Abstract
Purpose: This study's purpose is to develop a quantitative ultrasound technology to evaluate radiation-induced vaginal fibrosis. Radiation therapy (RT) is an important treatment modality for most gynecologic (GYN) malignancies. However, vaginal fibrosis is a common chronic side-effect, affecting 80% of women post vaginal or pelvic RT. Vaginal fibrosis leads to pain, sexual dysfunction, and poor quality of life.
Methods: The authors propose a novel ultrasound approach that combines conventional B-mode imaging with Nakagami parameter imaging to quantitatively evaluate post-RT vaginal injury. From the B-mode image, vaginal wall thickness and echo intensity were calculated to capture the anatomy and echogenicity of the vaginal wall. From Nakagami imaging, two statistical parameters, Nakagami probability density function (PDF) and Nakagami shape, were computed to measure the concentration and arrangement of vaginal tissue microstructures. This novel ultrasound imaging concept was investigated in a pilot study of 12 patients, who were previously diagnosed and treated for endometrial cancer. The 12 participants were stratified into two groups: (1) the control group consisted of 6 patients who received surgery (hysterectomy) alone and (2) the post-RT group consisted of 6 patients who received surgery plus radiotherapy, with a follow-up time of 12–38 months. Each participant underwent one transvaginal ultrasound study (6 MHz). Three transverse images of the anterior vaginal wall were acquired in a 2 cm step from the apex (vaginal cuff) to the introitus (vagina opening). The vaginal wall thickness, echo intensity, Nakagami PDF, and Nakagami parameter were calculated to evaluate radiation-induced vaginal fibrosis.
Results: Both B-mode and Nakagami methods showed significant differences in parameters between the post-RT and nonirradiated vaginal walls. Compared with the control group, the vaginal wall thickness of the post-RT group increased by 153.2% (p = 0.002), the echo intensity increased by 11.6% (p = 0.017), the Nakagami PDF increased by 72.3% (p < 0.001), and the Nakagami shape valued increased by 33.0% (p = 0.028).
Conclusions: Vaginal fibrosis appears to be associated with a thickened vaginal wall, higher echogenicity, as well as increased Nakagami PDF and shape parameters. This pilot study shows the authors’ quantitative ultrasound approach combining B-mode and Nakagami imaging is a promising imaging method to evaluate vaginal fibrosis. This imaging method may be useful as physicians try to address vaginal toxicities and sexual dysfunction in women after radiotherapy for GYN malignancies.
Keywords: vaginal fibrosis, ultrasound, Nakagami parameter imaging, radiation toxicity, gynecologic malignancy
INTRODUCTION
Radiation therapy (RT), either alone or in combination with surgery and chemotherapy, is an important treatment modality for most gynecologic (GYN) cancers. Unfortunately, high dose of radiation can cause severe and chronic vaginal toxicity.1 Vaginal fibrosis has been reported in up to 80% of women who have received pelvic or vaginal RT.2, 3 Vaginal fibrosis leads to stenosis (diminishing of vaginal length and width), pain, sexual dysfunction, and poor quality of life (QoL).4
Despite the significant incidence and severity, little research has been conducted to identify the pathophysiologic changes of vaginal toxicity. This paucity of data is largely due to the lack of an easy, reliable tool able to assess radiation-induced vaginal changes. In current clinical practice, vaginal toxicity is evaluated by physical examination involving subjective visual inspection and palpation. Thus far, imaging has played a negligible role in this area. An objective, noninvasive imaging method to measure vaginal fibrosis would be valuable as we try to prevent and treat this debilitating side effect.
The aim of this study is to develop a quantitative ultrasound technology to evaluate post-RT vaginal toxicity. We propose to explore a novel approach that combines conventional B-mode imaging with Nakagami parameter imaging to evaluate vaginal fibrosis. Due to its safe and cost-effective nature, transvaginal ultrasound is widely used in the obstetrics and gynecology (OB/GYN) clinics. Vaginal B-mode imaging provides visualization of anatomical structures within the pelvis. We have investigated two B-mode parameters-–vaginal wall thickness and echo intensity-–which could serve as surrogate biomarkers of vaginal fibrosis. To complement B-mode imaging, Nakagami-parameter imaging, a statistical model-based imaging technology, was utilized to capture the structural properties of the vaginal tissues. We have investigated two Nakagami parameters—Nakagami probability density function (PDF) and Nakagami shape factor-–which quantitatively measure the concentration and arrangement of vaginal tissue microstructures. The premise is that changes in the tissue microstructures often correlate with pathological phenomena, and can be an indicator of sequelae, such as vaginal fibrosis.
Ultrasound Nakagami parameter imaging is a relatively new concept in ultrasound imaging.5, 6, 7 Initially proposed to describe the statistics of radar echoes,8 the Nakagami method was used to analyze the statistical properties of ultrasonic echo signals, and showed early success in detection of organ abnormalities, such as those of the breast9, 10 and liver.11 The ultrasound-based Nakagami method utilizes radiofrequency (RF) signals obtained from the conventional ultrasound system to image tissue scatter statistics, which reveals the arrangements and distributions of scatterers (microstructures) inside the biological tissues. In this study, we adapted Nakagami-parameter imaging to measure radiation-induced vaginal changes, and expect the parameters to provide complementary information to the conventional B-mode images.
The remainder of the paper is organized as follows. Section 2 describes the ultrasound technologies based on B-mode images and Nakagami parameter imaging. Section 3 presents the results of the pilot study of 12 patients (6 post-RT and 6 nonirradiated patients). Section 4 discusses the experimental findings, study limitation, and future directions of this technology. Section 5 summarizes the preliminary investigation.
MATERIALS AND METHODS
Data acquisition–-Transvaginal ultrasound B-mode images and RF signals
Study participants
This study was approved by the Emory University Institutional Review Board. In this pilot study, 12 women, who had been diagnosed and treated with endometrial cancer, were enrolled. Written, informed consent was obtained from all participants. The mean age of the patients was 64 yr (range 41–75). The 12 participants were stratified into two groups: (1) the control group included 6 patients who had undergone surgery (hysterectomy) alone for the treatment of endometrial cancer and (2) the post-RT group included 6 patients who had received surgery plus RT. Currently, three main RT techniques exist to treat GYN malignancies: external beam radiation (1 patient, total dose 50.4 Gy), brachytherapy (2 patients, total dose 21 Gy), and external-beam radiation plus brachytherapy (3 patients, external beam dose 45 Gy plus brachytherapy 15 Gy). The median follow-up time between the completion of the RT and the ultrasound study was 21 months (range 12–38 months). This follow-up time was selected based on a previous report, by Bentzen et al., that fibrosis developed between 1 and 2 yr after RT and continued to progress over time.12
Ultrasound scans
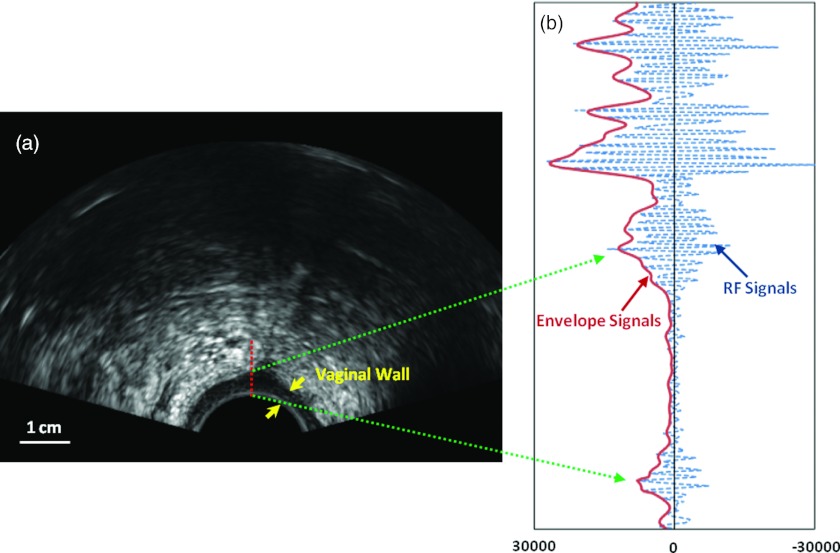
Each participant received one transvaginal ultrasound examination. A clinical ultrasound scanner, Sonix RP (Ultrasonix Medical Corporation, BC, Canada), with a 7.5-MHz biplane probe (BPC8-4/10) was utilized. The ultrasound probe has a cylindrical shape, with a 2 cm diameter and 20 cm length. The transvaginal scan was performed in the dorsal lithotomy (frog-legged) position. Three transverse ultrasound images of the anterior vaginal wall were acquired with 2 cm separation between consecutive slices, from the apex (vaginal cuff) to the introitus (vagina opening). All ultrasound data were acquired with the same settings: 6.0 MHz frequency, 1 cm focal length, 5.0 cm depth, 65% gain, 22 frames/s, and 80-dB dynamic range. Ultrasound B-mode images and RF echo signals were acquired simultaneously. At 6 MHz, the pulse length is approximately 0.51 mm and the −3-dB lateral beam width is 0.44 mm. For the B-mode image [shown in Fig. 1a], the pixel size is 0.192 mm (depth) × 0.193 mm (width). For RF data [shown in Fig. 1b], the RF sampling rate was 20 MHz and the number of bits per RF sample was 16. We chose to operate the probe at 6 MHz frequency to ensure adequate sampling for the RF data acquisition. Each RF frame consisted of 256 scan lines, and each scan line consisted of 1300 sample points. Each RF scan line was then demodulated using the Hilbert transform to obtain the envelope image and the B-mode image was formed based on the logarithm-compressed envelope image.
Figure 1.
Ultrasound B-mode image and RF signals. (a) Transvaginal ultrasound B-mode image. The vaginal wall is clearly visualized (arrows). (b) RF echo signals (in dotted line) and envelope signals (in solid line) of part of the central line. The Nakagami parameter method is based on analysis of the envelope signals.
Clinical assessment of vaginal fibrosis
Each participant received a physical examination at the time of the ultrasound scan. Radiation therapy Oncology Group (RTOG) late morbidity scoring scheme was used to evaluate vaginal fibrosis. In the RTOG scheme, physicians assign a score of 0 (absent) to 4 (necrosis) for the subcutaneous tissue toxicity, based on visual inspection and palpation. In this pilot study, a radiation oncologist (P.R.) evaluated all patients enrolled: all control patients had grade 0 (none) fibrosis, two post-RT patients had grade 2 (moderate) fibrosis, and the remaining 4 patients had grade 1 (mild) fibrosis. Ultrasound findings were compared with clinical assessment of vaginal toxicity in a blind study.
Data processing with B-mode image method
Our quantitative ultrasound evaluation of vaginal toxicity starts from the display of the B-mode image. Figure 1a shows a typical transvaginal B-mode image. In this study, a radiation oncologist (P.R.) contoured the vaginal walls on the 36 ultrasound images of the 12 patients. All the contours were then reviewed by a radiologist (S.T.) and a medical physicist (T.L.) and consensus was reached on the contours. Based on the contours, two ultrasound parameters, vaginal thickness and echo intensity, were subsequently calculated to capture the anatomical and echogenicity properties of the vaginal wall.
Data processing with Nakagami imaging method
The Nakagami analysis was applied to the backscattered envelope signals of the “raw” RF echo signals. Figure 1b shows the RF signal from the central line and the corresponding envelope signals. The theoretical framework for Nakagami imaging relates statistical parameters to properties of the examined tissue.13, 14, 15 The formulation treats the backscattered ultrasound envelope signals as random signals. Our analysis characterizes tissue structures in terms of a stochastic PDF under the Nakagami statistical model. The PDF of the ultrasonic backscattered envelope x under the Nakagami statistical model is defined as
| (1) |
where Γ(·) is the gamma function and W( · ) is the unit step function. The parameter ω is the scaling parameter, whereas u is the Nakagami parameter. Let E(·) denote the statistical mean, then scaling parameter ω and Nakagami parameter u associated with the Nakagami distribution can be obtained from the following equations:
| (2) |
| (3) |
| (4) |
| (5) |
where E(·) is the expectation value operator and T is a threshold.
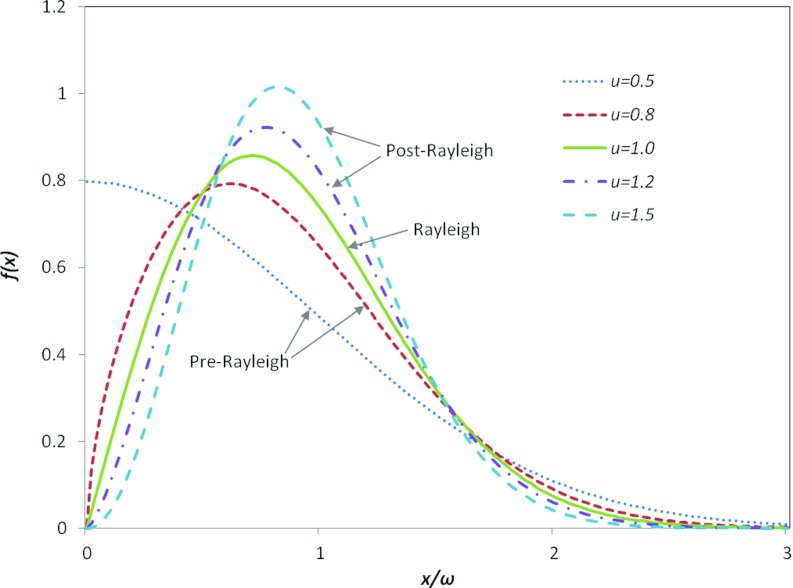
The Nakagami u is a shape parameter for the PDF. When u = 1, the Nakagami distribution reduces to a Rayleigh distribution.16 When 0 ⩽ u < 1, the envelope distribution is said to be pre-Rayleigh.8 When u > 1, the distribution conforms to post-Rayleigh,7 as shown in Fig. 2. This property makes the Nakagami distribution a general model for ultrasonic backscattering. When the region of interest (ROI) contains randomly located scatterers with varying scattering cross sections, the envelope statistics are likely to be pre-Rayleigh, and is typically between 0.5 and 1.17 Similarly, when some spatial periodicity exists among the scatterers within the resolution cell, then the envelope statistics are post-Rayleigh, and becomes larger than unity.7 Typically, u is used as a means to quantify the effective number of scatterers in the resolution cell. This interpretation can be obtained by noting that the random variable Y = X2 follows a gamma distribution, and interpreting the physical relationships between u and the effective number obtained from the gamma distribution.18
Figure 2.
Nakagami distributions for various Nakagami parameters.
Note that the window size determines the resolution of the Nakagami parameter image.19 The principle notion in image formation is the resolution which describes the ability of a system to resolve the smallest details in an object. There is a trade-off between resolution and statistical power. As the window size decreases, the resolution of the Nakagami u image is improved. However, a small window has fewer envelope data points, leading to an unstable estimation of the parameter ulocal. In the evaluation of radiation toxicity, the area of treatment is large, and the irradiated tissues typically range from 5 to 15 cm. Therefore, resolution is not a problem in the evaluation of post-RT tissues. The principle challenge in imaging radiation toxicity is to detect early changes that might be subtle. Hence, we selected a relatively big window size of 39 sample points (beam direction) and 15 scan lines (lateral direction) to improve the statistical power. At the probe surface, the actual window size is 1.50 mm (beam) × 1.26 mm (lateral). Because of the fan shape of the beam, the lateral width increases with depth.
Nakagami u image and Nakagami PDF image are generated using a 2D sliding window. The Nakagami parameters ω and u are commonly used parameters, which are computed from Eqs. 2, 3. From Eq. 1, we subsequently calculated a PDF value for the central point of the sliding window from the corresponding envelope intensity value xcenter, ω, and u. The sliding window is moved through the entire RF envelope image point by point and line by line to generate 2D Nakagami parameter images. This sliding window method could reduce the subresolvable effect at the locations of strong reflectors (e.g., tissue interface or point target) in a scattering medium. In other words, when the sliding window moves onto the strong reflector, the window not only covers the signals from the strong reflector but also contains those from the tissue background. Because there is a large difference in the echo intensity between the reflector and the background, the backscattered envelopes acquired by the window would tend to be extremely pre-Rayleigh distributed, rendering the Nakagami u parameter very small. From the Nakagami PDF and Nakagami u images, Nakagami PDF and u parameters were calculated based on physicians’ contours of the vaginal wall. For the Nakagami PDF image display, the threshold T is set to 0.001.
RESULTS
Figure 3 shows transvaginal B-mode images of the anterior vaginal walls of a control (nonirradiated) and a post-RT patient. The control patient in this example was a 66 yr old woman who underwent surgery for endometrial cancer 8 months prior to imaging. Measured from the B-mode images, the average and standard deviation of the vaginal thickness and intensity was 2.18 ± 0.85 mm and 34.8 ± 18.2 on the proximal scan, 1.86 ± 0.33 mm and 44.5 ± 19.2 on the mid scan, and 1.64 ± 0.50 mm and 43.9 ± 18.8 on the distal scan, respectively. The post-RT patient was a 64 yr old woman who received surgery and RT (external beam plus brachytherapy) 20 months prior to imaging. Clinical assessment revealed moderate vaginal fibrosis. Measured from the B-mode images, the average and standard deviation of the vaginal thickness and intensity was 3.41 ± 1.40 mm and 36.6 ± 17.9 on the proximal scan, 5.84 ± 0.80 mm and 45.1 ± 18.9 on the mid scan, and 5.48 ± 1.18 mm and 52.1 ± 22.5 on the distal scan, respectively. Therefore, compared with the control patient, increased vaginal wall thickness and echo intensity were observed in the post-RT patient.
Figure 3.

Transvaginal ultrasound images of a control and post-RT patients treated for endometrial cancer. Top row (control): a 66 yr old woman 8 months after surgery. Bottom row (post-RT): a 64 yr old woman 20 months post radiotherapy. Compared with the control patient, increased vaginal wall thickness and echo intensity were observed in the post-RT patient.
Figure 4 shows the corresponding Nakagami PDF and Nakagami u images of the distal scans of the two patients shown in Fig. 3. The Nakagami PDF value was 2.36 ± 0.79 × 10−4 and u parameter was 0.99 ± 0.66 for the control patient. The Nakagami PDF value was 3.21 ± 1.09 × 10−4 and u parameter was 1.35 ± 0.54 for the post-RT patient. Therefore, increased Nakagami PDF and u parameters were demonstrated in the post-RT vaginal wall. Microstructures in the normal vaginal wall seemed to exhibit Rayleigh distribution, while post-RT vaginal wall seemed to exhibit post-Rayleigh distribution.
Figure 4.
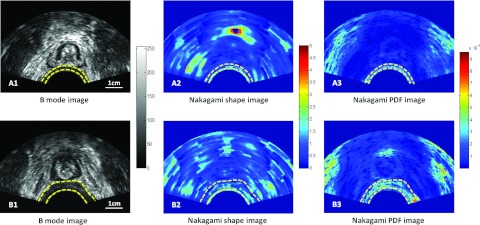
Comparison of vaginal B-mode and Nakagami parameter images of a control and post-RT patients. Top row (control): (A1) B-mode image of the distal scan, (A2) Nakagami shape image, and (A3) Nakagami. PDF parameter image. Bottom row (post-RT): (B1) B-mode image of the distal scan, (B2) Nakagami shape image, and (B3) Nakagami PDF parameter image. Compared with the control, increased Nakagami shape and PDF values were observed in the post-RT patient.
Overall, Fig. 5 shows the B-mode parameters and Nakagami parameters of all the participants. The separations on the scattergrams indicated that both B-mode and Nakagami methods were capable of distinguishing the control from the post-RT vaginal walls. While some overlaps do exist in the echo intensity and Nakagami u parameter, these two parameters still demonstrated significant differences between the control and post-RT groups. Overall, the vaginal wall thickness and the Nakagmi PDF parameters showed higher significant separation between the two groups.
Figure 5.
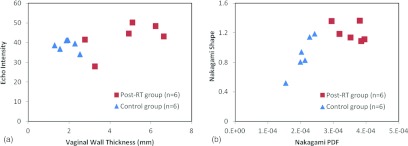
Comparison of the control and post-RT groups. (a) B-mode method: vaginal wall thickness and echo intensity, (b) Nakagami method: Nakagami PDF and Nakagami shape parameter.
Significant differences were observed in all parameters between the post-RT and control groups (Table 1). Compared with the control group, the vaginal wall thickness of the post-RT group increased by 153.2% (p = 0.002), the echo intensity increased by 11.6% (p = 0.017), the Nakagami PDF increased by 72.3% (p < 0.001), and the Nakagami shape valued increased by 33.0% (p = 0.028). Vaginal fibrosis appears to be associated with a thickened vaginal wall, higher echogenicity, as well as increased Nakagami PDF and shape parameters.
Table 1.
Ultrasound B-mode and Nakagami parameters of the control and post-RT groups.
| Groups | Wall thickness (mm) | Intensity | Nakagami Shape | Nakagami PDF (×104) |
|---|---|---|---|---|
| Control group (n=6) | 1.90 ± 0.45 | 38.55 ± 2.79 | 0.91 ± 0.25 | 2.06 ± 0.30 |
| Post-RT group (n=6) | 4.81 ± 1.55 | 43.04 ± 3.32 | 1.21 ± 0.12 | 3.55 ± 0.40 |
| p-value | 0.002 | 0.017 | 0.028 | <0.001 |
DISCUSSION
Our pilot study demonstrated the feasibility of using quantitative ultrasound to evaluate radiation-induced vaginal fibrosis. In this study, we proposed a novel ultrasound approach, combining B-mode and Nakagami parameter imaging, to assess post-RT vaginal toxicity in vivo. Conventional B-mode imaging, generated from the intensity of the backscattered echoes, provides the anatomy and intensity of the vaginal wall. In contrast, the Nakagami parameter image, constructed using the raw RF signals, reflects the arrangements and distributions of the vaginal tissue (microstructures).
To quantify the integrity of the vaginal wall, we investigated four ultrasound parameters: vaginal wall thickness and intensity derived from the B-mode method, and Nakagami PDF and u parameters derived from the Nakagami method. In this pilot study of 12 patients (6 post-RT and 6 control patients), significant differences were observed in all four parameters between the post-RT and control groups. These ultrasound parameters may serve as imaging signatures for the assessment of radiation-induced vaginal injury. Among them, post-RT changes in the vaginal wall thickness and Nakagami PDF appeared to have the highest significance.
The underlying cellular processes of radiation-induced fibrosis are complex and not well understood.20 The normal vagina is lined by stratified, nonkeratinized squamous epithelium that lies upon a loose connective tissue lamina. A layer of longitudinal smooth muscle fibers which contain collagen and large numbers of elastic fibers lies beneath the lamina propria.21 Although we have limited understanding of post-RT vaginal injury, it is known that normal tissue is replaced by mesenchymal cells20, 22 and extensive fibrosis could develop in the entire upper vaginal wall.21 Ultrasound backscattered signals are sensitive to tissue mechanical properties, in particular, stiffness. Fibrotic tissue in the post-RT vaginal wall is stiffer than normal tissues; therefore, post-RT vaginal wall exhibits higher echo intensity as well as increased Nakagami parameters.
Traditionally, vaginal toxicity is assessed through physicians’ visual and tactile examination. Such assessment is subjective and, hence, potentially inconsistent. Quantitative ultrasound is an attractive alternative that could provide clinicians with a simple visualization and quantitative assessment of the vaginal injury. However, imaging has played a negligible role in the evaluation of vaginal toxicity, and to the best of our knowledge, this is the first ultrasound study on post-RT vaginal fibrosis. In a study conducted by Panayi et al., the vaginal thickness of 25 nonirradiated women at the level of the bladder neck was 2.70 ± 0.61 mm.23 Their measurement is slightly higher than the average vaginal thickness of 6 control patients in our study. Such difference could be due to subject age (average age of 58 in Panayi's study vs 64 in our study) or measurement location variations between the two studies.
We should note that the B-mode image method and the Nakagami-parameter method are independent and complementary. The advantage of utilizing ultrasound B-mode image is its wide availability in the clinic. The Nakagami parameter image is constructed using the raw RF signals, and the same diagnostic ultrasound system is used in the current investigation. The Nakagami parameter estimated from the ultrasonic backscattered signals is only dependent on the statistical distribution of echo waveform and not affected by the echo amplitude and, thus, Nakagami imaging is less operator-dependent. However, most ultrasound systems do not provide RF data, but could easily be obtained by those clinics measuring vaginal health post RT.
The main limitation of the study is the small sample size of 12 participants. Nevertheless, the feasibility of utilizing quantitative ultrasound to evaluate vaginal fibrosis is demonstrated. Due to the small sample size, we were unable to further stratify the post-RT patients by their radiation dose, treatment techniques (external beam RT, brachytherapy, or both), or grades of fibrosis. Therefore, as a future direction, we will continue to develop this imaging tool, and conduct a clinical study with a larger cohort of patients to further understand the physiology of vaginal fibrosis.
Vaginal fibrosis is a common sequela of GYN and pelvic RT. Impairment of sexual functioning in women treated for cervical or endometrial carcinoma with RT has been reported in our own and other studies to range from 57% to 80%.1, 2 A detailed understanding of normal-tissue response is necessary for symptom management of post-GYN radiotherapy. Interventional research has been hampered by a lack of quantitative measures for tissue characterization. This imaging tool could provide insight into anatomical and structural changes that lead to vaginal toxicity, and potentially lay the groundwork for outcome metrics of interventions to prevent and treat vaginal toxicity.
CONCLUSION
In summary, this study demonstrated the feasibility of using ultrasound B-mode and Nakagami parameter imaging to assess radiation-induced vaginal toxicity. Significant differences in vaginal wall thickness, echo intensity, Nakagami PDF, and Nakagami shape parameter were demonstrated between control and post-RT groups. Ultrasound B-mode image provides anatomical and echogenicity features of the vaginal wall, while Nakagami parameter image provides structural features of the vaginal tissues. Together, these two imaging methods have the potential to serve as a functional imaging tool to study of vaginal fibrosis.
ACKNOWLEDGMENTS
This research was supported in part by the National Cancer Institute Grant No. CA114313 and the Georgia Cancer Coalition. Dr. Srini Tridandapani's work was supported in part by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) Grant No. K23EB013221. The authors report no conflicts of interest in conducting the research.
References
- Bruner D. W., Lanciano R., Keegan M., Corn B., Martin E., and Hanks G. E., “Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma,” Int. J. Radiat. Oncol., Biol., Phys. 27, 825–830 (1993). 10.1016/0360-3016(93)90455-5 [DOI] [PubMed] [Google Scholar]
- Nout R. A. and Creutzberg C. L., “Point: Vaginal brachytherapy should be a standard adjuvant treatment for intermediate-risk endometrial cancer,” Brachytherapy 10, 1–3 (2011). 10.1016/j.brachy.2010.11.002 [DOI] [PubMed] [Google Scholar]
- Nout R. A. and Creutzberg C. L., “Counterpoint: The role of adjuvant radiation in endometrial cancer. Inside, outside, or not at all? Rebuttal,” Brachytherapy 10, 436–438 (2011). 10.1016/j.brachy.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Bruner D. W. and Boyd C. P., “Assessing women's sexuality after cancer therapy: Checking assumptions with the focus group technique,” Cancer Nurs. 22, 438–447 (1999). 10.1097/00002820-199912000-00007 [DOI] [PubMed] [Google Scholar]
- Zimmer Y., Akselrod S., and Tepper R., “The distribution of the local entropy in ultrasound images,” Ultrasound Med. Biol. 22, 431–439 (1996). 10.1016/0301-5629(95)02064-0 [DOI] [PubMed] [Google Scholar]
- Wachowiak M. P., Smolikova R., Tourassi G. D., and Elmaghraby A. S., “General ultrasound speckle models in determining scatterer density,” Proc. SPIE 4687, 285–295 (2002). 10.1117/12.462164 [DOI] [Google Scholar]
- Shankar P. M., “A general statistical model for ultrasonic backscattering from tissues,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control 47, 727–736 (2000). 10.1109/58.842062 [DOI] [PubMed] [Google Scholar]
- Holfman H. C., Statistical Methods on Radio Wave Propagation (Pergamon/RAND Corporation, New York, 1960). [Google Scholar]
- Liao Y. Y., Tsui P. H., and Yeh C. K., “Classification of benign and malignant breast tumors by ultrasound B-scan and Nakagami-based images,” J. Med. Biol. Eng. 30, 307–312 (2010). 10.5405/jmbe.30.5.06 [DOI] [Google Scholar]
- Liao Y.-Y., Li C.-H., Tsui P.-H., Chang C.-C., Kuo W.-H., Chang K.-J., and Yeh C.-K., “Strain-compounding technique with ultrasound Nakagami imaging for distinguishing between benign and malignant breast tumors,” Med. Phys. 39, 2325–2333 (2012). 10.1118/1.3700167 [DOI] [PubMed] [Google Scholar]
- Ho M. C., Lin J. J., Shu Y. C., Chen C. N., Chang K. J., Chang C. C., and Tsui P. H., “Using ultrasound Nakagami imaging to assess liver fibrosis in rats,” Ultrasonics 52, 215–222 (2012). 10.1016/j.ultras.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Bentzen S. M., Thames H. D., and Overgaard M., “Latent-time estimation for late cutaneous and subcutaneous radiation reactions in a single-follow-up clinical-study,” Radiother. Oncol. 15, 267–274 (1989). 10.1016/0167-8140(89)90095-9 [DOI] [PubMed] [Google Scholar]
- Shankar P. M., “Ultrasonic tissue characterization using a generalized Nakagami model,” IEEE Trans. Ultrason. Ferr. 48, 1716–1720 (2001). 10.1109/58.971725 [DOI] [PubMed] [Google Scholar]
- Tsui P. H., Yeh C. K., Liao Y. Y., Chang C. C., Kuo W. H., Chang K. J., and Chen C. N., “Ultrasonic Nakagami imaging: A strategy to visualize the scatterer properties of benign and malignant breast tumors,” Ultrasound Med. Biol. 36, 209–217 (2010). 10.1016/j.ultrasmedbio.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Wagner R. F., Insana M. F., and Brown D. G., “Statistical properties of radio-frequency and envelope-detected signals with applications to medical ultrasound,” J. Opt. Soc. Am. A 4, 910–922 (1987). 10.1364/JOSAA.4.000910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamou J., Coron A., Oelze M. L., Saegusa-Beecroft E., Hata M., Lee P., Machi J., Yanagihara E., Laugier P., and Feleppa E. J., “Three-dimensional high-frequency backscatter and envelope quantification of cancerous human lymph nodes,” Ultrasound Med. Biol. 37, 345–357 (2011). 10.1016/j.ultrasmedbio.2010.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P. M., “A model for ultrasonic scattering from tissues based on the K distribution,” Phys. Med. Biol. 40, 1633–1649 (1995). 10.1088/0031-9155/40/10/006 [DOI] [PubMed] [Google Scholar]
- Shankar P. M., Dumane V. A., Reid J. M., Genis V., Forsberg F., Piccoli C. W., and Goldberg B. B., “Classification of ultrasonic B-mode images of breast masses using Nakagami distribution,” IEEE Trans. Ultrason. Ferr. 48, 569–580 (2001). 10.1109/58.911740 [DOI] [PubMed] [Google Scholar]
- Tsui P. H., Huang C. C., Sun L., Dailey S. H., and Shung K. K., “Characterization of lamina propria and vocal muscle in human vocal fold tissue by ultrasound Nakagami imaging,” Med. Phys. 38, 2019–2026 (2011). 10.1118/1.3562899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. M., Dische S., Gerber L., Saunders M., Leung S. F., and O’Sullivan B., “Measuring postirradiation subcutaneous soft-tissue fibrosis: State-of-the-art and future directions,” Semin. Radiat. Oncol. 13, 203–213 (2003). 10.1016/S1053-4296(03)00022-5 [DOI] [PubMed] [Google Scholar]
- Fajardo L. F., Berthrong M., and Anderson R. E., Radiation Pathology (Oxford University Press, Oxford, 2001). [Google Scholar]
- Rodemann H. P. and Bamberg M., “Cellular basis of radiation-induced fibrosis,” Radiother. Oncol. 35, 83–90 (1995). 10.1016/0167-8140(95)01540-W [DOI] [PubMed] [Google Scholar]
- Panayi D. C., Digesu G. A., Tekkis P., Fernando R., and Khullar V., “Ultrasound measurement of vaginal wall thickness: a novel and reliable technique,” Int. Urogynecol. J. 21, 1265–1270 (2010). 10.1007/s00192-010-1183-4 [DOI] [PubMed] [Google Scholar]