Abstract
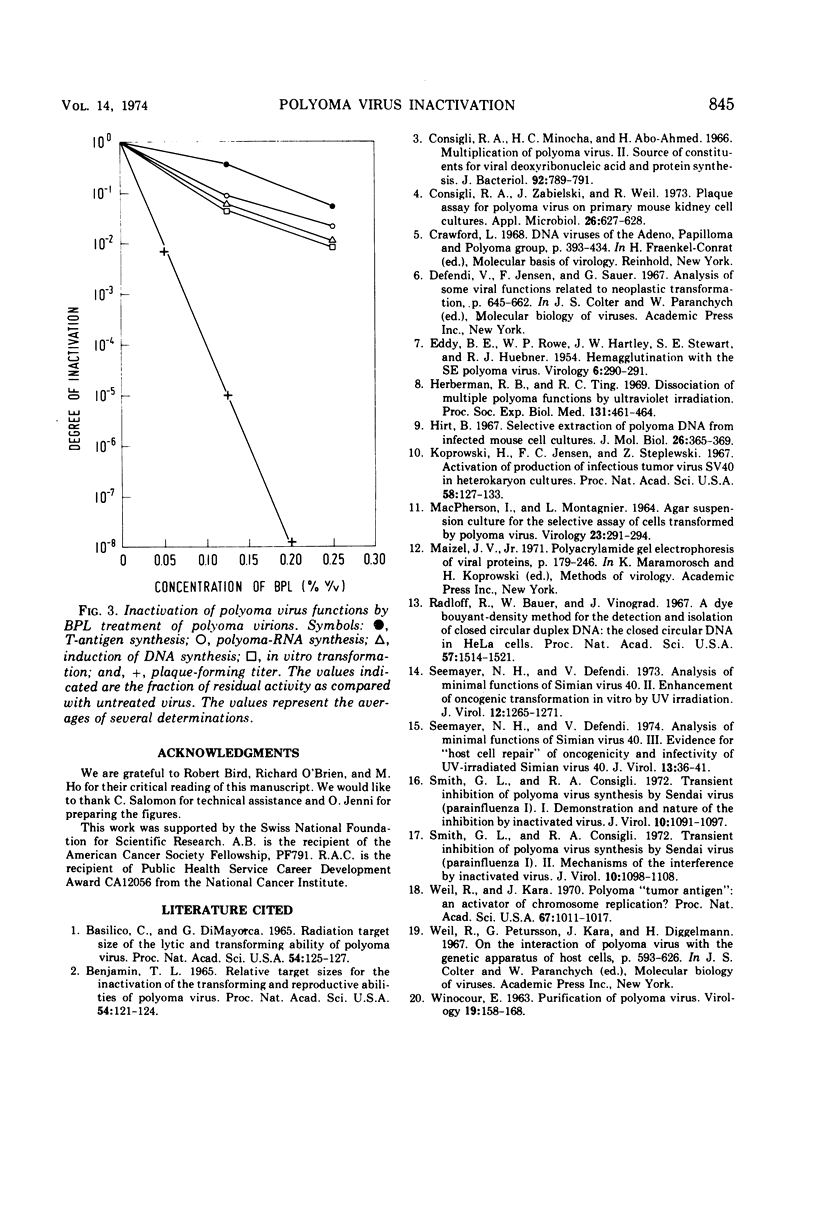
Polyoma virus was inactivated by treatment with β-propiolactone. T-antigen production, polyoma-RNA synthesis, induction of host DNA synthesis (measured by incorporation of labeled thymidine into the cell culture), and in vitro transforming ability were inactivated to a similar degree by various β-propiolactone concentrations (0.25% β-propiolactone reduced these functions approximately 96%), whereas plaque-forming ability and the ability of the virus to replicate its DNA and to synthesize capsid antigen were inactivated by a given concentration of β-propiolactone to a much greater degree (0.25% β-propiolactone led to a reduction of plaque-forming ability of over 8 logs). The significance of these data and their relationship to previously published experiments are discussed.
Full text
PDF
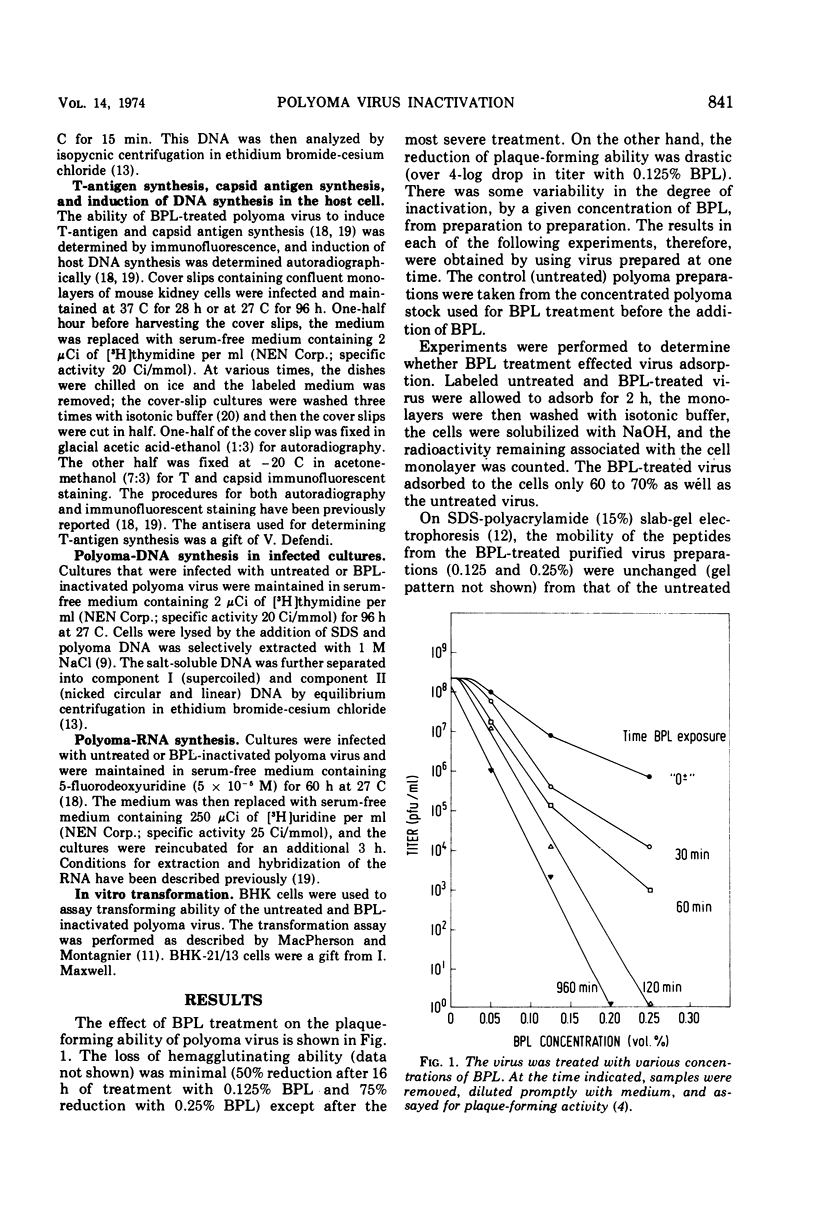
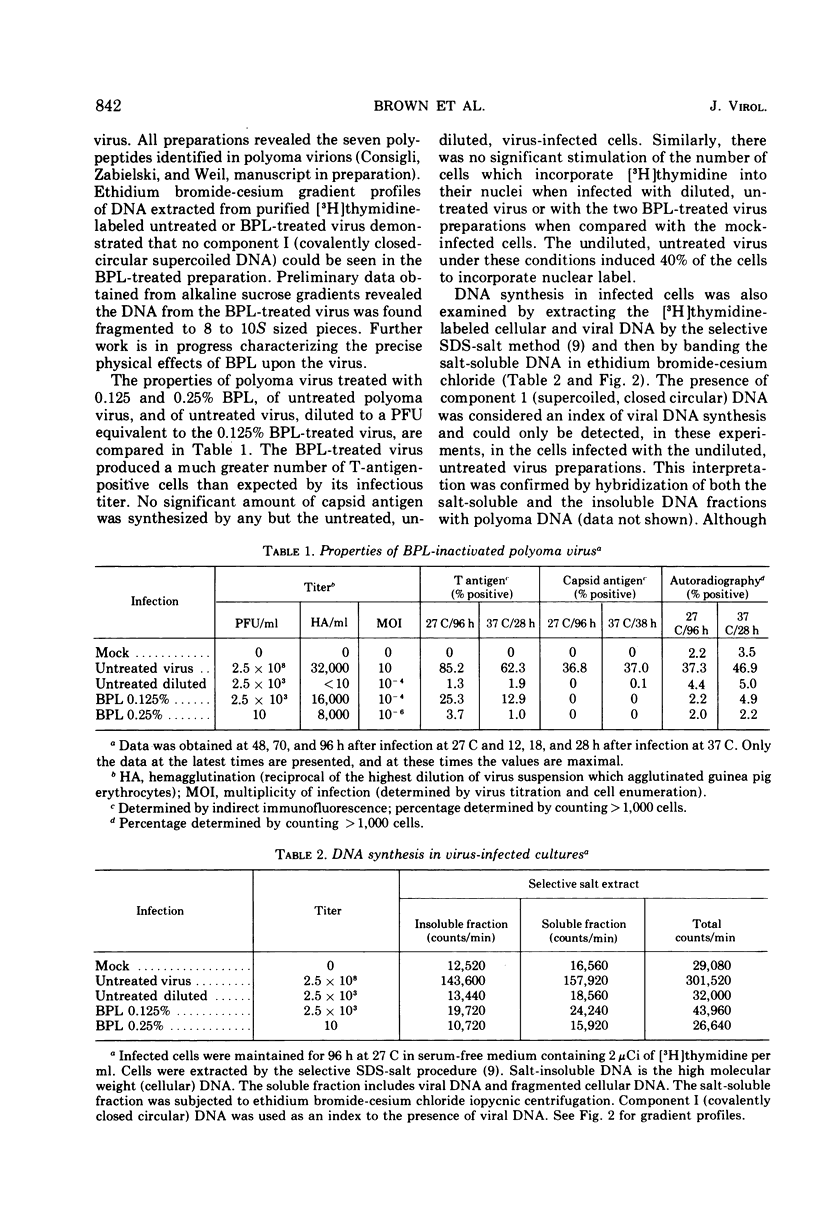
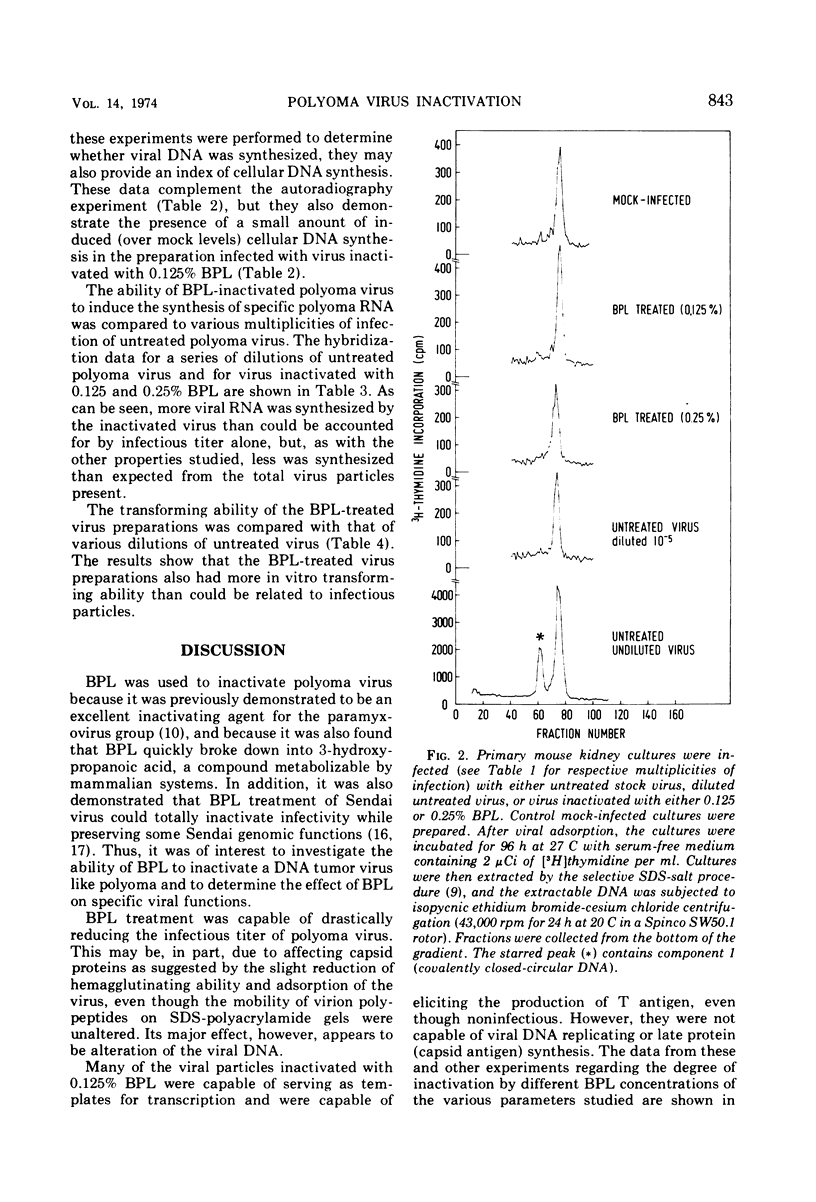
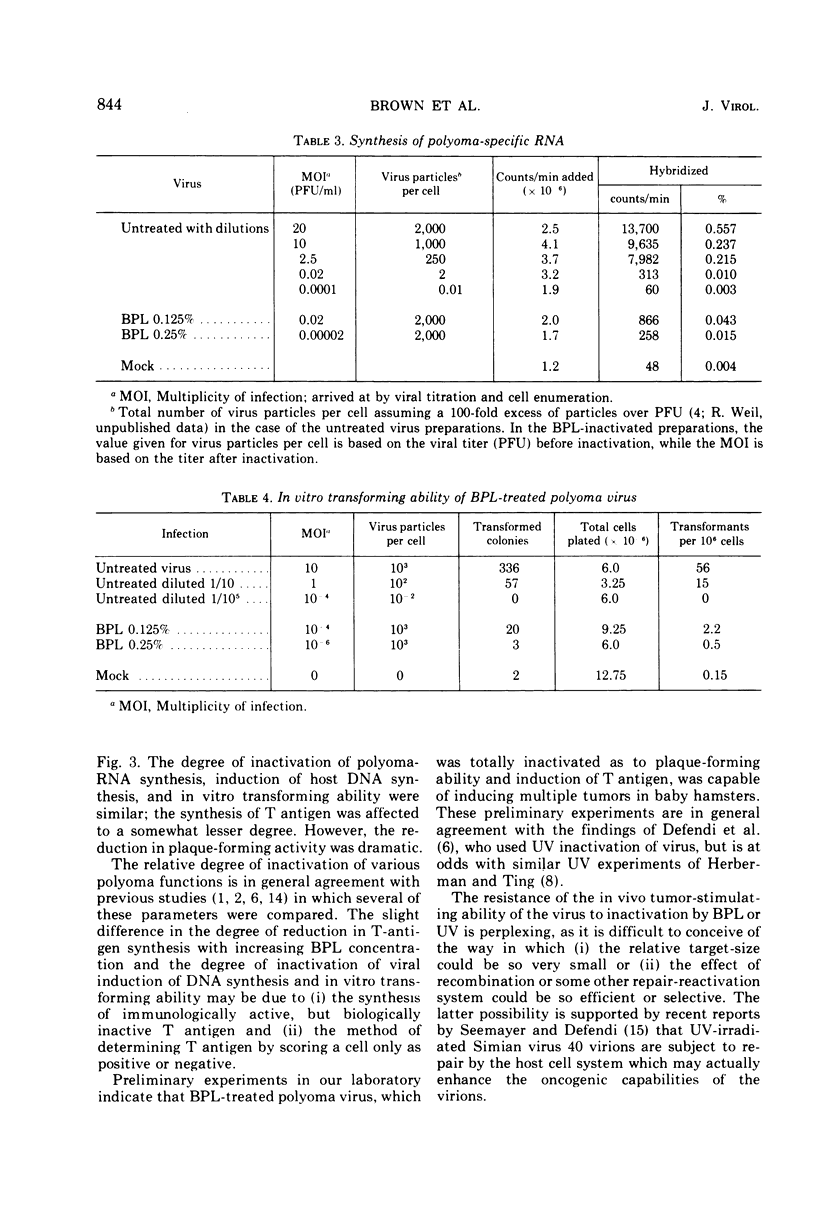
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Di Mayorca G. Radiation target size of the lytic and the transforming ability of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):125–127. doi: 10.1073/pnas.54.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Relative target sizes for the inactivation of the transforming and reproductive abilities of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):121–124. doi: 10.1073/pnas.54.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. Multiplication of polyoma virus. II. Source of constituents for viral deoxyribonucleic acid and protein synthesis. J Bacteriol. 1966 Sep;92(3):789–791. doi: 10.1128/jb.92.3.789-791.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Zabielski J., Weil R. Plaque assay for polyoma virus on primary mouse kidney cell cultures. Appl Microbiol. 1973 Oct;26(4):627–628. doi: 10.1128/am.26.4.627-628.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY B. E., ROWE W. P., HARTLEY J. W., STEWART S. E., HUEBNER R. J. Hemagglutination with the SE polyoma virus. Virology. 1958 Aug;6(1):290–291. doi: 10.1016/0042-6822(58)90078-3. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ting R. C. Dissociation of multiple polyoma virus functions by ultraviolet irradiation. Proc Soc Exp Biol Med. 1969 Jun;131(2):461–464. doi: 10.3181/00379727-131-33902. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemayer N. H., Defendi V. Analysis of minimal functions of simian virus 40. 3. Evidence for "host cell repair" of oncogenicity and infectivity of UV-irradiated simian virus 40. J Virol. 1974 Jan;13(1):36–41. doi: 10.1128/jvi.13.1.36-41.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemayer N. H., Defendi V. Analysis of minimal functions of simian virus 40. II. Enhancement of oncogenic transformation in vitro by UV irradiation. J Virol. 1973 Dec;12(6):1265–1271. doi: 10.1128/jvi.12.6.1265-1271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by sendai virus (parainfluenza I). II. Mechanism of the interference by inactivated virus. J Virol. 1972 Dec;10(6):1098–1108. doi: 10.1128/jvi.10.6.1098-1108.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Weil R., Kára J. Polyoma "tumor antigen": an activator of chromosome replication? Proc Natl Acad Sci U S A. 1970 Oct;67(2):1011–1017. doi: 10.1073/pnas.67.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]


