Abstract
The gastrointestinal tract is habitable by a variety of microorganisms and it is often a tissue inflicted by inflammation. Much discussion is raised in recent years about the role of microbiota in intestinal inflammation, but their role in intestinal cancer remains unclear. Here we discuss and extent our work on Drosophila melanogaster models of tumorigenesis and tumor cell invasion upon intestinal infection. In Drosophila midgut bacteria that cause enterocyte damage induce intestinal stem cell proliferation, which is diverted toward aberrant stem cell expansion upon oncogene expression to induce dysplastic tumors. In the hindgut though, oncogenes synergize with the innate immune response—not the bacterially mediated damage—to induce tumor cell invasion and dissemination to distant sites. Interestingly, our novel gene expression analysis of Drosophila hemocyte-like cells suggests commonalities with oncogenic hindgut cells in the innate immune response and the expression of matrix metalloproteinase 1 in response to bacterial infection.
Keywords: Drosophila, midgut, hindgut, innate immune response, inflammation, bacteria, microbiota, cancer
The intestinal tissue is easily accessible by pathogens, therefore it is structured to provide a physical barrier between the body and the environment. Its cells are held together with junctions that prevent the easy invasion of a potential pathogen. The epithelial surface is covered by a mucus layer, which protects the gut from harmful microbes and substances. In insects there is an additional layer, the peritrophic membrane that covers and further protects the intestinal cells.1 Nevertheless the mammalian and insect intestine hosts a variety of microbiota that when imbalanced via dietary or genetic changes can lead to gastrointestinal diseases and even cancer.2-4 However, despite the fact that bacterial components are well known to induce inflammation, which in turn is linked to cancer, the role of bacteria in carcinogenesis has not been demonstrated conclusively.3-5 Our studies have addressed this issue through the utilization of Drosophila melanogaster, the common fruit fly, as a model organism for bacterially-mediated intestinal inflammation and cancer. Primarily we assessed the effect of infection and oncogene expression in the rapidly dividing cells of the adult midgut. Recently, we also assessed the effect of intestinal infection and oncogene expression in the quiescent progenitor and mature cells of the adult hindgut. The fast turnover of midgut cells and the quiescent stem cells of the hindgut bear significant similarities with the mammalian intestinal crypt stem cells, some of which are rapidly dividing while other are quiescent.6 The most important advantage in using Drosophila's gut as a model for the study of human intestinal pathophysiology is the significant conservation between Drosophila and mammalian intestinal pathophysiology and regeneration via conserved signaling pathways.6 In addition, various human intestinal pathogens and changes in microbiota cause intestinal pathology in flies and in humans.6 Moreover we now have a variety of genetic tools and markers to study the role of bacterial pathogenicity in the intestinal tract of Drosophila melanogaster.7
Bacterially-Induced Enterocyte Damage as a Driver of Midgut Regenerative Inflammation and Intestinal Dysplasia
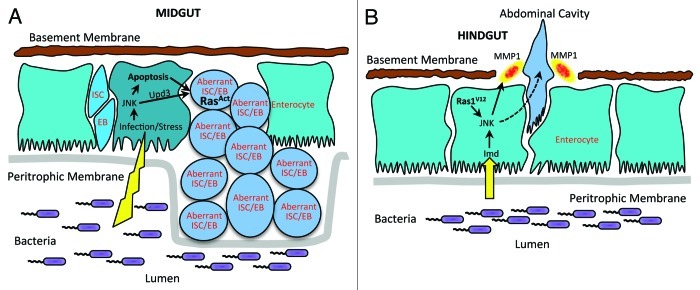
Our first study focused on the middle part of the Drosophila gastrointestinal tract, the midgut. We studied the intestinal cell changes after infection with Pseudomonas aeruginosa, an opportunistic human pathogen.8 We used two strains of P. aeruginosa, one virulent (PA14) and one avirulent (CF5), to investigate if virulence rather than the innate immune response can potentiate the induction of intestinal tumor formation. The results revealed that only the virulent PA14 strain induces damage and apoptosis of the mature intestinal cells, called enterocytes.8 To combat this effect, the intestinal stem cells operate in a compensatory mechanism to induce stem cell proliferation resulting in tissue hyperplasia, which is reversible after bacteria clearance. One factor that stimulates stem cell proliferation is pyocyanin, a toxin secreted by P. aeruginosa. To investigate the pathway responsible we investigated a usual suspect of apoptosis, the JNK pathway.9 Monitoring the expression of the JNK pathway reporter gene, puckered, we found that the JNK pathway is induced in PA14-infected mature midgut cells and not in the stem and progenitor cells, which seem to be resistant to apoptosis and JNK pathway activation.8 Additional testing revealed that enterocyte stress and apoptosis and not the innate immune response to infection induces stem cell proliferation (Fig. 1A). To examine the relationship and cooperation between infection and oncogenic predisposition we have expressed an oncogenic form of the Ras1 gene in the midgut stem cells and progenitors. Ras1 is a Drosophila ortholog of mammalian K-Ras, which is commonly mutated in human colorectal cancers.10 Under low Ras1 ongogene expression, the midgut tissue and stem cells appeared normal. Bacterial infection of these animals however induces intestinal dysplasia, typified by multilayering of the intestinal epithelium, changes in the apicobasal polarity and abnormal differentiation of cells, many of which have irregular nuclei and express progenitor cell markers, while endoreplicating.8 Importantly, clearance of the bacteria did not reverse these phenotypes, as opposed to wild type flies, suggesting that genetically predisposed stem cells retain their pathology. Moreover, mild increase of JNK activation level synergizes with Ras1 oncogene to induce supernumerary stem cells.8 All these findings suggest that virulent bacterial infection induces stem cell proliferation in the midgut, a result of mature cell stress and apoptosis by the JNK pathway in order to preserve intestinal homeostasis.9 Together with genetic predisposition, a synergism is evident where bacterial infection triggers cell polarity, contact and differentiation changes that lead to intestinal dysplasia.6
Figure 1. Mechanisms of cancer-like phenotypes upon bacterial infection in the midgut and hindgut.(A) Midgut stem cell compensatory mechanism upon enterocyte loss and its perturbation upon Ras oncogene expression. Enterocyte apoptosis and cytokine Upd3 expression - driven by JNK - lead to a compensatory mechanism that preserves intestinal homeostasis through proliferation of intestinal stem cells (ISCs) and their progeny, the enteroblasts (EB). Ras oncogenic predisposition leads to supernumerary but aberrant ISCs and EBs and a dysplastic tissue. (B) Hindgut model of cell invasion and dissemination. An immune response to intestinal bacteria induces the Imd pathway and activates JNK. Induction of JNK synergize with Ras1V12 oncogene to degrade the basement membrane (laminin layer) by MMP1, facilitating hindgut cell invasion and dissemination in the abdominal cavity.
Innate Immune Response as a Driver of Hindgut Cell Invasion and Dissemination
Drosophila infection can also inflict disease in the hindgut of oncogenic flies. Indeed in our recent study we developed an adult Drosophila model of tumor cell invasion expressing the Ras1 oncogene, Ras1V12, in the mature hindgut enterocytes and their progenitors.11 We noticed that strong expression of Ras1V12 for seven days induced the delamination of hindgut cells through the basal side of the epithelium. Additional analysis showed that high levels of matrix metalloproteinase 1 (MMP1) expression in the Ras1V12 hindgut epithelium could be responsible for the basement membrane degradation i.e laminin layer disruption in the gaps where hindgut epithelia cells and their cytoplasmic protrusions were mislocalized (Fig. 1B). Furthermore, we detected that Ras1V12 activated hindgut cells disseminate away from the hindgut to form foci in the abdominal cavity marked by the expression of hindgut enterocyte marker byn-GAL4-UAS-GFP. We devised a method to quantify the disseminating foci per fly, rank flies in categories of 0, 1–3, 3–10 and > 10 foci per fly and statistically analyze the percentage of flies in each category per condition. Importantly, the foci accumulation increase in penetrance and severity over time, suggesting that hindgut cell dissemination rate is higher than their death rate. Moreover, suppression of the JNK signaling as well as MMP1 activity reduced dissemination of Ras1V12 hindgut cells.
To test if bacterial infection cooperates with genetic predisposition to induce dissemination of hindgut cells, we fed flies expressing Ras1V12 at low levels with a virulent and an avirulent strain of P. aeruginosa. Both strains potentiated hindgut cell dissemination to a similar extend, revealing that an immune response rather than virulence is important for dissemination. To examine the mechanism of this synergism we inhibited the JNK pathway activity both genetically and pharmacologically, but also we induced JNK in the hindgut cells. We found that JNK induces MMP expression and is necessary for hindgut cell invasion.12,13 In addition, we observed that the JNK pathway reporter puckered was induced higher in bacterially fed Ras1V12 expressing flies than in uninfected controls. Since innate immunity against bacteria is also regulated by the Drosophila NF-kappaB pathway Immune Deficiency (Imd)14,15 we co-expressed Ras1V12 and Imd in hindgut cells and found a robust increase in MMP1 expression and enterocyte dissemination. On the contrary, co-expression of Ras1V12 and activated Rel, the NF-kappaB factor that is downstream of Imd, did not synergize to induce enterocyte dissemination. Epistasis analysis in infected flies via RNA interference and loss of function alleles of the Imd pathway genes revealed that the lmd-dTab2-dTak1-JNK but not the Imd-dDredd-Rel branch of the innate immunity synergises with Ras1V12 to induce enterocyte dissemination. Importantly, when Ras1V12 expression is kept at low levels enterocyte dissemination is not evident in uninfected flies. Under these conditions infection can induce and determine the onset of hindgut cell dissemination. In addition, clearance of P. aeruginosa reduced dissemination to the same levels as non-infected flies suggesting the requirement of constant infection in order to potentiate hindgut cell dissemination.
Commonalities and Differences Between Oncogenic Midgut and Hindgut in Tumor Formation and Tumor Cell Invasion Upon Intestinal Infection
Our studies investigated the effect of bacterial infection in genetically predisposed animal models (Fig. 2). The Drosophila midgut has been used in a number of studies to model actively dividing intestinal stem cells (ISCs) and their response to intestinal drugs and microbes.6 In this tissue damage is prominent and leads to regeneration via ISC proliferation and differentiation, which replenish intestinal cells in order to maintain tissue homeostasis.16,17 When microbes or drugs damage the intestinal midgut, various signaling ligands are secreted, which induce nearby ISC proliferation and differentiation.6 ISCs are dispersed throughout the adult Drosophila midgut epithelium.16 This however is not the case in the Drosophila hindgut. Hartenstein and colleagues have identified a narrow segment of cells in the anterior hindgut, the hindgut proliferation zone, where ISCs reside.18 Recent evidence shows that this zone contains quiescent stem cells, which can proliferate and generate new cells in response to cell loss from tissue damage.19 Thus, Drosophila hindgut can be used to provide information in studies of intestinal quiescent stem cells and the role of oncogenes and immune response to infection. Hence, we assessed the actively dividing and quiescent stem cell bearing tissues of the Drosophila midgut and hindgut, respectively, to investigate cancer-related phenotypes upon bacterial infection.
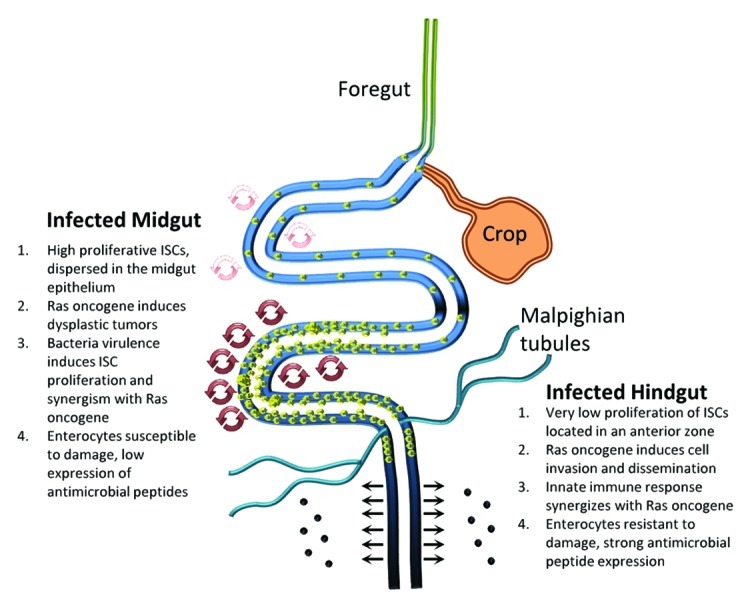
Figure 2. Drosophila midgut and hindgut comparison upon bacterial infection. A synergy is apparent between bacterial infection and Ras oncogene in both midgut and hindgut. In the midgut, the ability of virulent bacteria to provoke damage stimulates ISC mitosis (depicted as increased regeneration in the posterior as opposed to the anterior midgut) to maintain intestinal homeostasis. When ISCs are genetically predisposed (via Ras oncogene expression), they multiply aberrantly inducing intestinal dysplasia. However, the hindgut does not exhibit a similar regeneration mechanism upon infection. When progenitor and mature enterocytes are genetically predisposed (via Ras oncogene expression), bacterially-driven innate immune response induces invasion and dissemination of aberrant enterocytes into the abdominal cavity. Despite the differences, these two models describe a synergism of bacteria and oncogenes to induce cancer-related phenotypes.
Similarities between midgut and hindgut in the induction of inflammation and cancer-related phenotypes
In the midgut and the hindgut cancer-like phenotypes are apparent upon Ras1 oncogene expression. Indeed, Ras1 oncogene induces progenitor cell proliferation in both tissues, which results in tissue overgrowth. In addition, bacterial infection induces the JNK pathway in midgut and hindgut enterocytes, which in turn converges with Ras1 oncogene signaling to induce cancer-like phenotypes. Thus our findings reveal a synergistic effect between bacterial infection and genetic predisposition in carcinogenesis of both the midgut and the hindgut. In addition, the Wg and the JAK/STAT pathway are involved in the maintenance of both the midgut and the hindgut stem cells.18,20,21 The Upd3 cytokine that induces the JAK/STAT pathway is playing a major role in midgut cell inflammation, sending a signal from the stressed or dying enterocyte to ISCs20 (Fig. 1A) and it would be interesting to see if it plays a similar role in the hindgut.
Differences between midgut and hindgut in the induction of inflammation and cancer-related phenotypes
Despite these similarities, the bacterial factors and the host mechanisms leading to cancer-related phenotypes differ in the two intestinal tract regions (Fig. 2). In the midgut, the ability of bacteria to be virulent and induce damage of mature enterocytes is responsible for the induction of a regeneration mechanism that stimulates ISCs to proliferate and maintain intestinal homeostasis by replenishing enterocyte loss.8 The mechanism is different in the Drosophila hindgut, where bacteria infection does not induce proliferation of hindgut cells.11 In the midgut virulent bacteria damage and induce apoptosis of enterocytes, which in turn signals to ISCs to proliferate. In Ras1 oncogene expressing midguts bacterial infection induces ISC proliferation and abnormal differentiation forming dysplastic tumors characterized by alterations in cell polarity and tissue architecture, but they never invade basally or disseminate to other tissues.8 Upon hindgut infection though, Ras1 oncogene expression results in a different outcome.11 Challenged flies exhibit dissemination of hindgut cells away from the hindgut and form foci of one or more cells onto other tissues e.g the abdominal cavity trachea, fat body, nephrocytes and epidermis. Moreover, hindgut cells invade basally because they express MMP1 that degrades the extracellular matrix.11 This effect was visualized with both virulent and avirulent bacterial strains indicating that immune response rather than bacterial virulence is the mechanism utilized by the bacteria to promote hindgut cell invasion. In fact virulent bacteria can induce proliferation of the midgut cells, but they never induce proliferation of hindgut cells.11 On the contrary innate immune response to P. aeruginosa is not prominent in the midgut, but it is in the hindgut.11 Investigation of the major innate immune pathway Imd,22 shows that bacterial infection induces this pathway leading to JNK and MMP1 activation and hindgut cell invasion and dissemination (Fig. 1B). This can be further supported by previous findings on Drosophila imaginal disc cells and Ras cooperation with JNK in the induction of invasive tumors.13
Synergism Rather Than Antagonism Between Ras and Imd Pathways in the Hindgut
Interestingly, a recent study demonstrated that the Ras/MAPK signaling pathway represses the Imd pathway activity in the midgut, fat body and hemocytes.23 It shows that Ras/MARK activation regulates the expression of Pirk, a negative regulator of the Drosophila lmd pathway.24 Our investigation in the hindgut model gives a different picture since bacterial infection in the presence of Ras1 oncogene strongly induces cecropin expression, an antimicrobial peptide regulated by the Imd pathway.25 Additional experiments further demonstrate the synergistic effect of Imd and Ras, where RNA interference and loss of function alleles of Imd pathway components suppress hindgut cell invasion and dissemination induced by bacterial infection.11 A simple explanation for this discrepancy might be that the interaction between Ras and Imd pathways is tissue specific. It might be for example, that Pirk does not respond to Ras signaling in this tissue.
Ras-Ongogenic Hindgut Cells Share Commonalities with Hemocyte-Like Cells in Response to Bacterial Infection
Bacterial infection facilitates Ras-activated hindgut cells invasion and dissemination. This is reminiscent of the blood cell migratory potential. Thus Ras-activated hindgut cells and the blood cells might use common gene and cellular responses to infection in order to migrate.
Blood cells circulate in the body surveying the invading microorganisms and tissue damage. Microbes and damaged tissues release innate immunity stimulants, which lead to the recruitment of blood cells from surrounding tissues and the circulation to the infected or damaged tissue.26 One step in this process is the cytoskeleton remodelling where the cell polarizes and extents pseudopodia by actin polymerisation in order to move along surfaces. These protrusions are mainly regulated by the Rho GTPases.27 Attachment to adjacent cells or the extracellular matrix involves another type of proteins called integrins, which stabilize the cells by focal adhesions and further activate migration related signals.28 Eventually blood cells can migrate through pre-existing extracellular matrix pores, a method called ameboid, or utilize proteases such as matrix metalloproteinases (MMPs) to degrade the junctions between cells or the extracelllar matrix, where actin filaments engage with contractile proteins to move the cell in a mesenchymal migration mode.29 When blood cells arrive at the site of infection, a number of immune processes can occur, like phagocytosis and encapsulation of invading pathogens.30
When Ras1 oncogene is expressed in the hindgut cells we notice many of them breaking the bonds with the adjacent epithelial cells, producing cytoplasmic extensions, having irregularly shaped nuclei and producing MMPs that degrade the extracellular matrix. Then they move through the hindgut muscle layer and onto the trachea cells and eventually in other tissues of the fly body. Intestinal infection with P. aeruginosa plays a major role in facilitating this process by inducing the innate immune response. Interestingly, P. aeruginosa also induces MMPs in the mammalian lung epithelia, while Helicobacter pylori can induce MMPs and the migration of gastric tumorous epithelial cells via innate immune pathway genes.31 Moreover, H. pylori induces MMPs in macrophages and various bacteria have been shown to induce MMPs.31 Thus it appears that bacterially-mediated innate immune response can trigger MMP expression in blood cells and oncogenic epithelial cells alike.
Expression profiling of hemocyte-like cells and parallels with Ras-activated hindgut cell gene expression
To study this inflammatory mechanism of cells with the potential to migrate we investigated the gene expression of Drosophila S2 cells, a hemocyte-like cell line with phagocytic properties in response to bacterial challenge. We analyzed a previously published genome wide data set32 (Gene Expression Omnibus Database accession # GSE31564) focusing on the responses of S2 cells to a mixture of gram negative and gram positive bacteria at 30, 60, 90 and 180 min post challenge. From this analysis it becomes apparent that hemocytes and Ras-activated hindgut cells share common groups of genes and signaling pathways in response to bacterial infection and pertinent to their migration properties (Table 1). For example, in S2 cells transcriptional targets of the Imd and the JNK pathway are activated, such as the peptidoglycan recognition proteins PGRP-LB, PGRP-LF and PGRP-SB, the antimicrobial peptides Attacins, Cecropins and Drosocin, the Imd pathway component rel and the JNK pathway genes scarface and jun. Similarly in the Ras-activated hindgut, the Imd-JNK pathway is activated upon infection leading to the expression of the antimicrobial peptide Cecropin. Furthermore, the inflammatory pathway JAK/STAT is activated in the hindgut progenitor cell zone and its ligand, Upd3, is activated in the infected S2 cells.18
Table 1. Genes and signaling pathways induced in both Ras-activated hindgut cells and S2 cells.
| Process/Tissue | Ras-Hindgut | Hemocyte |
|---|---|---|
| Proteases |
MMP1 |
MMP1, Sras |
| |
|
|
| Imd pathway |
Imd, Tab2, Tak1, Cecropin |
Relish, PGRP-LB, PGRP-SB1, Cecropin, Attacin, Drosocin |
| JNK pathway |
JNK, puckered |
Scarface, Jun |
| JAK/STAT pathway |
STAT gene reporter |
Unpaired 3 |
| Cytoskeleton/Migration | Cytoplasmic extensions, invasion, dissemination | calpainA, RhoGEF3, Rhophilin, wunen, wunen 23, sulphated, dally |
Analysis of the expression data from hemocytes following challenge with bacteria have provided a panel of various induced genes and signaling pathways. S2 cell genes expressed 2–50 fold at 90 min of bacterial challenge include the induction of Imd, JNK and Jak/Stat pathway genes in S2. This is similar to the induction of these pathways in oncogenic hindgut cells. Strikingly, matrix metalloptoteinase 1 (MMP1) and severas (sras) shows a prominent 20-fold and 2-fold induction in S2 cells respectively. MMP1 is also expressed highly in the hindgut cells. In addition, the migratory phenotypes of hindgut cells can be paralleled to the cytoskeleton and cell migration gene expression of S2 cells.
A striking similarity though is the induction MMP1, which is induced by 20-fold in S2 cells in response to infection and is strongly activated in the Ras-activated hindgut. Induction of MMP1 is observed as early as 30 min in S2 cells and can last at least 3 h. Another metalloprotease, severas, is also induced 2 fold by infection in S2 cells. Matrix metalloproteases play key roles in cell migration e.g., by helping cells to move through tissues and breaking down the extracellular matrix.33 In addition, the S2 responses show induction of, calpain A, RhoGEF3, Rhophillin, wunen, wunen 2, sulphated, dally and other cytoskeleton and cell migration related genes. Further microarray studies of Ras-activated hindgut cells under bacterial infection could uncover more similarities between hemocytes and invading epithelial cells. Nevertheless, these data suggest that MMP1 and other innate immunity controlled genes could be responsible for the synergism of bacteria with blood cells and Ras1-activated hindgut cells that promotes their invasion and migration properties.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Christine Kocks for discussions and Chrysoula Pitsouli for the critical reading of our manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/22429
References
- 1.Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci U S A. 2005;102:11414–9. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743–7. doi: 10.1136/gut.48.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda) 2008;23:350–9. doi: 10.1152/physiol.00031.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selgrad M, Malfertheiner P, Fini L, Goel A, Boland CR, Ricciardiello L. The role of viral and bacterial pathogens in gastrointestinal cancer. J Cell Physiol. 2008;216:378–88. doi: 10.1002/jcp.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakatos PL, Fischer S, Lakatos L, Gal I, Papp J. Current concept on the pathogenesis of inflammatory bowel disease-crosstalk between genetic and microbial factors: pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take “toll”? World J Gastroenterol. 2006;12:1829–41. doi: 10.3748/wjg.v12.i12.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh SR, Mishra MK, Kango-Singh M, Hou SX. Generation and staining of intestinal stem cell lineage in adult midgut. Methods Mol Biol. 2012;879:47–69. doi: 10.1007/978-1-61779-815-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apidianakis Y, Pitsouli C, Perrimon N, Rahme L. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci U S A. 2009;106:20883–8. doi: 10.1073/pnas.0911797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda H, Miura M. Regulatory roles of JNK in programmed cell death. J Biochem. 2004;136:1–6. doi: 10.1093/jb/mvh098. [DOI] [PubMed] [Google Scholar]
- 10.Gisselbrecht S, Skeath JB, Doe CQ, Michelson AM. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;10:3003–17. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- 11.Bangi E, Pitsouli C, Rahme LG, Cagan R, Apidianakis Y. Immune response to bacteria induces dissemination of Ras-activated Drosophila hindgut cells. EMBO Rep. 2012;13:569–76. doi: 10.1038/embor.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–9. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–14. doi: 10.1016/S1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 15.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21:2568–79. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 17.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 18.Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–5. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- 19.Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5:290–7. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragab A, Buechling T, Gesellchen V, Spirohn K, Boettcher AL, Boutros M. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2011;30:1123–36. doi: 10.1038/emboj.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleino A, Myllymäki H, Kallio J, Vanha-aho LM, Oksanen K, Ulvila J, et al. Pirk is a negative regulator of the Drosophila Imd pathway. J Immunol. 2008;180:5413–22. doi: 10.4049/jimmunol.180.8.5413. [DOI] [PubMed] [Google Scholar]
- 25.Onfelt Tingvall T, Roos E, Engström Y. The imd gene is required for local Cecropin expression in Drosophila barrier epithelia. EMBO Rep. 2001;2:239–43. doi: 10.1093/embo-reports/kve048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 28.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 29.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 31.Vanlaere I, Libert C. Matrix metalloproteinases as drug targets in infections caused by gram-negative bacteria and in septic shock. Clin Microbiol Rev. 2009;22:224–39. doi: 10.1128/CMR.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung YS, Kocks C. Phagocytosis of bacterial pathogens. Fly (Austin) 2012;6:21–5. doi: 10.4161/fly.18497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vérollet C, Charrière GM, Labrousse A, Cougoule C, Le Cabec V, Maridonneau-Parini I. Extracellular proteolysis in macrophage migration: losing grip for a breakthrough. Eur J Immunol. 2011;41:2805–13. doi: 10.1002/eji.201141538. [DOI] [PubMed] [Google Scholar]