Abstract
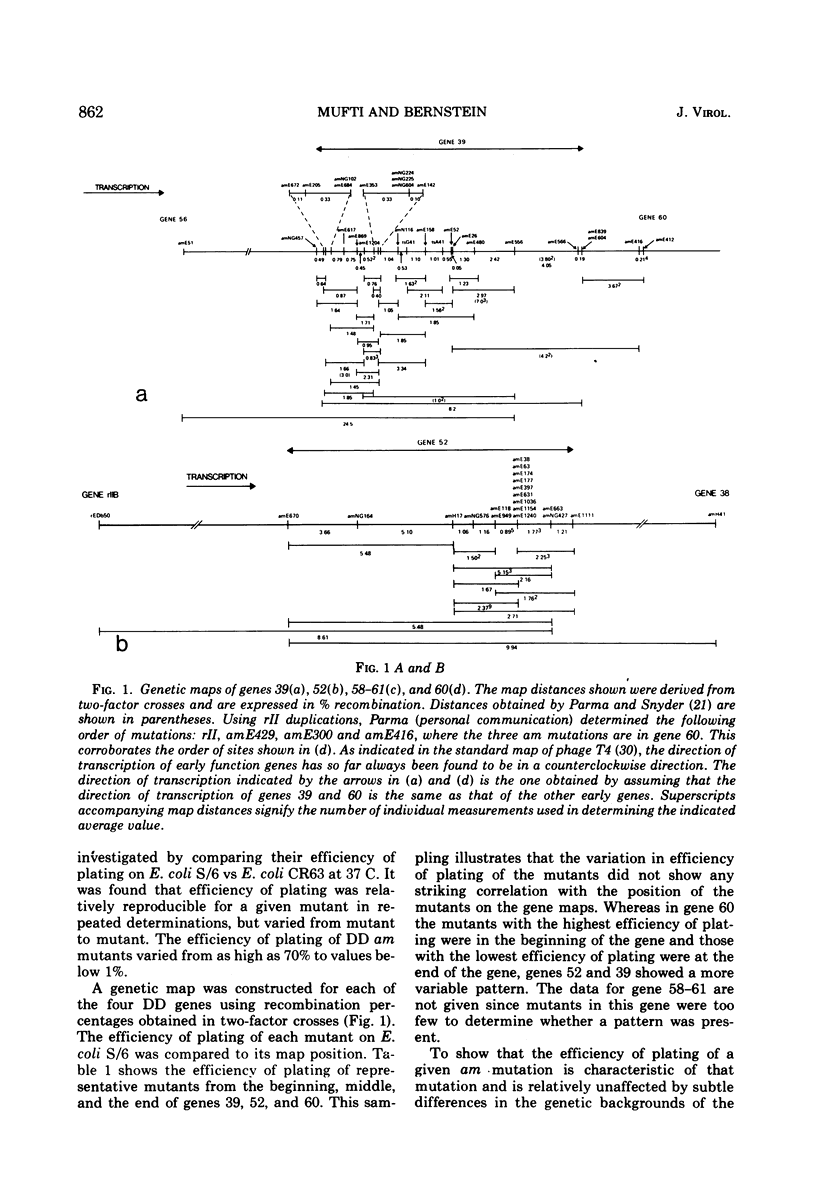
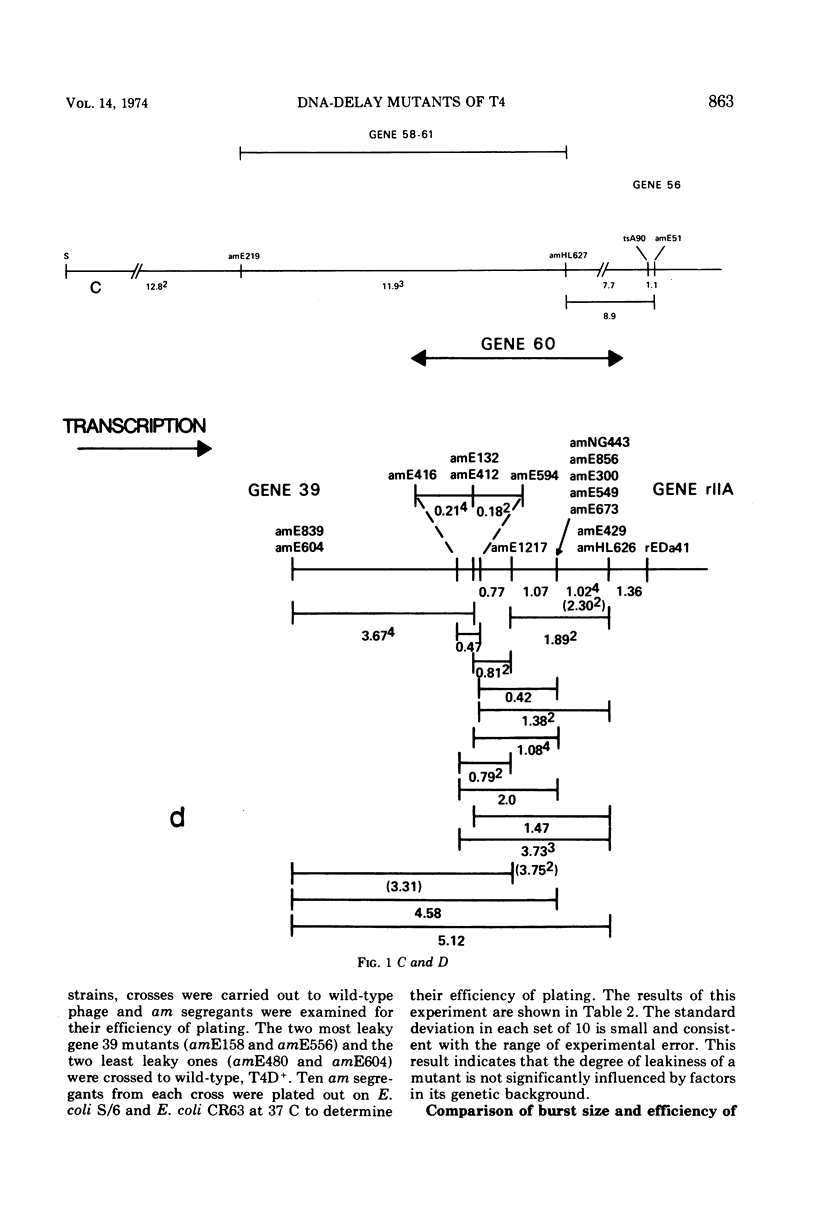
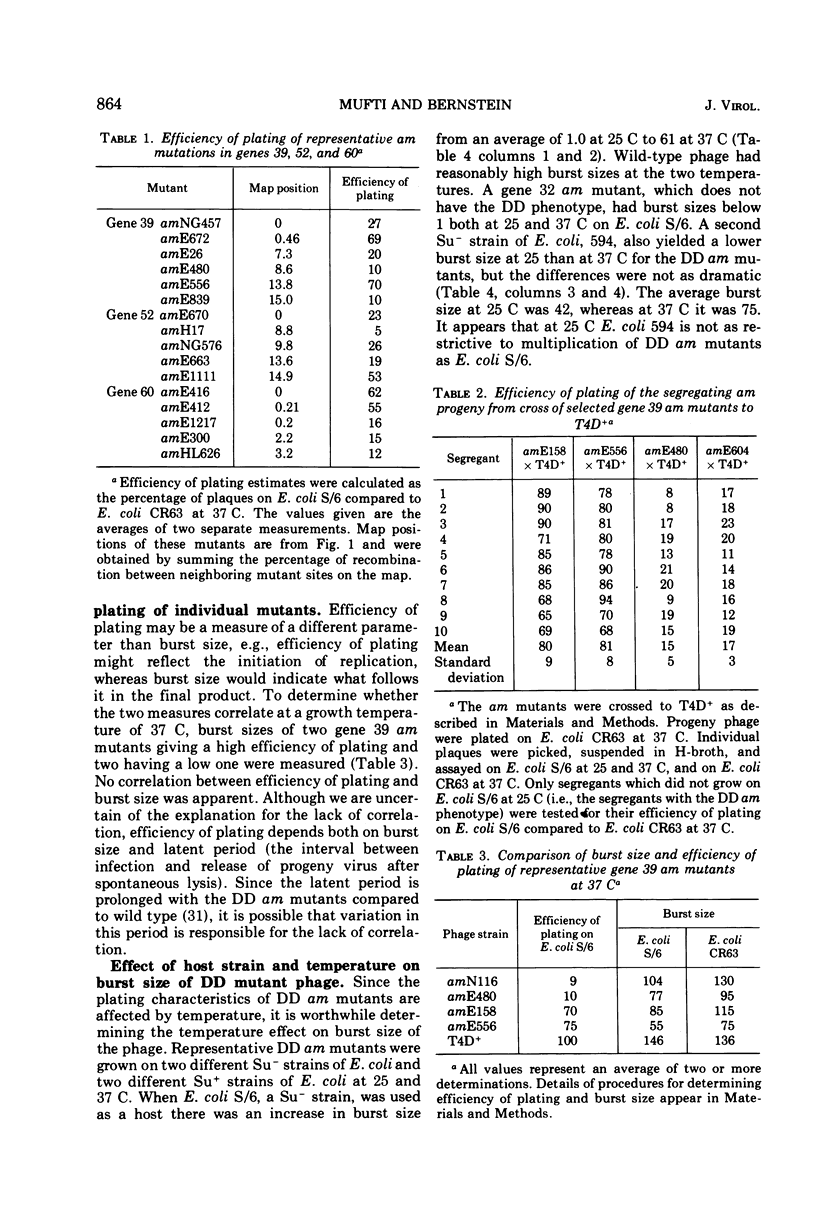
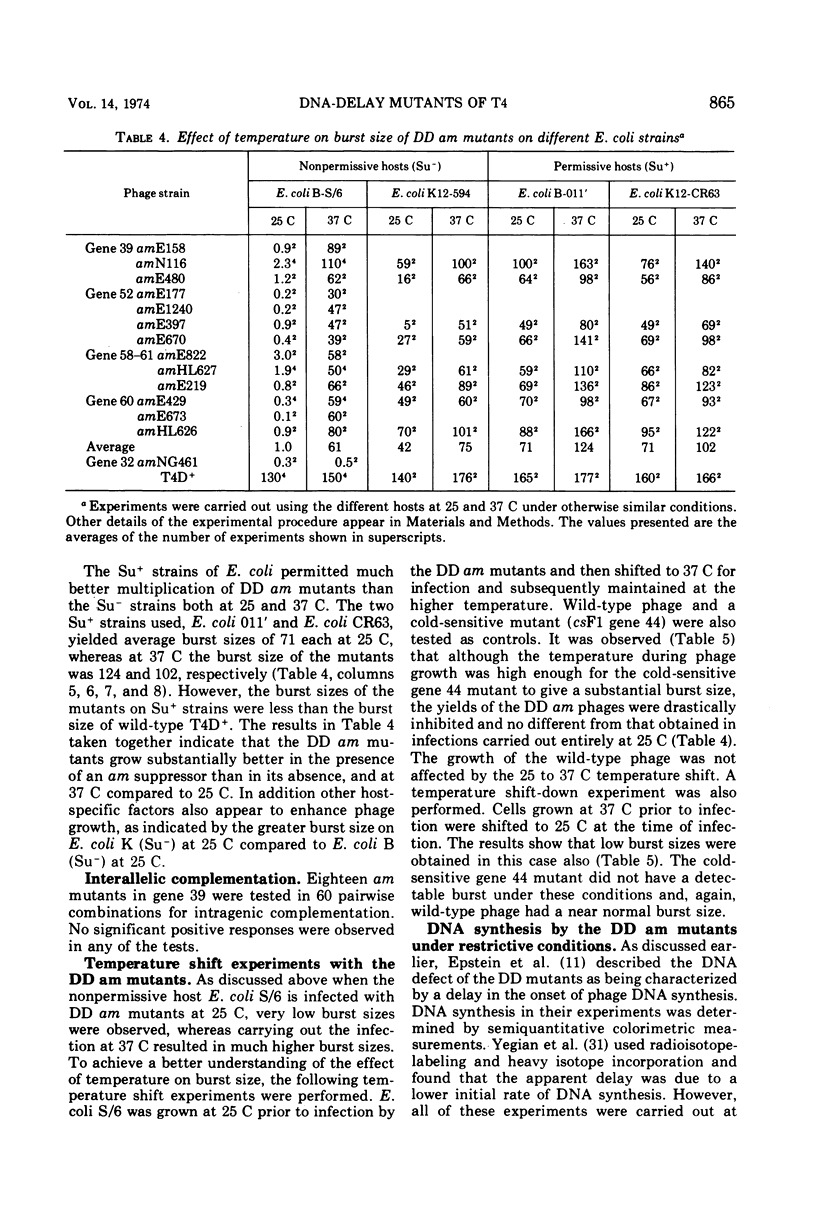
Mutants of phage T4 defective in genes 39, 52, 58-61, and 60 (the DNA delay or DD genes) are characterized by a delay in phage DNA synthesis during infection of a nonpermissive Escherichia coli host. Amber (am) mutants defective in these genes yield burst sizes varying from 30 to 110 at 37 C in E. coli lacking an am suppressor. It was found that when DD am mutants are grown on a non-permissive host at 25 C, rather than at 37 C, phage yield is reduced on the average 61-fold. At 25 C incorporation of labeled thymidine into phage DNA is also reduced to 3 to 10% of wild-type levels. Mutants defective in the DD genes were found to promote increased recombination as well as increased base substitution and addition-deletion mutation. These observations indicate that the products of the DD genes are necessary for normal DNA synthesis. The multiplication of the DD am mutants on an Su− host at 37 C is about 50-fold inhibited if prior to infection the host cells were grown at 25 C. This suggests that a compensating host function allows multiplication of DD am mutants at 37 C in the Su− host, and that this function is active in cells grown at 37 C prior to infection, but is inactive when the prior growth is at 25 C. Further results are described which suggest that the products of genes 52, 60, and 39 as well as a host product interact with each other.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD R. K., MCGREGOR J. F., BAIRD K. M. Mutation to extended host range and the occurrence of phenotypic mixing in the temperate coliphage lambda. Virology. 1956 Aug;2(4):565–574. doi: 10.1016/0042-6822(56)90012-5. [DOI] [PubMed] [Google Scholar]
- Berger H., Warren A. J., Fry K. E. Variations in genetic recombination due to amber mutations in T4D bacteriophage. J Virol. 1969 Feb;3(2):171–175. doi: 10.1128/jvi.3.2.171-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. Reversion of frameshift mutations stimulated by lesions in early function genes of bacteriophage T4. J Virol. 1971 Apr;7(4):460–466. doi: 10.1128/jvi.7.4.460-466.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- CHAMPE S. P., BENZER S. Reversal of mutant phenotypes by 5-fluorouracil: an approach to nucleotide sequences in messenger-RNA. Proc Natl Acad Sci U S A. 1962 Apr 15;48:532–546. doi: 10.1073/pnas.48.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines and the delay in deoxyribonucleic acid synthesis in some bacteriophage T4 infections. J Virol. 1971 Dec;8(6):925–927. doi: 10.1128/jvi.8.6.925-927.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR R. S., EPSTEIN R. H. Inactivation by ultraviolet light of an acriflavine-sensitive gene function in phage T4D. Science. 1961 Aug 4;134(3475):327–328. doi: 10.1126/science.134.3475.327. [DOI] [PubMed] [Google Scholar]
- Ennis H. L., Kievitt K. D. Association of the rIIA protein with the bacterial membrane. Proc Natl Acad Sci U S A. 1973 May;70(5):1468–1472. doi: 10.1073/pnas.70.5.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Guttman B. S., Begley L. Evidence for a magnesium pump induced by bacteriophage T4. Virology. 1968 Dec;36(4):687–690. doi: 10.1016/0042-6822(68)90203-1. [DOI] [PubMed] [Google Scholar]
- Haest C. W., de Gier J., van Deenen L. L. Changes in the chemical and the barrier properties of the membrane lipids of E. coli by variation of the temperature of growth. Chem Phys Lipids. 1969 Dec;3(4):413–417. doi: 10.1016/0009-3084(69)90048-6. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Yanofsky C. Restoration of enzymic activity by complementation in vitro between mutant alpha subunits of tryptophan synthetase and between mutant subunits and fragments of the alpha subunit. J Biol Chem. 1969 Sep 10;244(17):4539–4546. [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Michels C. A., Zipser D. Mapping of polypeptide reinitiation sites within the beta-galactosidase structural gene. J Mol Biol. 1969 May 14;41(3):341–347. doi: 10.1016/0022-2836(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Mosig G., Bowden D. W., Bock S. E. coli DNA polymerase I and other host functions participate in T4 DNA replication and recombination. Nat New Biol. 1972 Nov 1;240(96):12–16. doi: 10.1038/newbio240012a0. [DOI] [PubMed] [Google Scholar]
- Naot Y., Shalitin C. Defective concatemer formation in cells infected with deoxyribonucleic acid-delay mutants of bacteriophage T4. J Virol. 1972 Oct;10(4):858–862. doi: 10.1128/jvi.10.4.858-862.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Shalitin C. Role of gene 52 in bacteriophage T4 DNA synthesis. J Virol. 1973 Jun;11(6):862–871. doi: 10.1128/jvi.11.6.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma D. H., Snyder M. The genetic constitution of tandem duplications on the rII of bacteriophage T4D. Genetics. 1973 Feb;73(2):161–183. doi: 10.1093/genetics/73.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. F., Kievitt K. D., Ennis H. L. Membrane protein synthesis after infection of Escherichia coli B with phage T4: the rIIB protein. Virology. 1972 Nov;50(2):520–527. doi: 10.1016/0042-6822(72)90403-5. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabhai A., Brenner S. A mutant which reinitiates the polypeptide chain after chain termination. J Mol Biol. 1967 Jul 14;27(1):145–162. doi: 10.1016/0022-2836(67)90357-9. [DOI] [PubMed] [Google Scholar]
- Silver S. Acridine sensitivity of bacteriophage T2: a virus gene affecting cell permeability. J Mol Biol. 1967 Oct 14;29(1):191–202. doi: 10.1016/0022-2836(67)90190-8. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Vallée M., Cornett J. B. A new gene of bacteriophage T4 determining immunity against superinfecting ghosts and phage in T4-infected Escherichia coli. Virology. 1972 Jun;48(3):777–784. doi: 10.1016/0042-6822(72)90161-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Weintraub S. B., Frankel F. R. Identification of the T4rIIB gene product as a membrane protein. J Mol Biol. 1972 Oct 14;70(3):589–615. doi: 10.1016/0022-2836(72)90561-x. [DOI] [PubMed] [Google Scholar]
- Yegian C. D., Mueller M., Selzer G., Russo V., Stahl F. W. Properties of the DNA-delay mutants of bacteriophage T4. Virology. 1971 Dec;46(3):900–919. doi: 10.1016/0042-6822(71)90090-0. [DOI] [PubMed] [Google Scholar]



