Abstract
Background and the purpose of the study
The cervico-vaginal mucosa which is populated with microflora (mostly includes lactobacilli) is the portal of entry for sexually transmitted pathogens.
Methods
The in vitro anti-viral effect of vaginal and non-vaginal lactobacillus was evaluated using single cycle HIV-1 replication and HSV-2 plaque reduction assays. The XTT proliferation assay was used to monitor the cellular toxicity. The in vivo anti-HSV-1 activity was evaluated in BALB/c mouse model by monitoring skin lesion and immune response development.
Results and major conclusion
DMEM culture supernatant of L. Gasseri and L. fermentum (PH 7.3) did not show toxic effect but inhibited 50% of HIV replication at 12 and 31% concentrations, respectively. Co-culture of L. gasseri (1000 CFU/ target cell) showed mild cytotoxicity but inhibited 68% of HIV replication. The supernatant of L. crispatus inhibited 50% of HSV replication at 4% and also co-culture of L. gasseri, L. rhamnosus and L. crispatus revokes almost all of the HSV multiplication. Culture supernatants of L. gasseri and L. crispatus had significant virucidal effect against the HIV and HSV and inhibited HSV infection in a stage before viral entry to the target cells. Alive L. gasseri cells showed high potential for inhibiting HSV-1 infection in vivo condition. Current data indicates that lactobacilli supernatant encompasses components with neutralizing activity against HIV and HSV and it would be a determinant factor for viral diseases transmission and promising lead for anti-viral probiotic design.
Keywords: HIV, HSV, Sexually transmitted disease, Lactobacillus
Introduction
The cervico-vaginal mucosa is the portal of entry for sexually transmitted pathogenic microorganisms. In child-bearing age healthy females, the vaginal protective mucosa is populated with microflora that mostly includes lactobacilli. Vaginal health is positively associated with dominance of lactobacilli over pathogenic anaerobes [1]. Bacterial vaginosis (BV) is a situation with an imbalance of the vaginal microbial flora and is not caused by specific pathogenic microorganisms [2]. Reduction, absence or lack of antimicrobial properties (production of acid and H2O2) of lactobacilli and their replacement by anaerobic microbes such as Gardnerella vaginalis may be seen in BV [3].
Increasing data indicate that lacking lactobacilli or abnormal vaginal flora facilitates the transmission of viral sexually transmitted diseases (STD) [4]. There are some data reporting that HIV seropositivity correlates with BV independent of other behavior variabilities [5,6]. Recent prospective investigations demonstrated the association between vaginal flora alterations and the acquisition of Human immunodeficiency virus (HIV) infection [7]. Also, reduction in vaginal flora lactobacillus content was identified as a predisposing factor for infection of human papillomavirus (HPV) and herpes simplex virus type (HSV) [8-10]. Recent studies have revealed that abnormal vaginal flora triggers the shedding of HSV and cytomegalovirus (CMV) in women genital tract [11,12]. On the other hand genital herpes infection is a major risk factor for acquisition and transmission of HIV via sexual contact [13,14]. Moreover, HIV copy number in female genital-tract discharge inversely correlates with lactobacilli counts in bacterial flora [15].
Therefore lactobacilli exhibit an important role for viral infections in both the female health protection as well as reducing the risk of infection transmission to a healthy man. In vitro studies have disclosed that hydrogen peroxide production by L. acidophilus strain displays a virucidal effect against HIV-1 [16]. Additionally, it has been reported that pre-incubation with different lactobacillus strains reduces the infection titer of vesicular stomatitis virus (VSV) [17]. However the inhibition mechanism of lactobacilli against viral infections is poorly understood, but nevertheless metabolic products such as lactic acid, H2O2 and bacteriocins are possible mediators to account for the protection against viruses. These factors might act against viral particles by inactivation, epithelial cell attachment competition, mucin gel preservation or maintaining the appropriate innate immune response [18-20].
In spite of the clinical and epidemiological studies, the in vitro anti-viral activity of these probiotic bacteria in cell culture environment has not been investigated in detail. The purpose of this study is to evaluate anti-HIV and HSV activity of vaginal lactobacilli in vitro and in vivo conditions and determine the possible mode of action.
Materials and methods
Lactobacilli strains and growth condition
Vaginal lactobacilli strains; L. crispatus ATCC33820, L. gasseri ATCC33323, L. rhamnosus GG and L. fermentum ATCC14931 were used in this study as well as the non-vaginal ones; L. acidophilus LA-5 (Hansen company, USA), L. paracasei subsp. Casei ATCC25302, L. acidophilus NCFM and L. casei CRL431 (Hansen company, USA). All bacteria were stored at −70°C in 85% de Man- Rogosa-Sharpe (MRS) supplemented with 15% glycerol. Lactobacilli were inoculated from frozen glycerol vials onto MRS broth and incubated at 37°C for 48hs under anaerobic conditions [21]. Plating of serial lactobacilli 10-fold dilutions in MRS-agar was used to determine the colony forming units (CFU) titer. Colony counting was carried out after 48hs of incubation.
Culture supernatant
Lactobacilli were harvested from fresh MRS culture by centrifuging at 3 × 103 g and the pellet was washed with DMEM twice to eliminate the remnants MRS. Working stokes were prepared in DMEM (2 × 109 CFU/ml) and stored at 4°C. These working stokes were freshly (no more than 2 weeks) used for experiments. To prepare the culture supernatant (CS), lactobacilli (3.6 × 108 CFU) were inoculated onto each well of 6-wells plates containing 6 ml of high glucose DMEM and incubated for 20hs in a CO2 incubator. Culture supernatant (neutral pH phase) was harvested by clarification of the medium with 0.22 μm filters and used freshly for each experiment.
Cell culture and transfection
HEK293T (Human emberionic kidney), Hela (Cervix cancer) and vero (African green monkey kidney) cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 5 mM glutamine. The cells medium was supplemented with penicillin (100 IU/ml) and streptomycin (100 μg/ml) in particular experiments. HEK293T cells were transfected using polyfect transfection reagent (Qiagen, USA). Transfection was performed according to the manufacturer’s protocol in 6-wells plates [22].
Viruses
Single cycle replicable (SCR) HIV-1 (NL4-3) and HSV-2 viruses were used in this study. Plasmid mixture (2 μg) of PmzNL4-3 [23], pSPAX.2 and pMD2G was cotransfected into the HEK293T cells to prepare VSV surface glycoprotein (VSVG) pseudotyped SCR HIV-1 [24]. Virus containing supernatant was harvested and pooled 24, 48 and 72hs post transfection.
Vero cells were infected to supply the HSV-2 virus stokes. The Cells were seeded onto 6-wells plates (4.5 × 105 cells/well) and incubated for one day to reach 98% of confluence. One milliliter of virus supernatant was used to infect cells in each well and unbound virions were removed after 2hs. Virus containing supernatants were harvested and pooled every 24hs after infection until day 4.
HIV and HSV virus containing supernatants were clarified using 0.22 μm filters and 10mins centrifuging at 104 g. Viruses were stored at −70°C and assessed for infectious titer using replication and plaque reduction assays.
HIV replication assay
Single cycle replication assay was used to evaluate the inhibitory activity against replication of VSVG psudotyped SCR HIV-1 virions [24,25]. Hela cells were seeded in 96-wells plates (6 × 103 cells/well) and maintained for 24hs in antibiotic free medium. Lactobacilli cells or supernatant were added into the wells 2hs before infection. Cells were infected with SCR HIV-1 virions (600 ng P24) and incubated for 20hs to accelerate the virions adsorption [22]. After virus entry, the cells were washed two times with DMEM to remove unbound viral particles. Cells were fed with antibiotic containing medium and incubated for additional 48 hrs. Plates were centrifuged for 15mins at 3 × 103 g and P24 content of the cells supernatant was evaluated using P24 capture ELISA (Biomerieux, France).
HSV plaque reduction assay
The inhibitory effect of lactobacilli against multiplication of HSV was investigated using plaque reduction assay. Vero cells were placed into the 24-wells culture plates (Nunc, Denmark) at density of 4 × 105 cells per well and incubated for 24hs (in antibiotic free medium) to reach at least 98% of confluence. The cell monolayer was infected with 50pfu of HSV and washed after 1hs to remove unbound virions. The cell monolayer were then overlaid with DMEM supplemented by 1.5% of methylcellulose, 5% FBS and antibiotics. After 72hs, the overlay medium was removed and the cells were washed twice with DMEM and fixed by methanol. The formed plaques were counted after staining with 0.5% crystal violet.
Time-of-addition study
The inhibitory effect of L. gasseri and L. crispatus supernatant against different intervals of HSV replication was examined according to the previously described procedure with some minor modifications [26]. Vero cells monolayer was prepared by seeding the cells into 24-well plates (Nunc, Denmark) as describe above. Then, 20% concentration of L. gasseri and L. crispatus CS were added into the cells environment before (−6 and −2 hs), during (0 hs) or after (2 and 6 hs) time course of HSV-2 infection. Afterwards, the procedures similar to plaque reduction assay section were performed except that cell monolayer was washed twice by DMEM to eliminate the lactobacilli supernatant in pre-infection (−6, −2 and 0hs) groups. In other groups the lactobacilli CS was added after infection (2 and 6hs) and the concentration was kept constant until the end of the test.
Virucidal assay
The direct effect of L. gasseri and L. crispatus CS on HSV and HIV infectivity was evaluated by using virucidal assay. Different concentrations (2, 20 and 50%) of CSs were mixed thoroughly with HSV (5 × 103 pfu) and HIV (1.2 × 103 P24) virions, in final volume of 10 μl. The mixtures were incubated at 37°C for 2hs and then the fresh medium (90 μl) was added to each tube. Residual virus infectivity was determined by plaque reduction and HIV replication assays as described above.
In vivo anti-HSV assay
Animal consisted of pathogen-free, female BALB/c mice with 6–8 weeks of age-average 20 g of weight (purchased from Pasteur Institute of Iran, Iran) were handled according to the guidelines of the national institute of health guide and care for use of laboratory animal, Iran. The skin of BALB/c mice was shaved in right flank and scratched by needle (gauge 22). The naked and scratched skin was infected with 104 pfu of HSV-1 virions. Alive lactobacilli suspension (105 CFU in 20 μl of PBS) was added immediately after infection. Mice were maintained for six weeks and then evaluated for skin lesions and weight loss. The acyclovir cream (5%) was used as positive control. The immune response against HSV was also monitored by extracting the spleen cells and investigating their proliferative response to HSV antigens. Total spleen cells were treated with lysis buffer to remove red blood cells. Cell suspension was adjusted to 5.1 × 106 cells per milliliter and cultured (120 μl) in each well of 96-well plates with 103pfu of inactivated HSV for 72hs. Phytohemagglutinin-A (PHA 7 μg/ml; Gibco, USA) was used as control. The proliferation of the cells was measured by addition of 50 μl of XTT (sodium 3_-[1-(phenylaminocarbonyl)- 3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sulfonic acid; Roche, Germany) into each well. The plates were incubated at 37°C for 2hs and then read at test and references wavelengths of 450 and 630 nm, respectively.
Cytotxicity assay
The toxicity of lactobacilli cells and supernatant for Vero and Hela cells was determined using XTT (Roche, Germany) proliferation assay as previously is described. Vero cells were cultured in 96-wells plates (6 × 103/well) (Nunc, Denmark) contain lactobacilli cells (6 × 107 CFU/ml) and CS. Bacterial cells were removed after 1 hr and antibiotic containing medium was added into the wells. After 72hs, the medium was discarded and the cells were subsequently rinsed with phosphate buffered saline (PBS). To study the effect of lactobacilli cells and supernatant on Hela cells viability, HIV replication assay plates were subjected to the proliferation assay directly after washing cells with PBS. The XTT reagent (40 μl/well) was added into the plates and incubated at 37°C for additional 3hs. The optical densities (OD) were measured using enzyme immunoassay reader (stat fax2100, Awareness, USA) at test and reference wavelengths of 450 nm and 630 nm respectively.
Results and discussion
Anti-viral activity of lactobacilli supernatant
The DMEM culture supernatant (CS) of lactobacilli (pH of 7.3) was used to appraise the overall anti-HIV and HSV activity (Table 1). Virus multiplication was significantly decreased by all lactobacilli CS, although to different extents. L. gasseri, L. fermentum, L. acidophilus and L. crispatus inhibited 50% of HIV-1 virions replication at 12, 31, 46 and 48% concentration, respectively. Parallel experiments were performed to investigate the anti-HSV-2 activity of lactobacilli CS using plaque reduction assay. Lactobacilli CSs showed very high activity for inhibition of HSV virions replication. L. crispatus CS inhibited plaques formation by 50% in the very low concentration (4%) that reveals noteworthy anti-HSV activity of this strain. Other lactobacilli also showed moderate to strong anti-HSV activity. L. gasseri, L. acidophilus,L. rhamnosus and L. casei inhibited the HSV replication with IC50 value of 11, 14, 17 and 19%. L. crispatus showed significant inhibitory activity for HSV but was not effective for inhibition of HIV replication. This finding emphasizes the anti-HSV active components of this strain inhibit HSV in a specific manner.
Table 1.
The antiviral activity of lactobacilli supernatant
|
Lactobacilli strain |
Antiviral activity (%) |
|||
|---|---|---|---|---|
| |
HIV-1 Inhibitory |
HSV-2 Inhibitory |
||
| IC50(v/v) | CC50(v/v) | IC50(v/v) | CC50(v/v) | |
|
L. crispatus |
48.5 ± 4.4 |
NT |
4.2 ± 1.7 |
NT |
|
L. acidophilus (Hansen co.) |
46.3 ± 8.3 |
NT |
>50 |
NT |
|
L. paracasei subsp. Casei |
≥50 |
NT |
43.1 ± 3.6 |
NT |
|
L. rhamnosus |
≥50 |
NT |
17.7 ± 6.3 |
NT |
|
L. fermentum |
31.7 ± 4.1 |
≥50 |
>50 |
≥50 |
|
L. casei (Hansen co.) |
≥50 |
NT |
19.3 ± 1.6 |
NT |
|
L. gasseri |
12.2 ± 3.5 |
47.4 ± 2.1 |
11.2 ± 3.3 |
≥50 |
| L. acidophilus | ≥50 | NT | 14.9 ± 5.2 | NT |
IC50 is a concentration (v/v) which is able to inhibit 50% of virus multiplication; CC50 is a concentration (v/v) which is 50% toxic for target cells; NT, Not toxic.
The cytotoxicity results showed that the CS from vaginal and non-vaginal lactobacilli had no toxicity for target cells. This indicates the safety of these strains and selective activity of their CS for reducing of viral replication rather than cellular proliferation.
Moderate anti-HIV and strong anti-HSV activity of the lactobacilli supernatants demonstrate that the lactobacilli CS encompass molecules with considerable specific activity for inhibition of viral replication. These data also revealed notably difference between the anti-viral activity of vaginal and non-vaginal strains (Table 1). The anti-viral activity of lactobacilli due to disinfectant activity of MRS culture supernatants was previously reported [16,27]. Although in this study the neutral pH lactobacilli supernatant of lactobacilli in DMEM showed no cytotoxicity but significant anti-viral activity implying the presence of active compounds rather than disinfectant activity.
The anti-viral activity of co-cultured lactobacilli
The potential of lactobacilli for inhibition of HSV-2 and HIV-1 replication was studied by co-culturing the lactobacilli with cells. Lactobacilli were added into the cells environment considering physiological concentration in vaginal environment (100 CFU/cell) one hour before infection and removed by washing after viral adhesion to the cells. Antibiotics were added to the medium after infection to eradicate any growth of bacteria after viral entry. The cytotoxicity of lactobacilli was investigated in a parallel experiment. The vaginal lactobacilli strains (L. gasseri, L. crispatus and L. rhamnosus) showed higher potential for inhibition of HSV virion (Figure 1). L. gasseri blocked almost all of HSV virions infection however L. crispatus and L. rhamnosus inhibited this virus by 93 and 84 percent, respectively. Approximately 68 and 31% of HIV inhibition was observed by L. gasseri and L. fermentum co-culture, whereas little inhibition was shown by other lactobacilli strains investigated in this study. Our results demonstrated that presence of vaginal lactobacilli significantly reduces viral entry to the target cells although the non-vaginal ones were apparently lesser potent. All of investigated vaginal strains were active for blocking HSV however only two strains (L. gasseri and L. fermentum) had anti-HIV activity.
Figure 1.
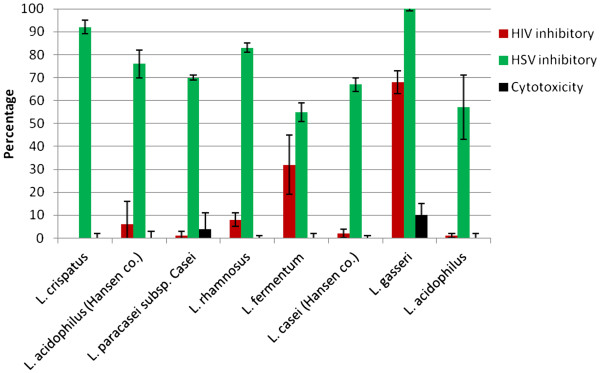
The anti-viral potential of lactobacilli co-culture against HSV-2 and HIV infection in co-cultures. Lactobacilli were added to the cells environment one hour before infection and removed by washing after viral adhesion to the cells. Cells were fed with antibiotic containing medium after infection.
Mode of anti-viral activity
Acording to the virucidal assay L. crispatus and L. gasseri inactivated 74 and 24%of HSV virions in 50% concentration, respectively (Table 2). The remarkable virucidal activity of lactobacilli CS is in consistent with the protective effect of lactobacilli against HSV and HIV virion in co-culture condition (Figure 1), suggesting that lactobacilli may secrete bacteriocins or other sort of molecules with viral neutralization activity. This mode of anti-viral activity is rather different from previously reported ones (due to H2O2 and H+ secretion) [27] since CS used in this study was in neutral pH and had no toxic effect for target cells.
Table 2.
The virucidal effect of lactobacilli supernatant
|
Lactobacillus Strain |
HIV virucidal effect (%) |
HSV-2 virucidal effect (%) |
||||
|---|---|---|---|---|---|---|
| 2%v/v | 20%v/v | 50%v/v | 2%v/v | 20%v/v | 50%v/v | |
|
L. crispatus |
1.1 ± 0.5 |
8.5 ± 0.6 |
14.3 ± 3.3 |
15.6 ± 8.7 |
28.6 ± 3.9 |
76.4 ± 7.2 |
|
L. gasseri |
4.3 ± 1.7 |
7.3 ± 1.5 |
15.4 ± 1.4 |
0.7 ± 0.2 |
6.3 ± 1.7 |
24.7 ± 3.2 |
| L. rhamnosus | 0.1 ± 0.02 | 2.1 ± 0.09 | 11.8 ± 0.01 | 4.2 ± 0.9 | 15.6 ± 2.1 | 38.3 ± 4.5 |
The culture supernatants of L. gasseri and L. crispatus (20%) were added at different intervals (before, during, and after) of HSV infection. The results showed that L. gasseri and L. crispatus CS restrain HSV infection when added −6 and -2hs before virion inoculation by 75 and 83%, respectively (Figure 2). However, the inhibitory rate declined to 40 and 65% or less when L. gasseri and L.crispatus CSs and virions were simultaneously added. This data indicated that the lactobacilli supernatants affect the initial stage of HSV-2 infection. The lactobacilli products may inhibit HSV through disturbing the adhesion of virions to the cells or neutralizing the viral particles. This is a fairly new proposed mode of anti-viral activity for lactobacilli which is different from held belief that lactobacilli inactivate viral particles by lowering PH and disinfectant agent secretion [27].
Figure 2.
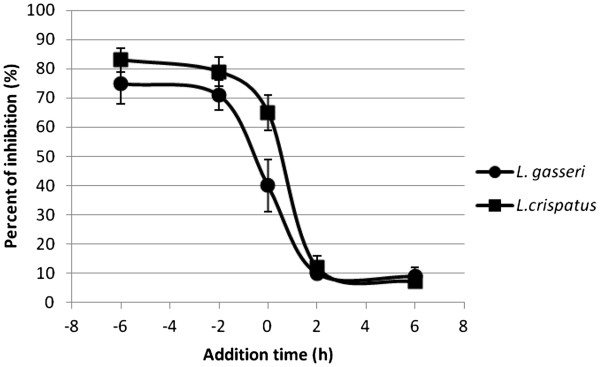
The inhibitory effect of L. gasseri and L. crispatus culture supernatants against different stages of HSV-2 infection. Culture supernatants (20%v/v) were added at different periods (before, during, and after) of HSV infection. The cells were washed twice by DMEM to eliminate lactobacilli supernatants prior to the inoculation of virions for pre-infection (−6, −2 and 0 h) groups.
The in vivo anti-HSV activity
The anti-HSV-1 activity of L. crispatus and L. gasseri, strains with highest potential in vitro experiments, was studied in BALB/c mice model. Alive L. gasseri cells showed significant activity for protecting mice against HSV-1 virions infection. As it can be seen in Figure 3, L. gasseri treated mice raised normal hairs while comparative hair loss can be seen in L. crispatus and control groups. The 6.5 and 8.8 g of weight loss was just observed in mice which received L. crispatus or no treatment, respectively (Table 3). To prove the inhibitory activity of L. gasseri against HSV infection the lymphocyte cells were extracted from mice spleen and evaluated for proliferation response to HSV antigens. Spleen cells from Acyclovir and L. gasseri treated mice showed no response to viral antigens with stimulation index (SI) values of 1.01 and 1.05 (Table 3). These data indicates that immune cells were not exposed to HSV antigens which unequivocally show inhibition of establishment of infection. The findings of this study lead to the conclusion that L. gasseri cells have comparable potential for inhibition of HSV infection in vivo condition to Acyclovir.
Figure 3.
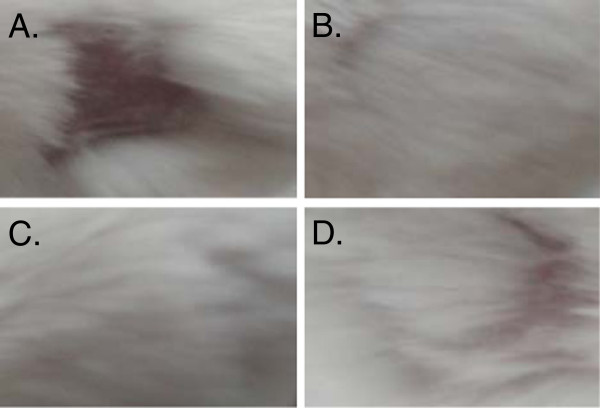
Skin lesions 6 weeks after cutaneous infection of HSV-1 in BALB/c mice. The shaved and scratch skin of right flank was infected with 104pfu of HSV-1 and the development of skin lesions was monitored after 6 weeks. The hair loss in the site of infection was clearly seen in infected mice (A) in comparison with ones treated with acyclovir cream (5%) (B). L. gasseri treated mice raised normal hairs (C) while comparative hair loss can be seen in L. crispatus (D).
Table 3.
The in vivo anti-HSV activity of lactobacilli
|
Lactobacilli strain |
Disease progress |
Immune response |
||
|---|---|---|---|---|
| Weight loss (g) | Hairless area (cm2) | Lymphocyte proliferation (OD) | Stimulation index (SI) | |
|
L. crispatus |
6.5 ± 0.4 |
1.7 ± 0.6 |
1.684 ± 0.43 |
1.9 ± 0.2 |
|
L. gasseri |
0 ± 0.1 |
0.07 ± 0.2 |
0.876 ± 0.12 |
1.05 ± 0.3 |
|
Negative control |
8.8 ± 0.3 |
4.1 ± 0.3 |
1.882 ± 0.62 |
2.1 ± 0.1 |
| Acyclovir | 0 ± 0.2 | 0.09 ± 0.01 | 0.793 ± 0.31 | 1.01 ± 0.2 |
Conclusion
The results of the present study reveal the anti-HSV and HIV effect of lactobacilli by production of anti-viral molecules and other possible mechanisms. Infection was significantly reduced with no cytotoxicity if HSV and HIV were cultured in the presence of living lactobacilli cells. The presence of lactobacilli cells was not necessary for inhibitory activity since virus replication is also inhibited in the presence of neutral pH supernatants. Vaginal lactobacilli strains were able to inhibit the first stages of HSV infection. The anti-viral activity of lactobacilli cells in the midst of herpes virus binding to the target cell was strain-dependent. L. gasseri showed inhibitory activity against HIV virions among investigated lactobacilli in this study. Numerous mechanisms may be involved in the inhibitory effect of lactobacilli against HSV and HIV such as: interfering with the early steps of virus infection, viral particle neutralizing and secretion of compounds with the ability of blocking the intracellular events of virus replication. The in vivo experiment confirmed the anti-viral activity of lactobacilli in mouse models. Potent lactobacilli could be promising probiotic candidates for protection against HSV and HIV or other viruses infections transmission.
Competing interest
The authors who have taken part in this study declare that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Authors’ contributions
RZ: Central contributions to conception, design and performing the experiments. Drafting and revising the manuscript. EM: Substantial contributions to conception, design and a part of experiments. SMS: Substantial contributions to conception, Analysis and interpretation of data and revising the article. ARA-S: Acquisition of data. SA-D: Acquisition of data. MHM: Corresponding author. Analysis and interpretation of data, revising the article. All authors read and approved the final manuscript.
Contributor Information
Rezvan Zabihollahi, Email: rezvan_z_m@yahoo.com.
Elahe Motevaseli, Email: e_motevaseli@tums.ac.ir.
Seyed Mehdi Sadat, Email: mehdi_sadat@yahoo.com.
Ali Reza Azizi-Saraji, Email: alireza_azizi8924@yahoo.com.
Sogol Asaadi-Dalaie, Email: sogol.asaadi@gmail.com.
Mohammad Hossein Modarressi, Email: modaresi@sina.tums.ac.ir.
Acknowledgement
This study was performed in Pasteur institute of Iran. This project was financially supported by Pasteur institute of Iran.
References
- Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- Donders G. Diagnosis and management of bacterial vaginosis and other types of abnormal vaginal bacterial flora: a review. Obstet Gynecol Surv. 2010;65:462–473. doi: 10.1097/OGX.0b013e3181e09621. [DOI] [PubMed] [Google Scholar]
- Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther. 2008;30:453–468. doi: 10.1016/j.clinthera.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Brotman RM, Erbelding EJ, Jamshidi RM, Klebanoff MA, Zenilman JM, Ghanem KG. Findings associated with recurrence of bacterial vaginosis among adolescents attending sexually transmitted diseases clinics. J Pediatr Adolesc Gynecol. 2007;20:225–231. doi: 10.1016/j.jpag.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CR, Duerr A, Pruithithada N, Rugpao S, Hillier S, Garcia P, Nelson K. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS. 1995;9:1093–1097. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/S0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpes TL, Meyn LA, Krohn MA, Hillier SL. Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora. Sex Transm Dis. 2003;30:405–410. doi: 10.1097/00007435-200305000-00006. [DOI] [PubMed] [Google Scholar]
- Watts DH, Fazzari M, Minkoff H, Hillier SL, Sha B, Glesby M, Levine AM, Burk R, Palefsky JM, Moxley M, Ahdieh-Grant L, Strickler HD. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005;191:1129–1139. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- Nagot N, Ouedraogo A, Defer MC, Vallo R, Mayaud P, Van de Perre P. Association between bacterial vaginosis and Herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm Infect. 2007;83:365–368. doi: 10.1136/sti.2007.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis. 2005;40:1422–1428. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- Ross SA, Novak Z, Ashrith G, Rivera LB, Britt WJ, Hedges S, Schwebke JR, Boppana AS. Association between genital tract cytomegalovirus infection and bacterial vaginosis. J Infect Dis. 2005;192:1727–1730. doi: 10.1086/497150. [DOI] [PubMed] [Google Scholar]
- Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, Magaret AS, Wald A, Corey L, Celum C. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- Sha BE, Zariffard MR, Wang QJ, Chen HY, Bremer J, Cohen MH, Spear GT. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ, Coombs RW. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991;174:289–292. doi: 10.1084/jem.174.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botic T, Klingberg TD, Weingartl H, Cencic A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int J Food Microbiol. 2007;115:227–234. doi: 10.1016/j.ijfoodmicro.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Reid G. Probiotic Lactobacilli for urogenital health in women. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S234–S236. doi: 10.1097/MCG.0b013e31817f1298. [DOI] [PubMed] [Google Scholar]
- Reid G, Dols J, Miller W. Targeting the vaginal microbiota with probiotics as a means to counteract infections. Curr Opin Clin Nutr Metab Care. 2009;12:583–587. doi: 10.1097/MCO.0b013e328331b611. [DOI] [PubMed] [Google Scholar]
- McGroarty JA. Probiotic use of lactobacilli in the human female urogenital tract. FEMS Immunol Med Microbiol. 1993;6:251–264. doi: 10.1111/j.1574-695X.1993.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Martin MC, Pant N, Ladero V, Gunaydin G. Krogh Andersen K, Alvarez B, Martinez N, Alvarez MA, Hammarstrom L. Appl Environ Microbiol: Marcotte H. Integrative expression system for delivery of antibody fragments by lactobacilli; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabihollahi R, Vahabpour R, Hartoonian C, Sedaghati B, Sadat SM, Soleymani M, Ranjbar M, Fassihi A, Aghasadeghi MR, Memarian HR, Salehi M. Evaluation of the in vitro antiretroviral potential of some Biginelli-type pyrimidines. Acta Virol. 2012;56:11–18. doi: 10.4149/av_2012_01_11. [DOI] [PubMed] [Google Scholar]
- Rezaei A, Zabihollahi R, Salehi M, Moghim S, Tamizifar H, Yazdanpanahi N, Amini G. Designing a non-virulent HIV-1 strain: potential implications for vaccine and experimental research. Journal of research in medical sciences. 2007;12:227–234. [Google Scholar]
- Zabihollahi R, Sadat SM, Vahabpour R, Aghasadeghi MR, Memarnejadian A, Ghazanfari T, Salehi M, Rezaei A, Azadmanesh K. Development of single-cycle replicable human immunodeficiency virus 1 mutants. Acta virologica. 2011;55:15–22. doi: 10.4149/av_2011_01_15. [DOI] [PubMed] [Google Scholar]
- Sadat SM, Zabihollahi R, Vahabpour R, Azadmanesh K, Javadi F, Siadat SD, Memarnejadian A, Parivar K, Khanahmad Shahreza H, Arabi Mianroodi R, Hekmat S, Aghasadeghi MR. Designing and biological evaluation of single-cycle replicable HIV-1 system as a potential vaccine strategy. 20th European Congress of Clinical Microbiology and Infectious Diseases. Austria: Clinical Microbiology and Infection; 2010. p. S334. [Google Scholar]
- Yang CM, Cheng HY, Lin TC, Chiang LC, Lin CC. Acetone, ethanol and methanol extracts of Phyllanthus urinaria inhibit HSV-2 infection in vitro. Antiviral Res. 2005;67:24–30. doi: 10.1016/j.antiviral.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Conti C, Malacrino C, Mastromarino P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol. 2009;60(Suppl 6):19–26. [PubMed] [Google Scholar]