Abstract
Prebiotics and probiotics are considered natural alternatives to dietary antibiotics in animal production. Plant extracts and yeast cell walls are mannose—rich products that can be used as substrate for adhesion of Gram-negative bacteria. We assessed whether the structure of these saccharides is relevant to develop their role as prebiotics and therefore, their suitability to be used as alternatives to antibiotics to prevent intestinal infections in pigs. The prebiotic functionality of β-galactomannan (βGM), mannanoligosaccharide from yeast Saccharomyces cerevisiae (Mannan SC) and monosaccharide D-Mannose were studied in porcine intestinal epithelial cells (IPI-2I) challenged with Salmonella enterica ser. Typhimurium. Results showed that in vitro challenge with Salmonella induces the secretion of proinflammatory cytokine IL6 and chemokine CXCL8 compared with control without infection. Both βGM and Mannan SC, attenuate Salmonella-induced secretion of IL6 and CXCL8. Interestingly, cells treated with D-mannose showed similar levels of proinflammatory IL6 and CXCL8 compared with the control of infection. These data suggest that prebiotic role of βGM is related to its oligosaccharide structure.
Keywords: Salmonella Typhimurium, mannanoligosaccharides, Saccharomyces cerevisiae, intestinal epithelial cells
Introduction
Livestock animals are subjected to a huge sort of stress factors, such as environmental conditions, animal management or diets that may disrupt the intestinal ecosystem and increase the risk of pathogenic infections. In the swine industry, transition from the liquid to a solid diet during weaning and post-weaning are critic processes due to an important decrease of feed intake, impairing animal performance, immune function, microbiota and therefore, gut homeostasis.1 Concerning the immune system, these performance stages lead to the end of transference of passive maternal immunity from the sows to the piglets which still have an immature or developing immune system.2 This means that piglets become highly susceptible to intestinal pathogens such as enterotoxigenic E.coli (ETEC) or Salmonella enterica ser. Typhimurium (hereafter Salmonella).
Antibiotic growth promoters (AGPs) have been extensively used as dietary additives to minimize the risk of infections, especially during the critical periods of animal production, like weaning. However, after European ban of AGPs (EC 1831/2003) several studies proposed the use of probiotics and prebiotics as natural alternatives to AGPs.1 In that sense, the health benefits of probiotics and prebiotics have been widely accepted as gut microbiota stabilizers because they are believed to avoid pathogenic colonization and therefore improving animal welfare.3,4
Our group recently published in vitro results that demonstrated the potential of probiotic Saccharomyces cerevisiae var Boulardii and prebiotic β-galactomannan (βGM) to prevent intestinal infections by E.coli5 and Salmonella6 in pigs. Here, we aimed to extend the knowledge about the prebiotic role of βGM against Salmonella challenge according to its polysaccharide structure.
Methods
Intestinal epithelial cell culture (IECs)
The porcine small intestine epithelial cell line IPI-2I (ECACC 93100622) was established from the ileum of an adult boar (SLAd/d haplotype).7 IPI-2I cells were maintained in DMEM glutamax (Invitrogen, Spain) supplemented with 10% FCS (Invitrogen), insulin 10 µg/ml (Sigma–Aldrich, Saint-Quentin, France). In all experiments, cells were cultured in 6-well plates (Nunc, Labclinics, Spain) to confluence. Before the addition of pre/probiotics and/or infection, cells were washed three times and the cell culture was replaced with DMEM media supplemented with insulin 10 µg/ml (Sigma–Aldrich). Cells were used between passages 30 and 70 and periodically tested to avoid Mycoplasma contamination (MycoAlert® Mycoplasma Detection Kit, Lonza).
Experimental treatments and prebiotic preparation
To assess the functionality of mannans according to their structure we compared the prebiotic βGM (Salmosan©, patent WO2009/144070 A2, licensed to Industrial Técnica Pecuaria, ITPSA, Barcelona, Spain) to another mannanoligosaccharide purified from Saccharomyces cerevisiae (Mannan SC, Sigma) and to D-Mannose monosaccharide (Sigma-Aldrich) in relation to a negative control (medium).
Prebiotic βGM, Mannan SC and D-Mannose were diluted in DMEM glutamax (1 mg/ml), vortexed and incubated 30 min at 37°C. Immediately before the Salmonella infection, βGM, Mannan SC and D-Mannose were respectively added to each well at 10µg/ml.
Host cell-pathogen assay
Pathogenic Salmonella enterica serovar Typhimurium (Salmonella) with antigenic formulae (4,12:i:1,2), resistant to ampicillin, chloramphenicol, streptomycin, sulfonamide and tetracycline was provided by Dr. Ignacio Badiola from Centre Recerca en Sanitat Animal (CReSA, IRTA-UAB, Bellaterra, Spain). Aliquots of Salmonella were provided in bacterial cryo-preservers (Technical Service Consultants, Ferrer International, Spain) and stored at -80°C until use. Before infection IEC cells, a single Salmonella cryo-preserver was added to 20 ml of Luria-Bertani media (LB) and cultured for 3–4h at 37°C with 180 rpm rotational agitation (Multitron HT, Infors). For infection, Salmonella was used during the exponential growth phase as determined by absorbance at 600 nm. Salmonella was used at MOI = 4 as previously described.6 After 3 h of host cell-pathogen assay performed as described before, 75µg/ml of Gentamycin (Sigma-Aldrich) were added to each well to avoid bacterial overgrowth. After 24 h, supernatants were sampled and stored at -80°C until analysis.
Determination of cytokine production
Cytokine protein determination in the culture supernatant was performed by enzyme-linked immunosorbent assays (ELISAs). Swine IL-6 and CXCL8 DuoSet ELISA kits (R&D Systems, Vitro SP) were used according to manufacturer’s recommendations.
Statistical analysis
All statistical analyses were performed using GLM procedure (PROC GLM) by SAS Software version 9.1.3 (SAS institute). Means for protein secretion were considered in a 2*3 factorial design (two infection levels * 3 experimental treatments) with Duncan’s post-test for group analysis. The P value ≤ 0.05 was considered to be significant. Superscript letters are used to designate statistical significance: Bars with different superscript letters are significantly different (p < 0.05) and bars with same superscript letters indicate no statistical difference (n.s).
Results and Discussion
Many intestinal pathogenic strains of Salmonella and E.coli share type I fimbriae, although from different evolutionary origin, which have high affinity for mannose residues of glycoproteins present on cell surface of the host.4,8,9 The recognition of these residues leads to bacterial adherence to enterocyte surface and intestinal colonization by the pathogen. Our recent articles showed how probiotic strain of Saccaromyces cerevisiae var Boulardii (Scb) and a recently developed prebiotic β-galactomannan (βGM), derived from fruit seed of the Ceratonia silliqua tree, inhibit in vitro adhesion of Salmonella6 and ETEC5 to pig intestinal cell line IPI-2I. Indeed, both products decreased the overall proinflammatory profile induced by ETEC or Salmonella, with the least expenditure of resources for innate immune response to deal with each pathogen.
Mannan derived products, such as Scb and βGM, are widely accepted to prevent Salmonella colonization by binding to the adhesin Fim H of the type I fimbriae. The interaction of probiotic Scb and prebiotic βGM was already confirmed in our previous work.6 However, enterotoxigenic E.coli K88, which is one on the most important causes of neonatal and post-weaning diarrhea (PWD) in piglets, bears type IV fimbriae or pilli.10 The latter has been described as mannose resistant to erythrocyte agglutination test in the presence of D-Mannose (5%).11 Nevertheless, the K88 adhesins (ab, ac and ad) bind to β-galactose residues of receptors of syalic glycoprotein and to neutral glycosphingolipids expressed on the surface of the intestinal mucosa.12 The presence of β-galactose in Scb and βGM may mimic host receptor for K88 adhesin4 and therefore inhibit ETEC attachement to cell surface.
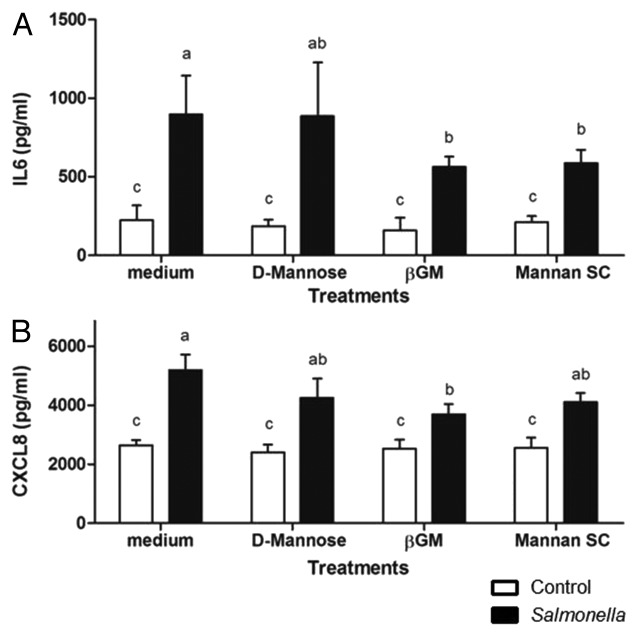
In addition to the residue composition, prebiotic functionality is determined by the size and molecular structure of the saccharides.13 Here, we evaluated functionality of βGM according to their polysaccharide structure. We compared βGM to monosaccharide D-mannose and to another mannan oligosaccharide purified from Saccharomyces cerevisiae yeast (Mannan SC) at a final concentration of 10 µg/ml. We observed that in vitro challenge with Salmonella induces the secretion of proinflammatory cytokine IL6 (4-fold increase: 224 vs. 897 pg/ml ; Figure 1A) and chemokine CXCL8 (2-fold increase: 2645 vs. 5192 pg/ml; Figure 1B) compared with control without infection. Both, βGM and Mannan SC showed a 40% reduction of Salmonella-induced secretion of IL6 (897 vs. 563 and 585 pg/ml, Figure 1A). Concerning CXCL8, βGM reduce chemokine secretion by 30% (5192 vs 3697 pg/ml; Figure 1B). However, Mannan SC shows a trend to reduce CXCL8 secretion induced by Salmonella (Fig. 1B). Interestingly, the secretion of proinflammatory IL6 and CXCL8 in IECs treated with monosaccharide D-mannose was similar to the control of infection (Fig. 1A and B). Present data confirms early results shown by Oyofo et al.14,15 and Vilà et al.16 who showed higher efficiency of oligomeric mannoses compared with monomeric D-mannose to inhibit in vivo Salmonella infection in other productive species like chickens. In pigs, the addition of a commercial source of mannanoligosaccharides in the diet increased weight gain during the starter period, improved feed efficiency in vivo together with a reduction of enterobacteria counts in the jejunum (see ref. 17). Indeed, mannanoligosaccharide additives have been shown to modify the composition of the gastrointestinal microbiota, resulting in increased populations of Bacteroidetes spp and Lactobacillus spp after Salmonella infection in vivo, leading to a greater compensatory weight gain of pigs (see ref. 18)
Figure 1. Effect of oligosaccharide structure on IL6 and CXCL8 secretion induced by Salmonella. Cytokine IL6 (A) and chemokine CXCL8 (B) concentration in supernatants from IPI-2I cells (1 × 106 cells/well) co-cultured for 24 h with Salmonella (MOI = 4) is decreased by βGM and Mannan SC (10 µg/ml). Bars with different superscript letters have statistically significant difference (p < 0.05). Data are representative of 3 independent experiments.
In this regard, our previous in vitro works showed that Scb has a higher anti-inflammatory role in ETEC infections.5 In contrast, βGM is a potent prophylactic agent against Salmonella infections.6 Therefore, the preference of the use of each probiotic or prebiotic additive as a dietary alternative to AGPs, should be determined, if possible, according to the specific conditions of each farm. This means the prevalence of ETEC or Salmonella of each productive facility or region. In that sense, considering that ETEC infections often occur during weaning,19 while Salmonella usually colonizes post-weaning and finishing growing pigs,20 to enrich diets with Scb at early stages to prevent ETEC infections and with βGM to reduce Salmonella prevalence at final growing stages should be considered. In addition, we hypothesize that the prebiotic βGM may enhance probiotic Scb survival and colonization of the intestinal tract improving host health.21 Future studies should determine the suitability to combine βGM and Scb to become a symbiotic additive to prevent intestinal infections .
Nowadays in EU, products such as Mannan SC and βGM are not authorized as zootechnical feed additives under the commission regulation (EC) 1831/2003.22 In the future, both products could be authorized following concepts described in the opinion of EFSA (see ref. 23) concerning potential new categories of zootechnical feed additives. In this case it would be additives which improve product quality as “Microbial contamination controllers “. However, today it is also possible to introduce them in the European feed legislation according to the regulation (EC) 767/200924 where there is a description of how ingredients can be used for particular purposes.
In conclusion, we demonstrated that oligosaccharide structure is crucial for mannans to develop their prebiotic role to inhibit pathogenic adhesion and therefore to reduce proinflamatory immune response induced by the pathogen.
Acknowledgments
This work was supported by grants from the Ministerio de Ciencia e Innovación AGL 2009–11936 (MICIIN, Spain). We gratefully acknowledge Dr I. Badiola (CReSA) for providing Salmonella Typhimurium strain.
Disclosure of Potential Conflicts of Interest
Joaquim Brufau is one of the inventors of the patent Salmosan WO2009/144070 A2 commercially licensed to Industrial Técnica Pecuaria (ITPSA). This does not alter the author’s adherence to all the Gut Microbes policies on data collection and analysis, preparation of the manuscript, or sharing data and materials. The other authors declare that they have no conflicting interests.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/22728
References
- 1.Gaggìa F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141(Suppl 1):S15–28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Levast B, de Monte M, Chevaleyre C, Melo S, Berri M, Mangin F, et al. Ultra-early weaning in piglets results in low serum IgA concentration and IL17 mRNA expression. Vet Immunol Immunopathol. 2010;137:261–8. doi: 10.1016/j.vetimm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Gibson GR, McCartney AL, Rastall RA. Prebiotics and resistance to gastrointestinal infections. Br J Nutr. 2005;93(Suppl 1):S31–4. doi: 10.1079/BJN20041343. [DOI] [PubMed] [Google Scholar]
- 4.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta. 2006;1760:527–37. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Badia R, Zanello G, Chevaleyre C, Lizardo R, Meurens F, Martínez P, et al. Effect of Saccharomyces cerevisiae var. Boulardii and β-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88) Vet Res. 2012;43:4. doi: 10.1186/1297-9716-43-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badia R, Brufau MT, Guerrero-Zamora AM, Lizardo R, Dobrescu I, Martin-Venegas R, et al. β-Galactomannan and Saccharomyces cerevisiae var. boulardii modulate the immune response against Salmonella enterica serovar Typhimurium in porcine intestinal epithelial and dendritic cells. Clin Vaccine Immunol. 2012;19:368–76. doi: 10.1128/CVI.05532-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaeffer B, Bottreau E, Velge P, Pardon P. Epithelioid and fibroblastic cell lines derived from the ileum of an adult histocompatible miniature boar (d/d haplotype) and immortalized by SV40 plasmid. Eur J Cell Biol. 1993;62:152–62. [PubMed] [Google Scholar]
- 8.Althouse C, Patterson S, Fedorka-Cray P, Isaacson RE. Type 1 fimbriae of Salmonella enterica serovar Typhimurium bind to enterocytes and contribute to colonization of swine in vivo. Infect Immun. 2003;71:6446–52. doi: 10.1128/IAI.71.11.6446-6452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capitani G, Eidam O, Glockshuber R, Grütter MG. Structural and functional insights into the assembly of type 1 pili from Escherichia coli. Microbes Infect. 2006;8:2284–90. doi: 10.1016/j.micinf.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Devriendt B, Stuyven E, Verdonck F, Goddeeris BM, Cox E. Enterotoxigenic Escherichia coli (K88) induce proinflammatory responses in porcine intestinal epithelial cells. Dev Comp Immunol. 2010;34:1175–82. doi: 10.1016/j.dci.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Nagy B, Fekete PZ. Enterotoxigenic Escherichia coli in veterinary medicine. Int J Med Microbiol. 2005;295:443–54. doi: 10.1016/j.ijmm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Sarabia-Sainz AI, Ramos-Clamont G, Candia-Plata MM, Vázquez-Moreno L. Biorecognition of Escherichia coli K88 adhesin for glycated porcine albumin. Int J Biol Macromol. 2009;44:175–81. doi: 10.1016/j.ijbiomac.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun. 2006;74:6920–8. doi: 10.1128/IAI.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyofo BA, DeLoach JR, Corrier DE, Norman JO, Ziprin RL, Mollenhauer HH. Prevention of Salmonella typhimurium colonization of broilers with D-mannose. Poult Sci. 1989;68:1357–60. doi: 10.3382/ps.0681357. [DOI] [PubMed] [Google Scholar]
- 15.Oyofo BA, Droleskey RE, Norman JO, Mollenhauer HH, Ziprin RL, Corrier DE, et al. Inhibition by mannose of in vitro colonization of chicken small intestine by Salmonella typhimurium. Poult Sci. 1989;68:1351–6. doi: 10.3382/ps.0681351. [DOI] [PubMed] [Google Scholar]
- 16.Vilà B, de Queiroz D, Badiola I, Pérez-Vendrell AM, Brufau J. Effects of carob bean gum on performance, nutrient digestibility and Salmonellaenterica var. Enteritidis colonisation in chickens. Food Res Int. 2012;45:1133–8. doi: 10.1016/j.foodres.2011.05.010. [DOI] [Google Scholar]
- 17.Castillo M, Martín-Orúe SM, Taylor-Pickard JA, Pérez JF, Gasa J. Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: Effects on microbiota and gut function. J Anim Sci. 2008;86:94–101. doi: 10.2527/jas.2005-686. [DOI] [PubMed] [Google Scholar]
- 18.Price KL, Totty HR, Lee HB, Utt MD, Fitzner GE, Yoon I, et al. Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J Anim Sci. 2010;88:3896–908. doi: 10.2527/jas.2009-2728. [DOI] [PubMed] [Google Scholar]
- 19.Nagy B, Fekete PZ. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet Res. 1999;30:259–84. [PubMed] [Google Scholar]
- 20.Meurens F, Berri M, Auray G, Melo S, Levast B, Virlogeux-Payant I, et al. Early immune response following Salmonella enterica subspecies enterica serovar Typhimurium infection in porcine jejunal gut loops. Vet Res. 2009;40:5. doi: 10.1051/vetres:2008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 22.European Commission. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off.J.Eur.U. L 268: 29-43.
- 23.Scientific Opinion Functional groups of additives as described in Annex 1 of regulation (EC) No 1831/2003. The EFSA Journal. 2008;920:1–19. [Google Scholar]
- 24.European Commission. Regulation (EC) No 767/2009 of the European Parliament and of the Council of 13 July 2009 on the placing on the market and use of feed, amending European Parliament and Council Regulation (EC) No 1831/2003 and repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC,93/74/EEC,93/113/EC and 96/25/EC and Commission Decision 2004/217/EC. Off.J.Eur.U. L 229:1-28.