relapse, as well as for stratification of patients before enrollment onto their treatment regimens. There is increasing evidence that epigenetic alterations, particularly inactivation of tumor suppressor genes or tumor-related genes through promoter hypermethylation, play a dominant role in various cancers including lung cancer [2], [3]. Promoter CpG island methylation is the most widely studied and best characterized epigenetic alteration in NSCLC, providing some of the most promising markers for early detection and prediction of prognosis or treatment response in NSCLC (see review article [4]).
The p16 INK4A gene is known as a tumor suppressor gene, which functions as negative regulator of the cell cycle progression through its inhibition of cdk4/6 and subsequent blockage of the cyclin-dependent phosphorylation of the Rb [5]. Promoter silencing of p16 INK4A through methylation lead to loss of control of the restriction point in the G1 phase of the cell cycle and favor cellular transformation [6], [7], [8]. Abnormal p16 promoter hypermethylation has been found in several types of tumor, and it is inactivated in 40% to 70% of NSCLC patients [8]. The contribution of p16 deregulation through methylation to the carcinogenic process has been extensively studied. Moreover, bulks of observational studies evaluating its prognostic value in NSCLC have been carried out in past ten years [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22].
The prognostic role of p16 methylation in NSCLC has been investigated over the past decade, with conflicting results from different literatures. Some studies concluded p16 hypermethylation was an independent prognostic factor for dismal outcome. Some other studies did not identify this association. In order to clarify this question, we sought to conduct a systematic review and meta-analysis to estimate the prognostic importance of p16 methylation status for overall survival (OS) and disease-free survival (DFS) in patients with NSCLC.
Materials and Methods
Literature Search Strategy
We performed a systematic search of the relevant literature using PubMed, Web of Science and Embase databases up to June 2012 without language restrictions. We used the following search terms and their combinations: “NSCLC,” “non-small-cell lung cancer,” “lung cancer,” “p16,” “p16 INK4a,” and “methylation.” Moreover, the references in all retrieved articles were screened to identify additional articles that were not identified in the initial literature search described above.
Selection Criteria
To be included in this meta-analysis, the studies should meet the following criteria: 1. full text publication with details of methods available; 2. Contained data on assessment of p16 methylation status; 3. Contained outcome data for NSCLC patients according to p16 methylation status (outcomes were overall survival and/or disease-free survival); 4. hazard ratio (HR) for OS or DFS according to p16 status either had to be reported or could be calculated from the sufficient data provided in the original article; 5. to avoid duplicated publications, we included the most recent report or the most complete one.
Data Extraction
The following information was drawn from each eligible study: first author, year of publication, number of patients participated, number of patients with p16 methylation status, TNM stage, histology, testing material, detecting methods, and data linking p16 methylation status and clinical outcome (OS and DFS). The whole process of data extraction was performed independently by two authors, and the disparities were solved by discussion.
Statistical Methods
The hazard ratio (HR) was abstracted or calculated to quantitatively evaluate the association between p16 methylation status and NSCLC prognosis. The summary HR for overall survival and disease-free survival were evaluated by calculating pooled Cox proportional hazard ratios and 95% confidence intervals (CI) as relevant effect measures using previously published methods. When related data were not available directly from the studies, we calculated the corresponding HR and its 95%CI using the method described by Tierney et al [23]. We then investigated the between-study heterogeneity by using the Cochran’s Q test, using a significance level of p value less than 0.1. The statistic I2 was also used to quantitatively evaluate the heterogeneity [24]. If I2 greater than 50% is considered a measure of severe heterogeneity, then the random-effects model was adopted to calculate HR according to the DerSimonian–Laird method [25]. Otherwise, the fixed-effects model (Mantel–Haenszel method) was used [26]. The assessment of sources of heterogeneity was undertaken by meta-regression analysis and subgroup analyses. One-way sensitivity analysis was performed to assess the stability of the results, namely, a single study in the meta-analysis was deleted each time to reflect the influence of the individual data set to the pooled HR [27]. We also used inverted funnel plots and the Egger’s test to examine the effect of publication bias (linear regression analysis) [28]. All p values were 2-sided and less than 0.05 was considered as significance. All analyses were carried out on STATA 11.0 software platform (Stata Corporation, College Station, TX).
Results
Selection and Characteristics of Studies
By the initial literature search, 145 records were identified regarding the association of p16 methylation status and NSCLC survival; 111 studies were excluded after screening the titles or abstracts because they were either review articles, abstracts, no on human being, duplicate publication, or studies irrelevant to the current theme (mainly on cancer early diagnosis). Thirty-four relevant studies were selected for detailed evaluation. Nine were excluded for the systematic review after full assessment (7 were lacking relevant survival data, another 2 were duplications). Of the 25 remained studies, eighteen studies were eligible for the meta-analysis [9], [12], [13], [14], [15], [18], [19], [22], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], 7 provided insufficient data for performing a quantitative aggregation [11], [17], [20], [39], [40], [41], [42].
The main features of the 25 studies eligible for the systematic review are shown in Table 1. The number of patients included across all studies varied from 43 to 335. Among the 25 studies, 13 reported that p16 hypermethylation was an unfavorable indicator of survival; the other 12 studies didn’t found this association. There were 18 studies containing 2432 patients had sufficient survival data for quantitative aggregation, among which, 14 studies used methylation-specific PCR (MSP) to assess gene methylation status, and 4 used quantitative MSP (Q-MSP) to determine its methylation status. Seventeen studies dealt with NSCLC of all histological subtypes, one with adenocarcinoma only [32]. Moreover, five trials reported the results of adenocarcinoma in stratified analysis [29], [30], [32], [33], [37]. Twelve trials detected p16 methylation by using fresh frozen tissue and 5 using formalin-fixed paraffin-embedded (FFPE) specimen, only one using peripheral blood as sample [14].
Table 1. The main characteristics and results of eligible studies evaluating p16 hypermethylation and NSCLC patients’ survival.
| First author | Year | Country | Number | Method | M | U | Stage | Material | Histology | Estimate | Results | OS | DFS |
| HR (95%CI) | HR (95%CI) | ||||||||||||
| Duk-Hwan Kim [22] | 2001 | USA | 185 | MSP | 51 | 134 | I–IV | fresh tissue | NSCLC | Cox | S | 4.72(1.02–21.85 ) | NA |
| Niklinska [42] | 2001 | Poland | 52 | MSP | 16 | 36 | I–II | fresh tissue | NSCLC | Cox | S | NA | NA |
| Fu [41] | 2003 | China | 64 | MSP | 36 | 28 | I–III | fresh tissue | NSCLC | NA | S | NA | NA |
| Harden [20] | 2003 | USA | 90 | MSP | 15 | 75 | I | fresh tissue | NSCLC | NA | NS | NA | NA |
| Maruyama [19] | 2004 | USA | 124 | MSP | 25 | 99 | I–IV | fresh tissue | NSCLC | Cox | NS | 0.81(0.43–1.52) | NA |
| Shimamoto [40] | 2004 | Japan | 45 | MSP | 17 | 28 | I–III | fresh tissue | NSCLC | K–M | S | NA | NA |
| Toyooka [29] | 2004 | Japan | 351 | MSP | 86 | 265 | I–III | fresh tissue | NSCLC | Cox | S | 1.82(1.10–3.00) | NA |
| 105 | fresh tissue | ADC | S | 2.57(1.15–5.76) | NA | ||||||||
| Wang [18] | 2004 | USA | 119 | MSP | 58 | 61 | I–IIIA | fresh tissue | NSCLC | K–M | S | 2.26(1.26–4.06) | NA |
| 49 | MSP | 24 | 25 | IIIA | fresh tissue | NSCLC | K–M | S | 2.72(1.14–5.13) | 1.46(1.14–6.81) | |||
| 70 | MSP | 37 | 33 | I–II | fresh tissue | NSCLC | K–M | S | 1.69(1.07–6.98) | 1.51(1.21–7.63) | |||
| Divine [17] | 2005 | 147 | MSP | 63 | 84 | I | FFPE tissue | ADC | K–M | NS | NA | NA | |
| Young Tae Kim [30] | 2005 | Korea | 61 | MSP | 41 | 20 | I–III | fresh tissue | NSCLC | Cox | NS | 0.390 (0.066–2.293) | 0.509 (0.164–1.577) |
| 72 | MSP | 60 | 12 | I–IV | fresh tissue | ADC | Cox | NS | 0.176 (0.029–1.074) | NA | |||
| Safar [31] | 2005 | USA | 105 | MSP | 41 | 64 | I–IV | FFPE tissue | NSCLC | Cox | NS | 0.73(0.43–1.23) | NA |
| Tanaka [32] | 2005 | Japan | 57 | MSP | 23 | 34 | I–III | FFPE tissue | ADC | K–M | S | 1.39(1.09–4.71) | NA |
| Gu [33] | 2006 | USA | 155 | Q-MSP | 34 | 121 | I–III | fresh tissue | NSCLC | Cox | S | 1.95(1.12–3.39) | NA |
| 18 | 59 | fresh tissue | ADC | Cox | NS | 1.24 (0.59–2.60) | NA | ||||||
| Jin Seuk Kim [38] | 2006 | Korea | 335 | MSP | 117 | I–IV | FFPE tissue | NSCLC | NA | NS | NA | NA | |
| 198 | MSP | I | FFPE tissue | NSCLC | Cox | S | 2.67(1.21–7.64) | 2.03 (1.09–6.23) | |||||
| Ota [15] | 2006 | Japan | 244 | Q-MSP | 87 | I–IV | FFPE tissue | NSCLC | Cox | S | 1.005(1.003–1.008) | NA | |
| Sugio [34] | 2006 | Japan | 224 | MSP | 49 | I–IV | fresh tissue | NSCLC | log–rank | NS | 2.31(0.64–9.82) | NA | |
| Fischer [14] | 2007 | Germany | 92 | MSP | 22 | 63 | IIIB–IV | blood sample | NSCLC | log-rank | NS | 1.47(0.61–3.57) | NA |
| Yanagawa [35] | 2007 | Japan | 101 | MSP | 27 | 74 | I–III | fresh tissue | NSCLC | Cox | NS | 0.93(0.33–2.62) | NA |
| Brock [36] | 2008 | USA | 187 | MSP | I | FFPE tissue | NSCLC | Cox | S | NA | 3.55(1.77–7.13) | ||
| Alaa [37] | 2009 | Japan | 88 | MSP | 30 | 58 | I–IV | fresh tissue | NSCLC | Cox | NS | 1.4(0.6–3.2) | NA |
| 43 | MSP | 10 | 33 | I–IV | fresh tissue | ADC | Cox | S | 2.4(1.8–69.7) | NA | |||
| Yoshino [13] | 2009 | Japan | 44 | MSP | 11 | 33 | IA | fresh tissue | NSCLC | K–M | S | 2.36 (1.14–9.90) | 2.18 (1.56–11.2) |
| Bradly [39] | 2010 | USA | 196 | MSP | I–II | fresh tissue | NSCLC | NA | S | NA | NA | ||
| Buckingham [12] | 2010 | USA | 132 | Q-MSP | I–II | fresh tissue | NSCLC | K–M | NS | 1.16 (0.67–2.02) | 1.35 (0.74–2.46) | ||
| Sasaki [11] | 2010 | Japan | 221 | MSP | 91 | I–IV | fresh tissue | NSCLC | NA | NS | NA | NA | |
| Kang [9] | 2012 | Korea | 80 | Q-MSP | 9 | I–III | fresh tissue | NSCLC | K–M | S | 2.31 (2.10–51.72) | NA |
MSP, Methylation-specific PCR; Q-MSP, quantitative methylation-specific PCR; FFPE, formalin-fixed paraffin-embedded; ADC, adenocarcinoma; S, significant; NS,not significant; NA, not available.
Meta-analysis Results
Using the methods described above, the overall survival and/or disease-free survival of 2432 patients in 18 studies were analyzed. The main results of this meta-analysis are summarized in Table 2.
Table 2. The results of meta-analysis on NSCLC overall survival and p16 methylation.
| Pts number | HR (95%CI) | Heterogeneity | |||
| chi-squared (d.f.) | p-value | I2 | |||
| All studies | 2432 | 1.36 (1.08–1.73) | 41.60 (d.f. = 18) | 0.001 | 56.7% |
| Subgroups | |||||
| Detecting method | |||||
| MSP | 1821 | 1.40 (1.02–1.92) | 26.69 (d.f. = 14) | 0.021 | 47.5% |
| Q-MSP | 611 | 1.26 (0.87–1.82) | 6.80 (d.f. = 3) | 0.079 | 55.9% |
| Stage | |||||
| I | 242 | 2.53(1.26–5.11) | 0.03 (d.f. = 1) | 0.865 | 0.0% |
| I–II | 202 | 1.28(0.79–2.06) | 0.46 (d.f. = 1) | 0.498 | 0.0% |
| I–III | 805 | 1.61(1.19–2.19) | 4.57 (d.f. = 5) | 0.470 | 0.0% |
| I–IV | 1091 | 1.00(0.71–1.41) | 11.39 (d.f. = 6) | 0.077 | 47.3% |
| Histology | |||||
| NSCLC | 2303 | 1.41(1.10–1.79) | 37.28 (d.f. = 16) | 0.002 | 57.1% |
| ADC | 412 | 1.56(0.84–2.88) | 9.76(d.f. = 5) | 0.082 | 48.8% |
| Sample type | |||||
| Fresh tissue | 1736 | 1.50(1.11–2.01) | 20.64(d.f. = 13) | 0.080 | 37.0% |
| FFPE tissue | 604 | 1.10(0.77–1.57) | 6.50(d.f. = 3) | 0.090 | 53.8% |
MSP, Methylation-specific PCR; Q-MSP, quantitative methylation-specific PCR; FFPE, formalin-fixed paraffin-embedded; ADC, adenocarcinoma; Pts, patients.
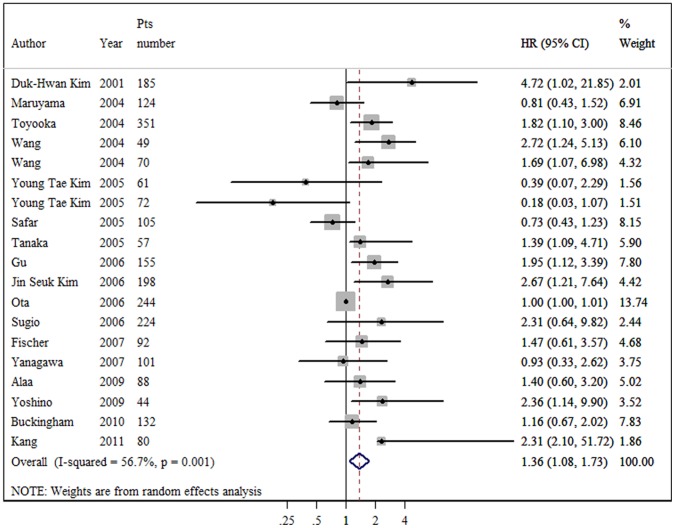
Overall survival: of the 17 evaluable studies for OS, the promoter hypermethylation of p16 was associated with a significant poor impact on NSCLC survival. However, the test for heterogeneity was highly significant (P = 0.001, I2 = 56.7%). Consequently, we applied a random-effect model in calculating the aggregated HR, which was 1.36 (95% CI: 1.08–1.73) (as Figure 1 shows). We also conducted subgroup analyses on sample type, detecting methods, histology, and tumor stage. When grouped according to the sample type of individual studies, the combined HRs of fresh frozen tissue and FFPE specimen were 1.50 (95% CI: 1.11–2.01) and 1.10 (95% CI: 0.77–1.57), respectively. When grouped according to the method of p16 methylation detection used, the combined HR was 1.40 (95% CI: 1.02–1.92) for MSP and 1.26 (95% CI: 0.87–1.82) for Q-MSP. Stratified by histology, the combined HR of the 16 studies reporting NSCLC as a whole indicated that patients with p16 hypermethylation had a risk of death 1.41 times greater than patients without p16 hypermethylation (HR = 1.41, 95% CI: 1.10–1.79). However, no such statistical significant death risk when pooling the 6 trials of adenocarcinoma subtype (HR = 1.56, 95% CI: 0.84–2.88). We also analyzed the data according to tumor stage. For the studies focusing on stage I and I-III, the aggregation produced statistically significant HRs without significant between-study heterogeneity (2.53 and 1.61, respectively). No significant association was found in I-II and I-IV subgroups.
Figure 1. Forest plot showing the association between p16 methylation and overall survival of NSCLC.
The summary HR and 95% CIs were shown (random-effects model analysis).
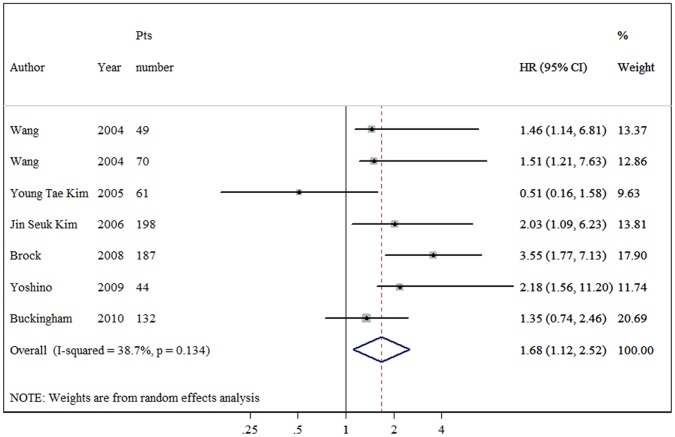
Disease-free survival: there were seven studies with 741 patients reported the association of p16 promoter hypermethylation and disease-free survival. NSCLC patients with p16 promoter hypermethylation were significantly correlated with shorter DFS, and the combined HR was 1.68 (95% CI: 1.12–2.52) without significant heterogeneity (p = 0.134, as figure 2 shows). In the stratified analyses, p16 methylation was significantly correlated with poor DFS according to MSP method, with a combined HR of 1.75 (95% CI: 1.07–2.87). When four trials using fresh tissue pooled, null association was observed (HR = 1.34, 95% CI: 0.92–1.96). The two studies with 358 cases using FFPE specimen were also combined, and the pooled HR was 2.86 (95% CI: 1.66–4.92).
Figure 2. Forest plot showing the association between p16 methylation and disease-free survival of NSCLC.
The summary HR and 95% CIs were shown (random-effects model analysis).
Sensitivity Analyses and Publication Bias
Sensitivity analyses were conducted by omitting one study per time in an attempt to check if individual study affected the final results of both OS and DFS. All the results were not materially altered. The visualization of the funnel plots for OS and DFS seemed asymmetrical. Egger’s test was used to provide statistical evidence for funnel plot symmetry. The results did not suggest significant evidence of publication bias: OS (Egger’s test, p = 0.05) and DFS (Egger’s test, P = 0.06).
Discussion
No consensus has been reached regarding whether p16 promote methylation is a prognostic marker in NSCLC patients, and it has attracted considerable attention. The present meta-analysis summarized for the first time all of the available researches on the impact of the promoter methylation of p16 on the survival of NSCLC. The overall pooled analyses of the associations between p16 methylation and NSCLC outcomes demonstrated negative impacts on patients with hypermethylation. As in the subgroup analyses for OS, the results suggested that p16 methylation was a poor prognostic indicator using MSP method, whereas those using Q-MSP method combined didn’t get a similar conclusion. Individual study selected various cutoff points of the ratio for p16 gene to dichotomize the patients into methylated and unmethylated groups might partly explain the disparity. When stratified analysis was conducted about different stages of NSCLC, the associations were also found in stages I to III, but not I-IV, with relatively larger sample pooled indicating that late-stage NSCLC patient confounded the observation of p16 methylation on survival. About test materials, we found that the association was significant by using fresh tissue, but not paraffin block. The reliability and reproducibility of the previously developed MSP method require high-quality DNA, often from a fresh frozen specimen. In most clinical settings, although paraffin specimens are available for easy transport and storage, it might cause false negative. One study reported that MSP can provide reliable gene methylation status on only two-thirds of formalin-fixed paraffin-embedded tumor specimens compared with fresh frozen tissues [43]. When histology type was taken into account, a dismal impact on survival was observed in NSCLC, but not in adenocarcinoma which was based on the data of 6 studies. As for DFS, cases with p16 promoter hypermethylation were significantly correlated with shorter DFS and no evidence of significant heterogeneity was found, suggesting a short recurrence. In subgroup analysis according to the different techniques used to detect p16 methylation, an adverse impact on DFS was observed with MSP method. Interestingly, such an association with DFS was found in the studies pooled using FFPE specimens, while fresh frozen tissues were not.
The search for a potential prognostic role of p16 methylation in NSCLC survival is based on its frequent hypermethylation in NSCLC and also on its potential interference with most pathways implicated in tumorigenesis. The role of p16 methylation in carcinogenesis has widely been investigated by in vitro experiments and by in vivo analyses based on animal models [44], [45], [46]. Hypermethylation of CpG islands located in the promoter regions of p16 is now firmly established as an important mechanism for gene inactivation, and the CpG island hypermethylation has been described in almost every tumor type [47]. The meta-analysis results suggested that p16 methylation status could be used as a stratified factor for NSCLC patient survival though with slight significant associations. Increasing data suggests that DNA methylation measurements of the promoter regions of specific genes have the potential to supply additional or superior information to that available from the existing cancer markers. These early finding now requires validation, initially in retrospective studies but ultimately in large prospective clinical studies. In addition to clinical validation, assays for methylated genes must be robust, simple, standardized, evaluated in external quality assurance schemes and made available at affordable costs. Only then, can patients expect to benefit from measurement of these markers [48].
To interpret the results of the present meta-analysis, some limitations should be taken into account. First, one limitation is that 7 trials had to be excluded from the meta-analysis because they did not provide sufficient data for aggregation. Four out of seven trials detected that p16 methylation positive related to poor prognosis. However, of the other 3 trials with negative results, two of which carried out the research using relatively larger samples (over 200 patients) still found null association. Moreover, it is known that negative studies are less frequently published or, if they are, with less detailed results, making them less assessable. Although Egger’s test didn’t reach statistical significant, publication bias may influence the results and leads to positive association. Second, eleven out of 18 (61.1%) studies reported HR and corresponding 95%CIs. In order to reduce the missing data, we managed to contact the authors and estimate the outcome with the help of a spreadsheet provided by Tierney et al [23]. In spite of the seven outcomes calculated from Kaplan–Meier curve or log-rank test may have introduced some imprecision, we felt this was a risk worth taking in view of the alternative. This situation also highlights the importance of a uniform reporting of study results about survival outcomes. Third, our analysis was based on literatures and not individual patient data. The unavailability of individual patient data could not allow us to correct the potential confounding factors. So, the multivariate analysis cannot be performed, and it cannot be exactly known whether p16 methylation is a prognostic factor, independently of as known clinical factors, such as age, sex, differentiation, stages, and performance status. Therefore, the results must be interpreted cautiously, because literature-based meta-analysis provides more bias and is less reliable than the individual patient data based analysis [49]. Finally, moderately significant heterogeneity was found in this meta-analysis (I2 = 56.7%) for overall survival. Most of the included studies were retrospective and differed in their study designs, such as patient selection, chemotherapeutic protocol and follow-up period. Some trial included patients receiving surgery or radiotherapy in addition to the platinum-based chemotherapy, thus adding the heterogeneity between studies. So we performed the analysis using random-effects model which considers the between-study heterogeneity. Moreover, stratified analyses would be helpful to reduce the heterogeneity and provide additional useful information.
On the basis of the results of this analysis, we support the hypothesis that NSCLC patients with the promoter hypermethylation of p16 have moderately risk of recurrence and death in all populations considered. Whether p16 methylation testing could move toward routine clinical application as a prognostic tool in NSCLC, it depends on further study validation. Future studies should have a more strict design, with large sample sizes to increase statistical power, a uniform way of analyzing survival outcomes, and a long and specified follow-up period.
Funding Statement
The authors have no support or funding to report.
References
- 1. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Sapari NS, Loh M, Vaithilingam A, Soong R (2012) Clinical potential of DNA methylation in gastric cancer: a meta-analysis. PLoS One 7: e36275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang B, Zhu W, Yang P, Liu T, Jiang M, et al. (2011) Cigarette smoking and p16INK4alpha gene promoter hypermethylation in non-small cell lung carcinoma patients: a meta-analysis. PLoS One 6: e28882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu F, Zhang HT (2011) DNA methylation and nonsmall cell lung cancer. Anat Rec (Hoboken) 294: 1787–1795. [DOI] [PubMed] [Google Scholar]
- 5. Serrano M, Hannon GJ, Beach D (1993) A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366: 704–707. [DOI] [PubMed] [Google Scholar]
- 6. Voorhoeve PM, Agami R (2003) The tumor-suppressive functions of the human INK4A locus. Cancer Cell 4: 311–319. [DOI] [PubMed] [Google Scholar]
- 7. Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, et al. (1995) 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med 1: 686–692. [DOI] [PubMed] [Google Scholar]
- 8. Esteller M (2008) Epigenetics in cancer. N Engl J Med 358: 1148–1159. [DOI] [PubMed] [Google Scholar]
- 9. Kang HJ, Kim EJ, Lee KM, Roh MS, Kwak JY, et al. (2012) Quantitative analysis of multiple gene promoter methylation in Korean non-small cell lung cancer patients and its association study with cancer risk factor and survival. Molecular and Cellular Toxicology 8: 25–34. [Google Scholar]
- 10. Sterlacci W, Tzankov A, Veits L, Zelger B, Bihl MP, et al. (2011) A comprehensive analysis of p16 expression, gene status, and promoter hypermethylation in surgically resected non-small cell lung carcinomas. Journal of Thoracic Oncology 6: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 11. Sasaki H, Hikosaka Y, Kawan O, Moiyama S, Yan M, et al. (2010) Methylation of the DLEC1 gene correlates with poor prognosis in Japanese lung cancer patients. Oncology Letters 1: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buckingham L, Penfield Faber L, Kim A, Liptay M, Barger C, et al. (2010) PTEN, RASSF1 and DAPK site-specific hypermethylation and outcome in surgically treated stage I and II nonsmall cell lung cancer patients. Int J Cancer 126: 1630–1639. [DOI] [PubMed] [Google Scholar]
- 13. Yoshino M, Suzuki M, Tian L, Moriya Y, Hoshino H, et al. (2009) Promoter hypermethylation of the p16 and Wif-1 genes as an independent prognostic marker in stage IA non-small cell lung cancers. International Journal of Oncology 35: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 14. Fischer JR, Ohnmacht U, Rieger N, Zemaitis M, Stoffregen C, et al. (2007) Prognostic significance of RASSF1A promoter methylation on survival of non-small cell lung cancer patients treated with gemcitabine. Lung Cancer 56: 115–123. [DOI] [PubMed] [Google Scholar]
- 15. Ota N, Kawakami K, Okuda T, Takehara A, Hiranuma C, et al. (2006) Prognostic significance of p16INK4a hypermethylation in non-small cell lung cancer is evident by quantitative DNA methylation analysis. Anticancer Research 26: 3729–3732. [PubMed] [Google Scholar]
- 16. Kim YT, Park SJ, Lee SH, Kang HJ, Hahn S, et al. (2005) Prognostic implication of aberrant promoter hypermethylation of CpG islands in adenocarcinoma of the lung. J Thorac Cardiovasc Surg 130: 1378. [DOI] [PubMed] [Google Scholar]
- 17. Divine KK, Pulling LC, Marron-Terada PG, Liechty KC, Kang T, et al. (2005) Multiplicity of abnormal promoter methylation in lung adenocarcinomas from smokers and never smokers. International Journal of Cancer 114: 400–405. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Lee JJ, Wang L, Liu DD, Lu C, et al. (2004) Value of p16INK4a and RASSF1A promoter hypermethylation in prognosis of patients with resectable non-small cell lung cancer. Clinical Cancer Research 10: 6119–6125. [DOI] [PubMed] [Google Scholar]
- 19. Maruyama R, Sugio K, Yoshino I, Maehara Y, Gazdar AF (2004) Hypermethylation of FHIT as a Prognostic Marker in Nonsmall Cell Lung Carcinoma. Cancer 100: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 20. Harden SV, Tokumaru Y, Westra WH, Goodman S, Ahrendt SA, et al. (2003) Gene promoter hypermethylation in tumors and lymph nodes of stage I lung cancer patients. Clinical Cancer Research 9: 1370–1375. [PubMed] [Google Scholar]
- 21. Zochbauer-Muller S, Fong KM, Virmani AK, Geradts J, Gazdar AF, et al. (2001) Aberrant promoter methylation of multiple genes in non-small cell lung cancers. Cancer Res 61: 249–255. [PubMed] [Google Scholar]
- 22. Kim DH, Nelson HH, Wiencke JK, Zheng S, Christiani DC, et al. (2001) p16INK4a and histology-specific methylation of CpG islands by exposure to tobacco smoke in non-small cell lung cancer. Cancer Research 61: 3419–3424. [PubMed] [Google Scholar]
- 23. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 25. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 26. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 27. Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127: 820–826. [DOI] [PubMed] [Google Scholar]
- 28. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Bmj 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toyooka S, Suzuki M, Maruyama R, Toyooka KO, Tsukuda K, et al. (2004) The relationship between aberrant methylation and survival in non-small-cell lung cancers. British Journal of Cancer 91: 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim YT, Lee SH, Sung SW, Kim JH (2005) Can aberrant promoter hypermethylation of CpG islands predict the clinical outcome of non-small cell lung cancer after curative resection? Annals of Thoracic Surgery 79: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 31. Safar AM, Spencer H, 3rd, Su X, Coffey M, Cooney CA, et al (2005) Methylation profiling of archived non-small cell lung cancer: a promising prognostic system. Clin Cancer Res 11: 4400–4405. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka R, Wang D, Morishita Y, Inadome Y, Minami Y, et al. (2005) Loss of function of p16 gene and prognosis of pulmonary adenocarcinoma. Cancer 103: 608–615. [DOI] [PubMed] [Google Scholar]
- 33. Gu J, Berman D, Lu C, Wistuba II, Roth JA, et al. (2006) Aberrant promoter methylation profile and association with survival in patients with non-small cell lung cancer. Clinical Cancer Research 12: 7329–7338. [DOI] [PubMed] [Google Scholar]
- 34. Sugio K (2006) The methylation status and protein expression of CDH1, p16INK4A, and fragile histidine triad in nonsmall cell lung carcinoma: Epigenetic silencing, clinical features, and prognostic significance. Cancer 106: 2190–2199. [DOI] [PubMed] [Google Scholar]
- 35. Yanagawa N, Tamura G, Oizumi H, Kanauchi N, Endoh M, et al. (2007) Promoter hypermethylation of RASSF1A and RUNX3 genes as an independent prognostic prediction marker in surgically resected non-small cell lung cancers. Lung Cancer 58: 131–138. [DOI] [PubMed] [Google Scholar]
- 36. Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, et al. (2008) DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med 358: 1118–1128. [DOI] [PubMed] [Google Scholar]
- 37. Alaa M, Suzuki M, Yoshino M, Tian L, Suzuki H, et al. (2009) Prostaglandin E2 receptor 2 overexpression in squamous cell carcinoma of the lung correlates with p16INK4A methylation and an unfavorable prognosis. International Journal of Oncology 34: 805–812. [DOI] [PubMed] [Google Scholar]
- 38. Kim JS, Kim JW, Han J, Shim YM, Park J, et al. (2006) Cohypermethylation of p16 and FHIT promoters as a prognostic factor of recurrence in surgically resected stage I non-small cell lung cancer. Cancer Res 66: 4049–4054. [DOI] [PubMed] [Google Scholar]
- 39. Bradly DP, Buckingham L, Coon J, Liptay M, Bonomi P, et al. (2010) Age-related CDKN2A (p16) promoter methylation and outcome in surgically treated stage I-II non-small cell lung cancer patients. Laboratory Investigation 90: 399A. [Google Scholar]
- 40. Shimamoto T, Ohyashiki JH, Hirano T, Kato H, Ohyashiki K (2004) Hypermethylation of E-cadherin gene is frequent and independent of p16INK4A methylation in non-small cell lung cancer: potential prognostic implication. Oncol Rep 12: 389–395. [PubMed] [Google Scholar]
- 41. Fu J, Zhang J, Zhang HW (2003) Abnormality of p16 gene and its clinicopathological significance in non-small cell lung cancer. Zhonghua bing li xue za zhi Chinese journal of pathology 32: 133–136. [PubMed] [Google Scholar]
- 42. Niklinska W, Chyczewski L, Laudanski J, Chyczewska E, Niklinski J (2001) Detection of p16 abnormalities in early-stage non-small cell lung cancer. Folia Histochemica et Cytobiologica 39: 30–32. [PubMed] [Google Scholar]
- 43. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, et al. (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352: 997–1003. [DOI] [PubMed] [Google Scholar]
- 44. Kato A, Shimizu K, Shimoichi Y, Fujii H, Honoki K, et al. (2006) Aberrant DNA methylation of E-cadherin and p16 genes in rat lung adenocarcinomas induced by N-nitrosobis(2-hydroxypropyl)amine. Mol Carcinog 45: 106–111. [DOI] [PubMed] [Google Scholar]
- 45. Honoki K, Tsujiuchi T, Mori T, Yoshitani K, Tsutsumi M, et al. (2004) Expression of the p16INK4a gene and methylation pattern of CpG sites in the promoter region in rat tumor cell lines. Mol Carcinog 39: 10–14. [DOI] [PubMed] [Google Scholar]
- 46. Patel AC, Anna CH, Foley JF, Stockton PS, Tyson FL, et al. (2000) Hypermethylation of the p16 (Ink4a) promoter in B6C3F1 mouse primary lung adenocarcinomas and mouse lung cell lines. Carcinogenesis 21: 1691–1700. [DOI] [PubMed] [Google Scholar]
- 47. Esteller M (2002) CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21: 5427–5440. [DOI] [PubMed] [Google Scholar]
- 48. Duffy MJ, Napieralski R, Martens JW, Span PN, Spyratos F, et al. (2009) Methylated genes as new cancer biomarkers. Eur J Cancer 45: 335–346. [DOI] [PubMed] [Google Scholar]
- 49. Stewart LA, Parmar MK (1993) Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 341: 418–422. [DOI] [PubMed] [Google Scholar]