Abstract
The brain-gut axis allows bidirectional communication between the central nervous system (CNS) and the enteric nervous system (ENS), linking emotional and cognitive centers of the brain with peripheral intestinal functions. Recent experimental work suggests that the gut microbiota have an impact on the brain-gut axis. A group of experts convened by the International Scientific Association for Probiotics and Prebiotics (ISAPP) discussed the role of gut bacteria on brain functions and the implications for probiotic and prebiotic science. The experts reviewed and discussed current available data on the role of gut microbiota on epithelial cell function, gastrointestinal motility, visceral sensitivity, perception and behavior. Data, mostly gathered from animal studies, suggest interactions of gut microbiota not only with the enteric nervous system but also with the central nervous system via neural, neuroendocrine, neuroimmune and humoral links. Microbial colonization impacts mammalian brain development in early life and subsequent adult behavior. These findings provide novel insights for improved understanding of the potential role of gut microbial communities on psychological disorders, most particularly in the field of psychological comorbidities associated with functional bowel disorders like irritable bowel syndrome (IBS) and should present new opportunity for interventions with pro- and prebiotics.
Keywords: probiotic, prebiotic, neurogastroenterology, immune, human trials, biomarkers, gastrointestinal tract, microbiota, microbiome
Introduction
Neurogastroenterology is a research area in the field of gastroenterology which regards the interactions of the central nervous system (CNS) (brain) and the gut—the so-called “brain-gut axis”. The brain-gut axis is a bidirectional communication system and includes the CNS, composed of the brain and spinal cord, and the enteric nervous system (ENS), involving nerves, hormones and other molecules such as neuropeptides and cytokines.1 The roles of the brain-gut axis are to monitor and integrate gut function and to mediate environmental effects such as hunger, stress and emotions on gut functions. Stress and emotions may be reflected by changes in gut physiology and gut symptoms.
Microbes in the gastrointestinal (GI) tract are represented by a wide variety of bacterial species. They can exert numerous effects on the intestinal neuroimmune system and influence a variety of host functions such as metabolic activity, immune response and physiological function.2 The gut microbiota (whose genes represent the intestinal microbiome) composition and activity is subject to a variety of influence including host physiology, immunology, diet, antibiotic usage and enteric infection. Microbial dysbiosis is associated with gastrointestinal and metabolic disorders. A growing body of evidence suggests that the host-microbial interaction may result in dysregulated neuroimmune functions, impacting behavior.3,4 Probiotic bacteria, “live microorganisms which when administered in adequate amounts confer a health benefit on the host,” and dietary prebiotics, “selectively fermented dietary ingredients that result specific changes, in the consumption and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health” (www.isapp.net), have been used in animal studies and human clinical trials to improve peripheral (gastrointestinal) and central (psychological) symptoms.
This review highlights the reviewed research and discussions by the expert group on “Probiotics and prebiotics in neurogastroenterology” at the 2011 annual meeting of the International Scientific Association for Probiotics and Prebiotics (ISAPP). Some of the main questions that the experts addressed were: What are the effects of the gut microbiota on the brain-gut axis? What is the impact of the brain-gut axis on the gut microbiota? What is the relevance of data obtained from in vitro, animal studies and clinical studies for human health?
The experts summarized the evidence related to the role of the interactions between the gut microbiota and the host’s brain-gut axis communications. Data were gathered from recent observations in animal studies (summarized in Table 1) and in human studies involving patients with functional bowel disorders and associated psychological comorbidities. The experts also reviewed recent evidence of the impact of the neuroimmune system on the composition of the microbiota (Fig. 1). Finally, recommendations are provided for future research on this topic as well as for advancing the translation of the data of the effects of probiotics and prebiotics observed in vitro or in animal models to clinical interventions in the area of functional bowel disorders.
Table 1. Summary of studies performed in animal models evaluating the effect of the gut microbiota and/or changes induced by probiotic and prebiotic interventions.
| Animal model | Test | GI microbiota | Effects | Notes | Effect | Reference |
|---|---|---|---|---|---|---|
| NMRI mice |
EPM Dark/light box |
GF vs SPF |
GF mice have increased motor activity and reduced anxiety compared with SPF with a normal microbiota. GF mice exposed to gut microbiota early in life display similar characteristics as SPF mice |
Commensal microbiota can affect the postnatal development of the HPA stress response in mice. |
CNS |
28 |
| Balb/c male mice |
Restraint stress |
GF vs SPF vs gnotobiotic mice (with B. infantis; with enteropathogenic E. coli with/without intimin receptor gene) |
GF male mice have a decrease in brain derived neurotrophic factor (BDNF)- a key neurotrophin involved in neuronal growth and survival -compared with SPF mice. They also have decreased expression of the glutamate NMDA receptor subunit 2a in the cortex and hippocampus. Effect was reversed in gnotobiotic animals colonized with B. infantis, but not with E. coli |
Measured by polymerase chain reaction and Western Blotting. Commensal microbiota can affect the postnatal development of the HPA stress response in mice. |
CNS |
29 |
| Swiss Webster female mice |
EPM |
GF vs SPF mice |
GF mice have a more pronounced anxiolytic behavior and increased expression of BDNF mRNA in the dentate gyrus of the hippocampus compared with SPF mice. They have reduced serotonin 1A receptor mRNA expression in the dentate gyrus of the hippocampus |
Measured by in situ hybridization |
CNS |
27 |
| Recombinase activating gene (RAG) Ko mice |
EPM |
|
Reduced inflammation is linked to reduced anxiety |
|
Brain-gut communication |
30 |
| Male Balb/c mice or AKH mice infected with T. muris. |
Light/dark test |
L. rhamnosus NCC4007 and B. longum NCC3001 |
Infection with T. muris induced anxiety like behavior and decreased level of BNDF. Treatment with B. longum reverses the effect and normalizes BDNF level. |
Vagatomy did not have an effect on the probiotic treatment |
|
86 |
| Male Wistar rats (gnotobiotic model) |
Conditioned defensive burying test |
L. helveticus R0052 + B. longum RO07 Vs. placebo vs. diazepam |
Probiotics have anxiolytic effect compared with placebo |
Same probiotics were also administered in 75 healthy volunteers with stress symptoms in another study |
|
35 |
| Sprawley rats |
Colorectal distension,tail flick and paw pressure tests |
L. reuteri ATCC 23272 |
Live, killed probiotic bacteria or conditioned medium inhibited the constitutive cardioautonomic response to colorectal distension in rats through effects on enteric nerves. |
|
|
16 |
| Balb/c mice |
EPM Swim test |
L. rhamnosus (JBI) |
Increase of corticosterone Increase of GABA |
Alter mRNA expression of GABA receptor Vagotomy prevented these effects |
ENS/CNS |
31 |
| Balb/c mice and NIH mice |
|
SPF + antibiotic vs. GF mice |
Antibiotic decreased anxiety behaviors, Interspecies gut microbiota transplantation changed species specific associated phenotype |
|
|
87 |
| CF1 mice |
|
Citrobacter rodentidum infection |
Increase anxiety like in pathogen infected mice |
|
CNS |
36 |
| C57BL/6 mice |
|
Citrobacter rodentidum infection |
Increase memory dysfunction in pathogen infected mice |
|
CNS |
37 |
| Maternal deprivation in offspring’s rats |
|
L. paracasei NCC 2461 |
Probiotic reverse rectal hyperalgesia |
Animal model for IBS |
CNS and brain-gut axis |
21 |
| Maternal deprivation in male offspring’s rats |
|
|
Plasma PUFA different in maternally separated animal vs controls |
Animal model for IBS |
CNS and brain-gut axis |
88 |
| Maternal deprivation in male offspring’s rats |
|
Lipopolysaccharide challenge |
Plasma corticosterone increase in maternally separated animals. Increase in immune response after LPS challenge Alteration in fecal microbiota vs control group |
Animal model for IBS |
CNS and brain-gut axis |
89 |
| Maternal deprivation in offspring’s rats | Swim test | B. infantis | Probiotic treatment resulted in normalization of the immune response, reversal of behavioral deficits peripheral interleukin (IL)-6 release and amygdala corticotrophin-releasing factor mRNA level) and restoration of basal noradrenaline concentrations in the brainstem | Animal model for IBS | CNS and brain-gut axis | 24 |
Abbreviations: ANS, autonomous nervous system; CNS, central nervous system; ENS, enteric nervous system; EPM, Elevated plus maze; GF, germ-free; HPA, hypothalamic-pituitary-adrenal; IBS, irritable bowel syndrome; SPF, specific pathogen free.
Figure 1. Interaction of the gut microbiome, probiotics and prebiotics on the brain gut axis. Modified from reference 85.
Effects of the Intestinal Microbiome on the Brain-Gut Axis
Effects of the intestinal microbiome and probiotics on the ENS
The ENS, often referred to as “the second brain”, is a part of the peripheral nervous system and a division of the autonomic nervous system which controls the function of the gastrointestinal tract. The ENS is embedded within the wall of the digestive tract and extends between the esophagus and anus. It contains thousands of ganglia and approximately 400 million neurons, more than any other peripheral organ and about the same number of neurons as the spinal cord. This system is capable of autonomous function, controlling the digestive system local physiological state. The ENS is involved in several functions including: control of gastrointestinal motility, sensation, regulation of fluid exchange, local blood flow, gastric and pancreatic secretion, gastrointestinal endocrine functions, defense reactions and entero-enteric reflexes.
Embedded within the wall of the GI tract, the ENS is “protected” from the luminal content by the intestinal barrier. The intestinal barrier function can be viewed in different ways. On one level barrier function is the prevention of diffusion of ions and small solutes across the single layer of epithelial cells that protects underlying compartments, including the ENS, from the noxious contents of the lumen. Tight junctions provide this level of protection as a result of the many transmembrane proteins including occludins, various claudins, junctional adhesion molecules (JAMs) and cingulin. Homotypic and/or heterotypic interactions between these proteins as well as charge differences protect movement across the paracellular space.5 Much of the inflammation-associated increase in intestinal permeability is now recognized to be driven by the impact of proinflammatory cytokines, including tumor necrosis factor-α (TNF), on signaling pathways, specifically myosin light chain kinase (MLCK), that govern tight junction permeability.5 In this case, MLCK phosphorylates myosin light chain, which interacts with actin, driving cytoskeletal contraction of the perijunctional actomyosin ring that underlies tight junctions thus, increasing paracellular permeability. On a more inclusive level, other factors contributing to intestinal barrier function including mucus layers, glycocalyx, secretory IgA, antimicrobial peptides, chloride and water secretion and the intestinal microbiota.
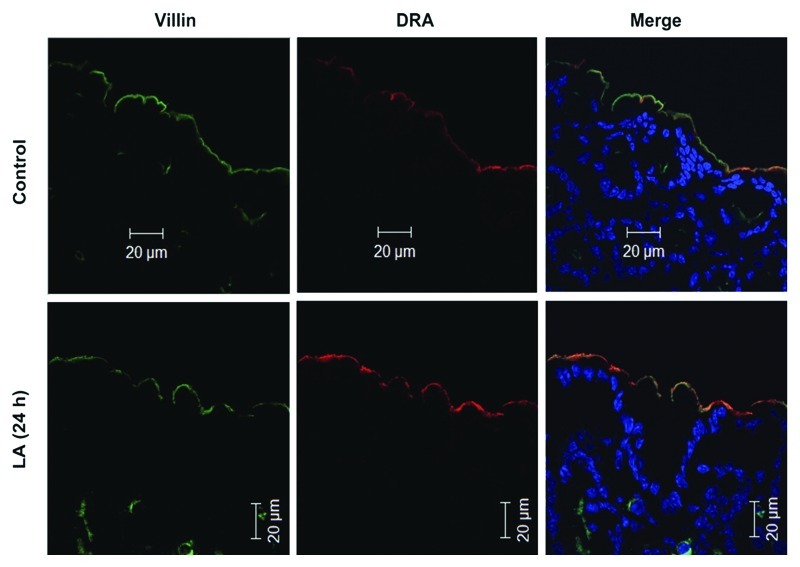
The gut microbiota6 and certain probiotics7 can promote the development of the intestinal barrier. Some specific probiotic strains altered the expression of tight junction proteins including occludin, cingulin, claudin-2 and ZO-2.8,9 Evidence also suggests that commensal and probiotic organisms bolstered additional aspects that contribute to intestinal barrier function6,8,10 including increased IgA production, mucin expression, prevention of intestinal epithelial cell apoptosis,11 inhibition of colonization by enteric pathogens and the immune response. Specific Lactobacillus strains upregulated the expression of key intestinal epithelial transporters such as the apical anion exchanger SLC26A3 (DRA) responsible for the bulk of Cl- absorption by the gut12,13 (Fig. 2) suggesting a mechanism for therapeutic benefit in the treatment of diarrhea. Treatment with Saccharomyces boulardii reduced calbindin 28k myenteric neurones in pig’s jejunum receiving S. boulardii. (S. cerevisiae HANSEN CBS 5926).14
Figure 2. In vivo effect of Lactobacillus acidophilus (LA) on the major apical Cl−/OH- exchanger (DRA) expression.12 Immunofluorescent localization of DRA (red) and villin (green) in the colonic epithelium of control mice and 24h-LA treated mice. Control colon shows a modest expression of DRA on the epithelial surface whereas LA-treated colon shows enhanced expression of the DRA as visualized from the increased intensity of the yellow color in the merge picture. Published with permission from Am J Physiol.
In addition to their effect on the intestinal barrier, pathogens or probiotics can have an impact on the ENS. Microbes or their byproducts directly target intestinal sensory nerves. Cholera toxin releases 5-HT from the mucosa that acts on 5-HT3 receptors on sensory nerves.15 Conversely, certain probiotics can have opposite effects and reduced pain induced by colorectal distension in rats.16 L. acidophilus NCFM induced analgesic effect by increasing expression of intestinal mucosa opioid and cannabinoid receptors in rats.17 A variety of other probiotics, (i.e., L. farciminis), prevented stress-induced hypersensitivity18 or reduced the excitability of enteric neurons in models of mice colitis or of gut dysfunction.19,20 L. paracasei NCC 2461 reversed rectal hyperalgesia in a model of maternal deprivation in rats.21 Interestingly, these effects have been observed not only with live strains, but also with dead (heat-killed) or even conditioned probiotic medium.22
Effects of the intestinal microbiome and probiotics on brain-gut communications
The autonomic nervous system (ANS) consists of sensory and motor neurons that run between the CNS and different internal organs, including the GI tract. Its actions are largely involuntary. The ANS is divided into the sympathetic and parasympathetic system.23 The neurotransmitters of the pre- and post-ganglionic neurons are acetylcholine and noradrenaline [or norepinephrine (NE)] respectively. Activation of the sympathetic branch of the autonomic nervous system helps prepare and adjust the body for emergencies and acute change in homeostasis including the inhibition of gut peristalsis. The parasympathetic system returns the body functions to normal after they have been altered by sympathetic stimulation. Additionally, communication between the immune system (i.e., cytokines) and the nervous system cytokines play important roles in brain-gut communications.
Effect of probiotics on various mediators of the brain-gut axis have been demonstrated with several probiotics: Bifidobacterium infantis decreased pro-inflammatory cytokines in maternally deprived offspring rats, an animal model that has been used as a proxy for IBS.24 The same strain increased polyunsaturated fatty acids (PUFAs) and exhibited anti-inflammatory effects when co-administered with α-linoleic compared with α-linoleic supplementation alone.25,26 It is believed that the microbiota, as well as pro- and prebiotics, can have a significant impact on the gut-brain axis, but further research in this area is needed to reveal the magnitude, mechanisms and clinical relevance of these effects.
Effects of the intestinal microbiome and probiotics on the CNS
The CNS consists of the brain and the spinal cord, with more than four hundred million neurons. Behavior is an excellent read-out of CNS function. Studies have been conducted with a view to establishing a behavioral phenotype and neurochemical profile that might be associated with the gut microbiota. To date, the most consistent data have been in relation to indices of anxiety. Neufeld and colleagues reported a less anxious phenotype for germ-free (GF) animals in the elevated plus maze test.27 This behavioral phenotype was replicated in another study with germ-free animals in both the elevated plus maze test and the light dark box and was associated with an altered gene expression profile in relevant brain regions.28 Changes in CNS gene expression of plasticity-related genes have been reported; however the findings have been inconsistent from different laboratories, perhaps due to strain and sex differences in the animals. A decrease in brain derived neurotrophic factor (BDNF), a key neurotrophin involved in neuronal growth and survival and expression of the glutamate NMDA receptor subunit 2a (NR2a) were measured in the cortex and hippocampus of male GF Balb/C mice compared with SPF controls.29 More recently, Neufeld et al.27 demonstrated an increased expression of BDNF mRNA in the dentate gyrus of the hippocampus and reduced serotonin 1A receptor mRNA expression in the dentate gyrus of the hippocampus in female GF Swiss Webster mice,27 a molecular signature that is consistent with the observed reduced anxiety-like behavior in GF mice.27,28 If anxiety-like behavior in GF mice is considered in the context of inflammation, reduced inflammation leads to reduced anxiety-like behavior. Immunocompromised mice had reduced anxiety-like behavior, as observed in the recombinase activating gene (RAG-1) knockout model. Increased exploration in the open field and decreased open arm avoidance in the elevated plus maze test are characteristic of these mice.30 In contrast, increased inflammation leads to increased anxiety-like behavior in other animal models.19
Probiotic treatment can also alter brain neurochemistry. Chronic treatment with L. rhamnosus (JB-1) induced region-dependent alterations in GABAB1b mRNA in the brain with increases in cortical regions and concomitant reductions in expression in the hippocampus, amygdala and locus coeruleus, in comparison with control-fed mice.31 This strain reduced stress-induced corticosterone anxiety and depression related behavior. These changes were not observed in mice that had underwent vagotomy suggesting that the microbiota-brain changes observed were mediated by the vagus nerve.
Effects of the Central and Peripheral Nervous Systems on the Intestinal Microbiome
Although several studies in animals have demonstrated an effect of the brain on microbiota composition, investigators have focused primarily on changes for specific bacterial groups (i.e., lactobacilli, bifidobacteria and pathogens) observed in stressful conditions. Stressful conditions are known to impact gastrointestinal motility, permeability, immune function and release of specific hormones and mediators in the GI tract.
Stress hormones, such as NE, stimulate the growth of specific pathogens; however the underlying mechanisms remain poorly understood. Studies with Escherichia coli suggest that NE acts by inducing de novo synthesis of various bacterial virulence factors or that cognate bacterial receptors utilize NE as a growth-activating ligand.32 Besides NE, other neurotransmitters (GABA, catecholamines, serotonin and acetylcholine) and hormones normally associated with the nervous and endocrine systems, could also play this role. An impact of stress on microbial colonization has been observed in monkeys exposed to stress in early life.33 The biochemical elements used by the nervous, endocrine and other systems for intercellular communication in vertebrates may originate from unicellular organisms and be conserved.32 A high affinity GABA system in Pseudomonas fluorescences with binding properties similar to those of a brain receptor has been reported.34
Conversely, stress response can be modulated by gut microbes. Microbes play a critical role in the development of an appropriate stress response later in life. A pivotal window in early life where gut microbial colonization takes place must occur to ensure normal development of the core stress axis, the hypothalamic-pituitary-adrenal (HPA) axis. Gut microbes also are able to modulate exaggerated stress responses: In GF mice, a mild restrained stress induced an amplified release of corticosterone and adrenocorticotropin hormone (ACTH) compared with specific pathogen-free (SPF) controls. This response was reversed by recolonization with fecal matter from SPF animals and by monoassociation with B. infantis in a time dependent manner. Other probiotic administration studies also support a role for the microbiota in alleviating anxiety-like behaviors. Administration of L. helveticus R0052 and B. longum R0175 taken in combination reduced anxiolytic-like activity in rats.35 A strategy employing antibiotic induced dysbiosis of the microbiome reduced anxiety-like behaviors measured by the step down box and the light/dark box tests. Altered protein levels of BDNF in the amygdala and hippocampus and discontinuation of an antibiotic cocktail restored the normal behavioral profile of the animals.19 Similarly, perturbation of the microbiota by means of an infectious agent like Citrobacter rodentium increased anxiety like behavior in mice 7–8 h post infection36 and produced stress induced memory dysfunction 10 and 30 d post infection.37 Memory dysfunction was prevented by daily administration of a probiotic cocktail.37 A role for the gut microbiota in pain perception has also been proposed.38
The Association Between Intestinal Microbiome and the Brain-Gut Axis in Humans
Effects on human behavior
Little information is available regarding the role of the intestinal microbiome on human behavior. Only a few studies have investigated the anxiolytic effects of probiotics as primary clinical endpoints in clinical trials. In one study, positive effects of the probiotics L. helveticus R0052 and B. longum R0175 in an animal model were confirmed in adult human volunteers in a randomized double blind trial.39 The active treatment reduced psychological distress, measured by the Hopkins Symptom Checklist and the Hospital Anxiety and Depression Scale. A reduction of urinary cortisol was concomitantly observed.
An emerging area of interest is the role of the microbiota in autism spectrum disorders (ASD). ASD are a group of neurodevelopmental disorders including autism, Asperger's Disorder, Rett's Disorder, childhood disintegrative disorder, pervasive developmental disorder, not otherwise specified.40 GI disturbances are prevalent in children with autism and the number of GI symptoms is shown to be associated with the severity of autism.41 Several studies have now reported changes in microbiota profile in patients with autism.41-47 While this area of research is new and consensus across studies has not yet been established, several interesting observations are worth noting. In one recent study, an increased diversity of microbiota was observed in patients with severe autism compared with matched controls; and in particular Bacteroidetes was significantly increased in patients with autism, whereas Firmicutes was observed to be higher in controls.47 In contrast, another study that compared patients with autism and GI problems (ASD+GI) to patients with GI problems alone, showed reduced Bacteroidetes and increased Firmicutes and Betaproteobacteria (Sutturela) presence in ASD+GI patients.43 It also has been speculated that secretion of bacterial (neuro) toxins produced by clostridia may affect behavior and stress in autistic subjects.45 Furthermore, urinary metabolite phenotypes—mostly derived from gut bacterial fermentation—differentiated children with autism from their unaffected siblings.48 Clearly, more research is needed to clarify the gut microbial dysbiosis and determine the relationship between microbiota and autism.
Studies considering possible mechanisms for gut-brain communication in autism suggest that an altered metabolic phenotype in association with gut dysbiosis may contribute to ASD.43,49 Interestingly, short-term treatment with antibiotics has been reported to improve behavioral symptoms in some patients with autism.50 Probiotics and novel therapies targeted at gut dysbiosis show promise for treatment of ASD, however, more studies are needed to better define these novel therapeutic strategies.51,52
Effects in functional gastrointestinal disorders
Functional gastrointestinal disorders (FGIDs) are defined as chronic or recurrent gastrointestinal symptoms not explained by structural or biochemical abnormalities. Multinational working teams known as the Rome Committees have defined and categorized FGIDs based on clusters of clinical symptoms and suggested symptom based criteria for their diagnosis in children and adults.53 It has been proposed that in some cases, FGIDs involve a continuum starting in infancy and continuing into adolescence and adulthood. A long-term follow up study has demonstrated that 25% of children who have FGIDs have symptoms as adults.54 FGIDs are heterogeneous disorders with pathophysiologic mechanisms that require better clarification. They are currently understood as an integration of multiple factors (genetic, physiological, psychological, microbial, environmental and dietary) that contribute to the overall clinical presentation and outcome.55 To illustrate this complexity, up to 60 potential genes thought to play a role in FGIDs have been identified in the aminergic pathway (serotonin, noradrenaline) and in alterations of intestinal immunity and barrier function.56
Many reports associate intestinal dysbiosis and a common FGID, IBS, although a cause and effect relationship has not been demonstrated.57,58 Next generation sequencing technologies recently have revealed in more detail gut dysbiosis in different IBS cohorts in both children, teenagers and adults. In one study performed in the pediatric population,59 the IBS microbiome was characterized by greater abundance of the family Gammaproteobacteria, a family that includes several potential pathogenic bacteria. Hemophilus parainfluenza was found to be present in higher abundance in IBS patients. Additionally, bacterial genera that have not been previously linked with IBS, such as Dorea, were found to be associated with the disease in IBS adult subjects as well.60 Recent studies of the microbiota in adults with different IBS subtypes have also confirmed specific gut bacterial dysbiosis, with a decrease in bifidobacteria in IBS-D patients and increase in Gammaproteobacteria.61-63 Furthermore, several species have now been correlated with a lower or higher severity as well as frequency of pain in children (Fig. 3) and warrant further investigation.59 Although findings of compositional changes at the species levels are intriguing, individual species and the metabolic function of the gut microbiota may be more important, as most species of a genus may not contribute to disease.
Figure 3. Different distribution of bacterial taxa in children with irritable bowel syndrome was correlated with the relative frequency of abdominal pain.59 Bacterial taxa (specified in leftmost column) were defined by randomForest and confirmed by feature selection using Boruta. The list is sorted first by Mann-Whitney U score followed by the largest disparity in medians for each group. Taxa represent the lowest taxonomic depth (Genus) that is labeled by the Ribosomal Database Project Classifier. Red rectangles display the HM (high-medium) abdominal pain phenotype. Light blue rectangles display the L0 LO (low-none) abdominal pain phenotype. Boxes represent the first quartile, median and third quartile of the OTU distributions for each pain group. Empty circles represent outliers that are 1.5 greater than the respective interquartile ranges. (A) OTUs with greater abundance in patients with HM vs L0 abdominal pain phenotypes. (B) OTUs with reduced abundance in patients with HM vs L0 abdominal pain phenotypes. Published with permission from Gastroenterology.
The role of gut microbiota in IBS also is supported by the positive effect of the manipulation of gut microbiota on IBS symptoms with probiotics,64 prebiotics65 and antibiotics.66 More than 40 probiotic intervention studies performed in IBS patients have been published and recently reviewed.67-70 In most studies, a strong placebo effect was observed, underscoring the important role that subjective psychological factors play in the response to treatment in these patients. Nevertheless, many of these studies have demonstrated efficacy in improving functional GI symptoms in IBS patients.
Gut barrier function is vital in maintaining gut health, but this barrier is impaired in IBS. 15–50% of patients with IBS, depending on the cause and the particular subtype, have increased intestinal permeability as measured by sugar permeability tests and by changes in the expression of tight junction proteins.71 While in vitro work to identify important candidate effector molecules continues, the effects on the enteric nervous system in human intervention studies with probiotics are sparse. A short-term improvement in mucosal barrier function measured by a triple sugar test in patients with IBS was observed after administration of a probiotic fermented milk (Streptococcus thermophilus, Lactobacillus bulgaricus, Lactobacillus acidophilus and Bifidobacterium longum) compared with a milk beverage containing no bacteria.72 As noted in other studies with prebiotics and probiotics, effects on gut barrier function varied among strains and effector molecules.73-75 While intestinal permeability may be altered in a subset of IBS patients, the relationship with dysbiosis requires further study. Commensal and probiotic organisms can regulate intestinal barrier function through a variety of mechanisms and hence contribute to the development or perpetuation of IBS. However, such a clear causative link has not been yet demonstrated.
Prebiotics are “selectively fermented food ingredients that allow specific changes, both in the composition and/or activity in the gastrointestinal microbiota that confer benefits upon host health”.65 Inulin-type fructans and lactulose modulate gut transit, decrease putrefactive activity within the gut lumen,76 prevent GI infections77 and mitigate inflammatory responses.78,79 Hypothetically, some of the alterations in the composition or function of the intestinal microbiota in IBS may be corrected or counteracted by prebiotics’ abilities to modulate the gut microbiota and their function. Few studies have investigated the effect of prebiotics in patients with IBS. The study by Olesen and Gudmand-Hoyer80 tested a large dose (20 g) of inulin during 12 weeks. The authors hypothesized that IBS symptoms may be provoked by large quantities of fermentable carbohydrates in the colon. After 4–6 weeks on treatment, IBS symptoms worsened, as expected, in patients on 20 g inulin per day and improved in patients on placebo. However, continuous treatment for 12 weeks resulted in adaptation and no difference was found between groups: symptoms improved in 58% of the inulin group vs 65% of the placebo group and symptoms worsened in 8% of the inulin group vs 13% of the placebo group. Large doses of highly fermentable carbohydrates should not be recommended to IBS patients.81 Nevertheless, two other published studies of adequate size have reported beneficial effects of a prebiotic in IBS.82,83 In the double blind placebo controlled study by Paineau et al.82 that included 105 IBS subjects, treatment with a short chain inulin-type fructan at 5 g/day over 6 weeks reduced the incidence and intensity of GI symptoms as compared with the placebo. Prebiotic treatment also improved functional digestive disorders related quality of life. Another study tested a galactooligosaccharide (GOS) in a double blind placebo controlled trial with 44 IBS subjects randomized into 3 groups, either receiving 7 g/day placebo, 3.5 g/day GOS and 3.5 g/day placebo or 7 g/d GOS for 6 weeks.83 The prebiotic significantly improved flatulence, bloating and the composite score of symptoms, as well as subjective global assessment. It also increased the proportion of bifidobacteria in faecal samples. In summary, evidence accumulated so far in clinical studies testing prebiotics in IBS subjects is limited, but suggests possible benefits of specific prebiotics (inulin, GOS) at moderate doses. Further studies with adequate methodology are warranted.84
Conclusions and Future Research
The knowledge gained in recent years about the ability of gut microbes to influence and contribute to host health and wellbeing opens new windows of opportunity for nutritional and pharmacological tools to improve host-microbe symbiosis, potentially using probiotics and prebiotics. The most innovative and exciting area for future applications derives from the evidence accumulated on the role of the gut microbiota in the brain-gut axis. Translation of the effects of microbiota/probiotics on the brain-gut axis found in laboratory and animal studies and further understanding of how these effects might improve physiological (e.g., GI) and psychological (e.g., anxiety and depression) disturbances are certainly a major challenge for research in forthcoming years.
Researchers need to better understand how to assess and manipulate the potential microbial impact on the brain-gut axis amidst a large number of bacterial, host and environmental influences. An integrated approach must consider a number of parameters including deep GI microbiome analysis, markers of immune and neural functions and responses to environmental conditions (e.g., stress and diet). A systemic characterization of the GI microbiome composition should be considered. New technologies, such as 16S rRNA metagenomics sequencing and bacterial metagenomics and metatranscriptomics combined with metabolomics likely will help to study segregate groups and disease subtypes. Characterization of the microbiome in other sites (oral cavity, small intestine) of the GI tract also may help to understand better these complex host-microbiome interactions. Finally, studies linking microbial colonization, brain development and behavior during early life in infants, children and adolescents are of paramount relevance to further develop this field. International initiatives such as the International Human Microbiome Consortium provide access to massive amounts of data linking microbial sequences with human phenotype. These bioinformatics tools certainly will assist researchers to segregate better different populations and target potential groups for interventions.
Investigators face numerous challenges including the demonstration and articulation of specific beneficial effects and associated mechanisms of probiotics and prebiotics. Additionally, the involvement of neuroimmune interactions in the GI tract is an understudied area with respect to probiotics and prebiotics. Before using probiotics in interventional studies, many factors remain to be considered. Continuing technological advances, enhancing knowledge of the immune system and state of the art investigation of the gut microbiota are essential to make human interventional studies with probiotics and prebiotics meaningful.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank ISAPP organizers for gathering the different international experts to attend this workshop.
Glossary
Abbreviations:
- ACTH
adrenocorticotropin hormone
- ANS
autonomous nervous system
- ASD
Autistic Spectrum Disorder
- BNDF
brain-derived neurotrophic factor
- CNS
central nervous system
- ENS
enteric nervous system
- EPM
Elevated plus maze
- FGID
functional gastrointestinal disorder
- GOS
galactooligosaccharides
- GF
germ-free
- GI
gastrointestinal
- HPA
hypothalamic-pituitary-adrenal
- IBS
irritable bowel syndrome
- IgA
Immunoglobulin A
- ISAPP
International Scientific Association for Probiotics and Prebiotics
- JAMs
junctional adhesion molecules
- MLCK
myosin light chain kinase
- NE
norepinephrine
- PUFA, polyunsaturated fatty acids
RAG-1, recombinase activating gene 1
- SPF
specific pathogen-free
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/22973
References
- 1.O’Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl) 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- 2.O’Hara AM, Shanahan F. Gut microbiota: mining for therapeutic potential. Clin Gastroenterol Hepatol. 2007;5:274–84. doi: 10.1016/j.cgh.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24:405–13. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 4.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 6.Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110–20. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27:496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–76. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 9.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140–9. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 10.Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, et al. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1(−/−);Nod2(−/−) Mice. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.22848. [DOI] [PubMed] [Google Scholar]
- 11.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–53. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, et al. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;298:G395–401. doi: 10.1152/ajpgi.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, et al. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr. 2008;138:1355–9. doi: 10.1093/jn/138.7.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamm K, Hoppe S, Breves G, Schröder B, Schemann M. Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterol Motil. 2004;16:53–60. doi: 10.1046/j.1365-2982.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 15.Fung C, Ellis M, Bornstein JC. Luminal Cholera Toxin Alters Motility in Isolated Guinea-Pig Jejunum via a Pathway Independent of 5-HT(3) Receptors. Front Neurosci. 2010;4:162. doi: 10.3389/fnins.2010.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55:191–6. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–7. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 18.Ait-Belgnaoui A, Han W, Lamine F, Eutamene H, Fioramonti J, Bueno L, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55:1090–4. doi: 10.1136/gut.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–9. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdú EF, Bercík P, Bergonzelli GE, Huang XX, Blennerhasset P, Rochat F, et al. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004;127:826–37. doi: 10.1053/j.gastro.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, et al. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901–7. doi: 10.1093/jn/137.8.1901. [DOI] [PubMed] [Google Scholar]
- 22.Duncker SC, Kamiya T, Wang L, Yang P, Bienenstock J. Probiotic Lactobacillus reuteri alleviates the response to gastric distension in rats. J Nutr. 2011;141:1813–8. doi: 10.3945/jn.110.136689. [DOI] [PubMed] [Google Scholar]
- 23.Azpiroz F. Intestinal perception: mechanisms and assessment. Br J Nutr. 2005;93(Suppl 1):S7–12. doi: 10.1079/BJN20041338. [DOI] [PubMed] [Google Scholar]
- 24.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–88. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Clarke G, Fitzgerald P, Hennessy AA, Cassidy EM, Quigley EM, Ross P, et al. Marked elevations in pro-inflammatory polyunsaturated fatty acid metabolites in females with irritable bowel syndrome. J Lipid Res. 2010;51:1186–92. doi: 10.1194/jlr.P000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall R, Ross RP, Shanahan F, O’Mahony L, Kiely B, Quigley E, et al. Impact of administered bifidobacterium on murine host fatty acid composition. Lipids. 2010;45:429–36. doi: 10.1007/s11745-010-3410-7. [DOI] [PubMed] [Google Scholar]
- 27.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–64, e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 28.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–75. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM. Neurobehavioral changes resulting from recombinase activation gene 1 deletion. Clin Diagn Lab Immunol. 2003;10:13–8. doi: 10.1128/CDLI.10.1.13-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyte M. The role of microbial endocrinology in infectious disease. J Endocrinol. 1993;137:343–5. doi: 10.1677/joe.0.1370343. [DOI] [PubMed] [Google Scholar]
- 33.Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38:414–21. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie GD, Nicholson-Guthrie CS. gamma-Aminobutyric acid uptake by a bacterial system with neurotransmitter binding characteristics. Proc Natl Acad Sci U S A. 1989;86:7378–81. doi: 10.1073/pnas.86.19.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–64. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 36.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89:350–7. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–17. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 38.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 39.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–61. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 40.Association AP. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC, 2000. [Google Scholar]
- 41.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3 doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–65. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–91. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 46.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35(Suppl 1):S6–16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 47.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–53. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res. 2010;9:2996–3004. doi: 10.1021/pr901188e. [DOI] [PubMed] [Google Scholar]
- 49.MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–69. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 50.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–35. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 51.Kałużna-Czaplińska J, Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition. 2012;28:124–6. doi: 10.1016/j.nut.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Critchfield JW, van Hemert S, Ash M, Mulder L, Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract. 2011;2011:161358. doi: 10.1155/2011/161358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol. 2005;100:1868–75. doi: 10.1111/j.1572-0241.2005.41893.x. [DOI] [PubMed] [Google Scholar]
- 55.Ringel Y, Drossman DA. Irritable Bowel Syndrome. In: MA RMaG, ed. Netter’s textbook of Internal Medicine: Sauders elsevier, 2009:419-25. [Google Scholar]
- 56.Saito YA. The role of genetics in IBS. Gastroenterol Clin North Am. 2011;40:45–67. doi: 10.1016/j.gtc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ringel Y, Carroll IM. Alterations in the intestinal microbiota and functional bowel symptoms. Gastrointest Endosc Clin N Am. 2009;19:141–50, vii. doi: 10.1016/j.giec.2008.12.004. [vii.] [DOI] [PubMed] [Google Scholar]
- 58.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–30, e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–91. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 61.Parkes GC, Rayment NB, Hudspith BN, Petrovska L, Lomer MC, Brostoff J, et al. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:31–9. doi: 10.1111/j.1365-2982.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- 62.Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:513–20, e246-7. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 63.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–32. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 65.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 66.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. TARGET Study Group Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 67.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033–49, quiz 1050. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 68.Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome--focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35:403–13. doi: 10.1111/j.1365-2036.2011.04965.x. [DOI] [PubMed] [Google Scholar]
- 69.Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. doi: 10.1186/1471-230X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ringel Y, Ringel-Kulka T. The rationale and clinical effectiveness of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2011;45(Suppl):S145–8. doi: 10.1097/MCG.0b013e31822d32d3. [DOI] [PubMed] [Google Scholar]
- 71.Martínez C, Lobo B, Pigrau M, Ramos L, González-Castro AM, Alonso C, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2012 doi: 10.1136/gutjnl-2012-302093. In press. [DOI] [PubMed] [Google Scholar]
- 72.Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 73.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–34. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 74.Nissen L, Chingwaru W, Sgorbati B, Biavati B, Cencic A. Gut health promoting activity of new putative probiotic/protective Lactobacillus spp. strains: a functional study in the small intestinal cell model. Int J Food Microbiol. 2009;135:288–94. doi: 10.1016/j.ijfoodmicro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 75.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–97. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Preter V, Vanhoutte T, Huys G, Swings J, Rutgeerts P, Verbeke K. Baseline microbiota activity and initial bifidobacteria counts influence responses to prebiotic dosing in healthy subjects. Aliment Pharmacol Ther. 2008;27:504–13. doi: 10.1111/j.1365-2036.2007.03588.x. [DOI] [PubMed] [Google Scholar]
- 77.Lewis S, Burmeister S, Brazier J. Effect of the prebiotic oligofructose on relapse of Clostridium difficile-associated diarrhea: a randomized, controlled study. Clin Gastroenterol Hepatol. 2005;3:442–8. doi: 10.1016/S1542-3565(04)00677-9. [DOI] [PubMed] [Google Scholar]
- 78.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, et al. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–9. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casellas F, Borruel N, Torrejón A, Varela E, Antolin M, Guarner F, et al. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25:1061–7. doi: 10.1111/j.1365-2036.2007.03288.x. [DOI] [PubMed] [Google Scholar]
- 80.Olesen M, Gudmand-Hoyer E. Efficacy, safety and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am J Clin Nutr. 2000;72:1570–5. doi: 10.1093/ajcn/72.6.1570. [DOI] [PubMed] [Google Scholar]
- 81.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657–66, quiz 667. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 82.Paineau D, Payen F, Panserieu S, Coulombier G, Sobaszek A, Lartigau I, et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br J Nutr. 2008;99:311–8. doi: 10.1017/S000711450779894X. [DOI] [PubMed] [Google Scholar]
- 83.Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508–18. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- 84.Brownawell AM, Caers W, Gibson GR, Kendall CW, Lewis KD, Ringel Y, et al. Prebiotics and the health benefits of fiber: current regulatory status, future research and goals. J Nutr. 2012;142:962–74. doi: 10.3945/jn.112.158147. [DOI] [PubMed] [Google Scholar]
- 85.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 86.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–12, e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 87.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609, 609, e1-3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 88.Clarke G, O’Mahony SM, Hennessy AA, Ross P, Stanton C, Cryan JF, et al. Chain reactions: early-life stress alters the metabolic profile of plasma polyunsaturated fatty acids in adulthood. Behav Brain Res. 2009;205:319–21. doi: 10.1016/j.bbr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 89.O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–7. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]