Abstract
Background
Septimeb is a new herbal-derived remedy, recently approved for its potential immunomodulatory effects. Regarding the key role of immune system in the pathogenesis of severe sepsis and lack of any standard treatment for improving survival of these patients; we evaluated the effect of Septimeb -as an adjutant to standard treatment-on inflammatory biomarkers and mortality rates in patients with severe sepsis.
Methods
In this multicenter, randomized, single-blind trial, we assigned patients with severe sepsis and Acute Physiology and Chronic Health Evaluation (APACHE II) score of more than 20 to receive standard treatment of severe sepsis (control group) or standard treatment plus Septimeb. This group was treated with Septimeb for 14 days then followed up for another14 days. APACHE score, Sequential Organ Failure Assessment (SOFA) and Simplified Acute Physiology Score (SAPS) were calculated daily. Blood samples were analyzed for interleukin 2 tumor necrosis factor-α, total antioxidant power, platelet growth factor and matrix metalloproteinase 2.
Results
A total of 29 patients underwent randomization (13 in control group and 16 in Septimeb group). There was significant difference between the Septimeb and control group in the 14 days mortality rate (18.8% vs. 53.85 respectively, P=0.048). Compared to control group, Septimeb was significantly effective in improving SAPS (P= 0.029), SOFA (P=0.003) and APACHE II (P=0.008) scores. Inflammatory biomarkers didn’t change significantly between the two groups (P>0.05).
Conclusion
Septimeb reduces mortality rates among patients with severe sepsis and it could be added as a safe adjutant to standard treatment of sepsis.
Keywords: Severe sepsis, Immunomodulation, Septimeb, ICU
Introduction
Sepsis is one of the most prevalent and fatal diseases in Intensive Care Units (ICU) resulted in 30-60% mortality [1,2]. Sepsis is a systemic inflammatory response to an infection leading to endothelial dysfunction, impairment of microcirculation, tissue hypoxia, apoptosis and finally multiple organ failure and death [3]. Nowadays the role of inflammatory cytokines, oxidative stress and immune system in the pathogenesis of sepsis is obvious. Continuous elevated levels of various cytokines in severe sepsis could result in uncontrolled inflammation [4-7]. Breaking of inflammatory cascade may lead to improving of survival [8]. A significant association exists between inflammatory cytokine levels in the first 72 hours of severe sepsis and severity of tissue hypoxia, organ dysfunction and mortality [9]. IL-6 and Acute Physiology and Chronic Health Evaluation (APACHE) II score are used respectively in prediction of mortality and clinical evaluation of severe septic patients [10]. Therefore modulation of inflammation and immune system response could be one of the strategic plans to manage sepsis.
Several immunomodulatory therapies have been evaluated in sepsis. Corticosteroids [11,12], anti Tumor Necrosis Factor α (TNF α) antibodies [13,14], Anti Interleukin 1 antibodies [15], Platelet Aggregating Factor (PAF) antagonist [16,17], antioxidants especially selenium [18,19], modulation of nutrition [20], modulation of coagulation [21] and complement pathway [22] are examples of immunotherapy in sepsis, but none of them were effective on survival of patients. Procedures such as plasmapheresis and hemofiltration slightly improve prognosis of sepsis by reducing circulating levels of inflammatory cytokines but couldn’t decrease mortality rate [23].
Analysis of current immunomodulating strategies indicates that monovalent approaches in isolation are unlikely to restore immunostasis or attain status of complete therapy. It is likely that multiple immunomodulating strategies will be necessary to achieve clinical success owing to complex interplay between pathways. Botanical extracts provide cytoprotective, antiinflammatory, and antimicrobial activities in addition to immunoregulatory activity [24].
Septimeb (a more potent form of IMODTM) is a herbal extracts including Tanacetum vulgare (tansy), Rosa canina and Urtica dioica (nettle) in addition to selenium, flavonoids and carotenes. Tanacetum vulgare may relieve anti-inflammatory symptoms and Rosa canina defers blood glucose and cholesterol elevation. Extracts from Urtica dioica may prevent maturation of myeloid dendritic cells and reduce T cell responses [25]. Septimeb can regulate TNF-α, interferon-γ (IFN-γ) and IL-2 [26]. IMOD has been patented in Europe with code of WO/2007/087825 for its immunomodulator and anti-TNF-α capacities and improving CD4 in HIV positive patients [27,28]. The pre-clinical safety studies of IMOD in animals and phases I and II trials have been successfully conducted, showing optimistic results [29,30]. As sepsis is an immune mediated disease and modulating of immune system might be effective in improving prognosis and survival, we aim to evaluate Septimeb’s effect on plasma levels of inflammatory biomarkers, and mortality rates of patients with severe sepsis.
Methods
Patient selection
In all cases, informed consents were received from patients or their legal guardian. The study procedure and protocol were approved by the ethical committee of Tehran University of Medical Sciences (TUMS). Our clinical trial was registered in Australian Newzeland Clinical Trial Organization with code number of (ACTRN012607000376448).
Study subjects
Between March 2009 and April 2011, 29 patients with severe sepsis and Acute Physiology and Chronic Health Evaluation (APACHE) scores of more than 20, admitted to general ICU of “Sina” Hospital of TUMS and “Shohada” Hospital of Tabriz University of Medical Sciences were enrolled in this study. Exclusion criteria for all patients were ages below 18 and more than 65 years, pregnancy, breast feeding, over 24 hours of sepsis diagnosis and death probability within 28 days due to nonspecific reasons (such as renal or liver failure).
Patients were randomized via block randomization to receive one of the following treatments: standard treatment for severe sepsis (control group) and standard treatment plus Septimeb (intervention group) for 14 days and were also followed up for another 14 days. According to latest international guidelines [31], standard treatments for severe sepsis were implemented as following: early resuscitation within the first 6 hours of admission, appropriate diagnosis studies to ascertain causative organisms before starting antibiotics, early administration of broad spectrum antibiotic therapy, hypotension control with avasopressor, fluid administration to target Central Venous Pressure (CVP) of 8–12 mmHg and central venous oxygen saturation (Sa O2) > 95%, low dose steroid, glycemic control(blood glucose <150 mg/dl), target hemoglobin values of 7–9 g/dl in absence of coronary artery disease or acute hemorrhage, lung protection ventilation and standard prophylactic measures for deep vein thrombosis (DVT) and Stress-related Mucosal Damage (SRMD).
Patients in intervention group received 240mg (4 ml) of Septimeb in 150 ml of DW 5%, infused over 1.5 hours on the first day. All hemodynamic data were monitored during the infusion and in case of any negative hemodynamic deterioration, dermal rash, urticaria or anaphylactic reaction, the infusion discontinued. Then 360mg (6 ml) of the drug was infused in 150 ml of DW5%, everyday for 14 days. The time gap between the beginnings of treatment with intervention and the diagnosis of sepsis was also noted to evaluate possible impact of the onset of intervention on mortality.
Demographic data and clinical information were obtained at the beginning (Table 1). APACHE scores, sequential organ failure assessment (SOFA), and simplified acute physiology score (SAPS) were calculated daily. Higher scores indicate more severe illness and a higher number of therapeutic interventions.
Table 1.
Demographic and baseline characteristic of patients
| Control group (N:14) | Septimeb group (N:16) | P value | |
|---|---|---|---|
| Age (year) |
45.69 ± 20.67 |
39.56 ± 16.46 |
0.38 |
| Male, No. |
10 |
15 |
0.18 |
| APACHE II |
24.76 ± 4.14 |
27.56 ± 7.39 |
0.23 |
| SOFA |
8.92 ± 1.97 |
9.25 ± 2.32 |
0.69 |
| SAPS | 47.69 ± 11.61 | 43.68 ± 11.21 | 0.35 |
Data are mean ± SD. APACHE: Acute Physiologic And Chronic Health Evaluation. SAPS: Simplified Acute Physiologic Score. SOFA: Sequential Organ Failure Assessment.
Mortality during ICU stay and mortality during 14 days were recorded. The standard treatment of sepsis was continued throughout ICU stay of patients.
Sample collection and handling
All patients had central venous catheters and arterial lines for blood sampling. For all patients there were seven scheduled time points to assess IL-2, TNF-α, total antioxidant power (TAP), Matrix Metalloproteinase2 (MMP2) and Platelet Growth Factor (PGF). The levels were analyzed via commercially available enzyme-linked immunosorbent assay kit (BenderMed system, Vienna, Austria). The first sample was taken on ICU admission and prior to initiation of the therapy. Other samples were obtained on days of 1, 2, 3, 7, 10, and 14 post therapies. Blood samples were collected into vacutainer tubes containing EDTA.
The samples were spun at 3000 × g for 15 minutes in order to remove cells and cellular debris. The cell free supernatant and plasma were stored at −80°C until the time of analysis.
Statistical analysis
All data were expressed as mean ± SD. Univariate comparisons of baseline characteristics were performed by the independent student’s t-test for parametric data and Mann-Whitney’s t-test for non-parametric data. To assess differences between the time points in each treatment group, repeated-measure analysis of variance was done to analyze changes in levels of the biomarkers. Pearson’s chi square and Kaplan-meier (Log rank) tests analyzed the differences of survival rates among the two groups. P-values less than 0.05 were considered statistically significant.
Result
Thirty patients with severe sepsis and APACHE scores of over 20 were enrolled in this study (13 patients in control group and 16 in intervention group). Demographic and baseline values for APACHE, SOFA and SAPS scores have been summarized in Table 1. The differences in age and sex were not significant between two groups (P= 0.38 and 0.18, respectively). Differences in baseline values for APACHE, SOFA and SAPS scores were not statistically significant either (Table 1).
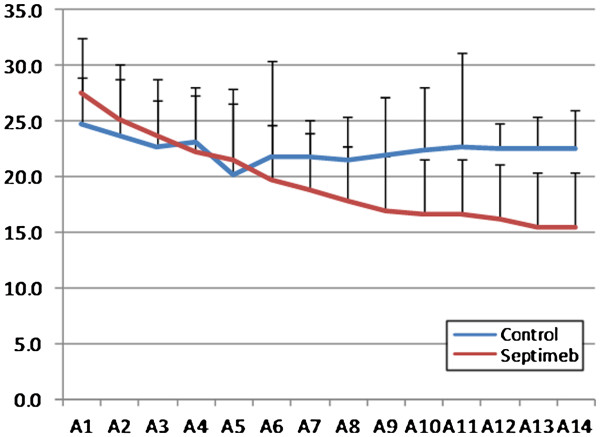
Compared to controls, Septimeb was effective in improving APACHE (P= 0.229), SOFA (P=0.335) and SAPS (P= 0.059) scores in 14 days but the trend was not statistically significant (Figures 1, 2 and 3). But the score differences between the 14th and first day by LOCF method (Last Observation Carried Forward), showed that adding Septimeb was significantly more effective than standard treatment (Table 2).
Figure 1.
Changes of patients APACHE II score in different days of study. Difference between two groups is not significant (P= 0.266). A: APACHE (Acute Physiologic And Chronic Health Evaluation) 1–14: day 1–14.
Figure 2.
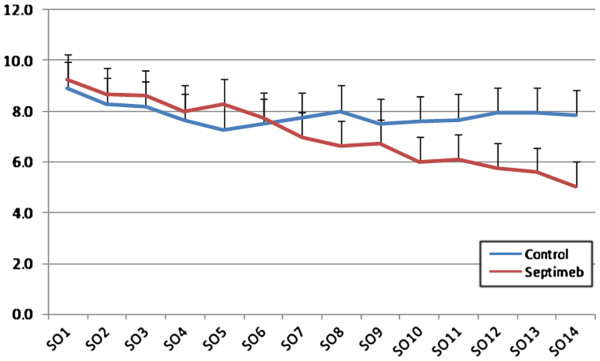
Changes of patients SOFA score in different days of study. Difference between two groups is not significant (P= 0.335). SO: SOFA (Sequential Organ Failure Assessment) 1–14: day 1–14.
Figure 3.
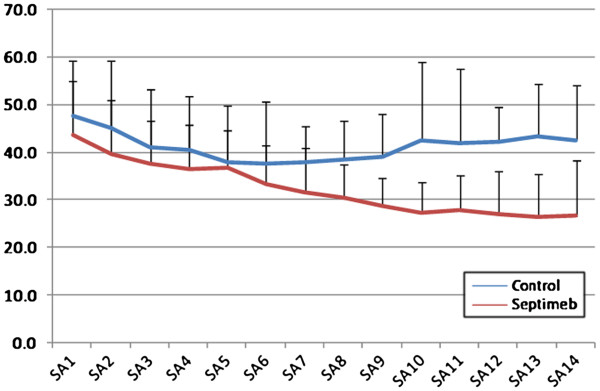
Changes of patients SAPS score in different days of study. Difference between two groups is not significant (P= 0.059). SA: SAPS (Simplified Acute Physiologic Score) 1–14: day 1–14.
Table 2.
Change in patient's APACHE, SAPS and SOFA score from 1st day to 14th day study
| Control group (N:14) | Septimeb group (N:16) | P value | |
|---|---|---|---|
| APACHE mean difference between 14th and first day±SD |
−1.7(±9.8) |
−12.7(±7.9) |
0.003 |
| SOFA mean difference between 14th and first day |
−0.5(±2.3) |
−4(±3.3) |
0.013 |
| SAPS mean difference between 14th and first day | −3(±12.9) | −18(±16.6) | 0.003 |
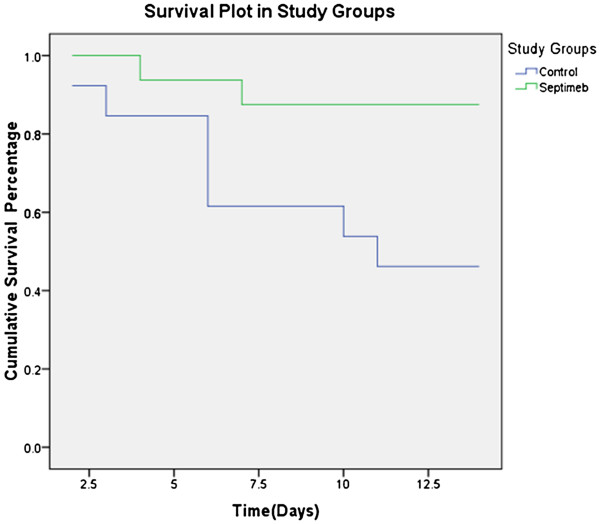
The mean survival time was 9.84 (7.32-12.36, CI: 95%) days in control group and 12.93 (11.49-14.3, CI: 95%) days in Septimeb group. The difference in survival time was significant (P=0.019) (Figure 4). In the intervention group, the mortality rate was 18.8% during 28 days while it was 53.8% in control group, the difference was statistically significant (P=0.048).
Figure 4.
Changes of patient’s survival during study period. The differences in survival time was significant (P=0.019).
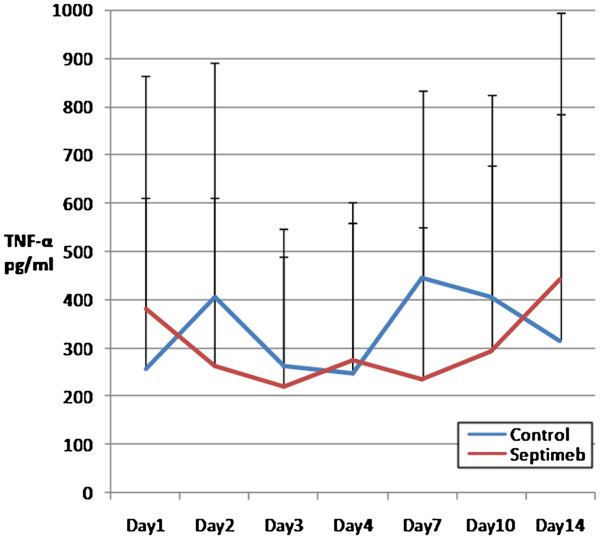
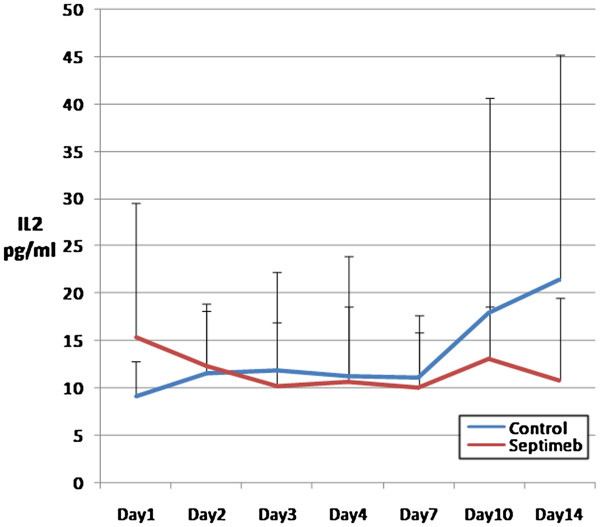
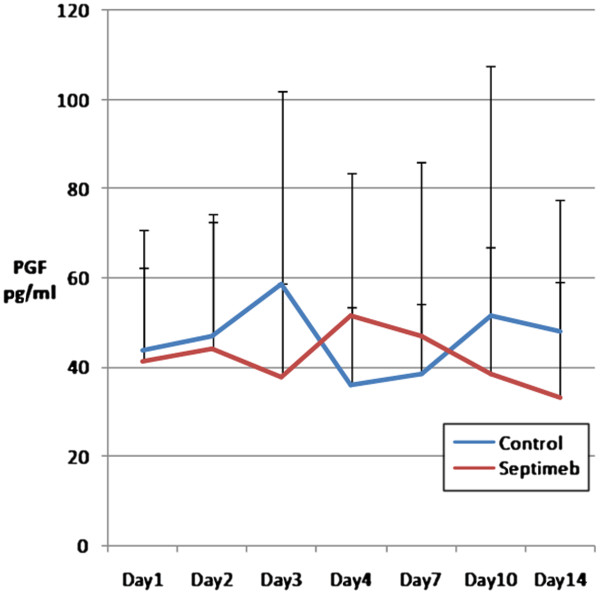
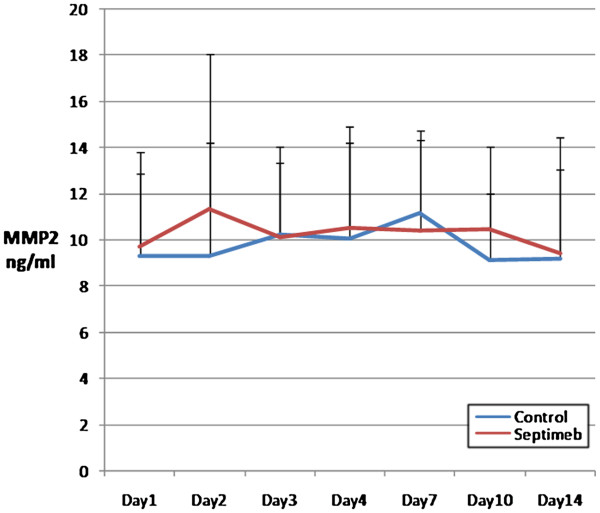
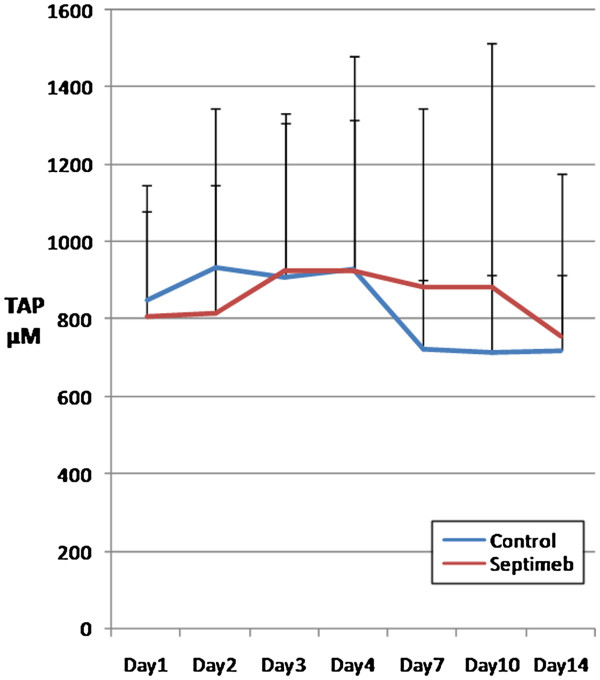
The changes in the levels of biomarkers including IL-2 (P= 0.35), TNF- α (P= 0.66), TAP (P= 0.92), MMP 2 (P= 0.62) and PGF (P= 0.45) were not different among two groups (Figures 5, 6, 7, 8 and 9). None of the time points (0,1, 2, 3, 7,10 and 14 days) showed significant differences between two groups (p>0.05).
Figure 5.
Changes in blood TNF-α level in different days of study. Data are mean ± SE. **Difference between two groups is not significant at P>0.05.
Figure 6.
Changes in blood IL-2 level in different days of study. Data are mean ± SE. **Difference between two groups is not significant at P>0.05.
Figure 7.
Changes in blood PGF levels in different days of study. Data are mean ± SE. **Difference between two groups is not significant at P>0.05.
Figure 8.
Changes in blood MMP-2 level in different days of study. Data are mean ± SE. **Difference between two groups is not significant at P>0.05.
Figure 9.
Changes in blood TAP level in different days of study. Data are mean ± SE. **Difference between two groups is not significant at P>0.05.
Discussion
The results of this study show that Septimeb, as an adjutant to standard treatment of severe sepsis could reduce mortality rates in patients, but has not significant anti-inflammatory effects.
Despite great advancements in understanding the pathophysiology of sepsis and development of novel therapeutic approaches, mortality of sepsis remains unacceptably high [32]. As mentioned earlier, immune system and inflammatory cytokines have a key role in pathogenesis of sepsis and several studies evaluated the effect of different immunomodulatory treatment on severe sepsis, but fail to improve survival of patients [11-19,21-23,33]. Among immunomodulatory agents, the activated protein C and corticosteroids are the only approved treatment for sepsis. According to the results of PROWESS trial [34,35] and a Meta-analysis [36] about the ineffectiveness of activated protein C on survival of patients with sepsis, Food and Drug Administration (FDA) withdrew this drug from the market and therefore currently there is no specific treatment for severe sepsis.
Previous studies have shown that monovalent therapies could not restore immunostasis in severe sepsis, so the need of drugs with polyvalent activities such as botanical immune-drugs seems inevitable [24,37]. Botanical immunodrugs e.g. Chinese remedy and green tea have been found to have dose-dependent attenuate bacterial endotoxin-induced HMGBI release [38,39]. Septimeb is a new herbal-derived remedy that has been suggested to be able to stimulate lymphocytes in order to produce cytokines in HIV positive patients and also inhibit TNF alpha and increase interferon gamma and Interleukin 2 [40,41]. The phase I and II trial studies with IMODTM shows this drug is safe and has no mutagenic or allergenic potentials. Based on the results of this study, Septimeb (a more potent form of IMOD) reduced APACHE, SOFA and SAPS scores, as quantitative indices of clinical status [29,30]. Improvements of these scores by Septimeb confirm the potential of this drug in alleviation of the severity of sepsis and consequently reduced mortality rates of patients.
The results of previous study [30] did not show any improvement in survival of septic patients, but in the current study Septimeb decreased mortality rate significantly, this could be because of larger sample size in this study.
Immunotherapy must be applied early during the development of immune-dysfunction following trauma or acute onset of infection, to ensure that all cellular components of immune system will be protected. There is a much earlier temporal relationship between inflammation and the onset of organ dysfunction. Mean biomarker levels during 72 hours were significantly greater in hospital non-survivors [42]. Supporting evidence showed that the initial inflammatory response directly correlates to early, but not late sepsis mortality [8]. A complex interaction of cytokines and cytokine-neutralizing molecules probably determines the clinical presentation and course of sepsis. Intervening in this sequence of events to modify the host inflammatory responses may be a beneficial treatment strategy for sepsis, but currently tested anticytokine therapies have been largely unsuccessful [20]. Levels of inflammatory biomarkers didn’t change significantly by Septimeb and positive effect of Septimeb on mortality rate might be due to other mechanisms. The main problems in severe sepsis are O2 extraction and O2 consumption. The critical importance of tissue oxygenation was addressed by a study in which patients in the earliest stages of sepsis were treated by aggressive management with fluids, blood transfusion and inotropic agents to optimize hemodynamic function [43]. After macrocirculatory resuscitation, many patients still demonstrate multisystem organ dysfunction and shock. The recent landscape of sepsis research is focusing on two separate mechanisms that may explain the persistence of the shock state. The first is the role of the microcirculation in the body, and the mixed response of various circulatory beds to the sepsis. The second is that the cells and mitochondria receiving adequate perfusion and oxygenation may still have a decreased ability to properly utilize energy to form ATP, referred to as cytopathic hypoxia [44], that can lead to profound metabolic alterations and the inability to supply energy to the tissues. These two represent attractive targets for novel adjunctive resuscitative agents in the treatment of septic shock. Therefore improving tissue oxygenation could be a possible mechanism of Septimeb in reduction of sepsis mortality.
Fortunately, Septimeb showed no adverse effects on coagulation factors like platelet count, prothrombin time, partial thromboplastin time, fibrinogen, and D-dimer. Hemodynamic instability and anaphylactic reactions were not observed during infusion. It could be a safe adjutant to other standard treatments of sepsis [45].
Conclusion
Despite several limitations of this study, especially the small sample size, Septimeb shows positive effects on survival of patients with severe sepsis, considering the withdrawal of activated protein C from markets. Additional studies with adequate sample size is recommended to be performed with Septimeb, but regarding the results and also limited side effects of Septimeb, it could be considered as an adjutant along with other standard measures for treatment of severe sepsis.
Competing interests
The authors have no financial interest to declare. There is no conflict of interest to declare.
Authors’ contribution
EK: study design, patient selection, Sample collection and handling, sample analyzing via commercially available enzyme-linked immunosorbent assay kit, draft the manuscript. MA: study design, patient selection, Sample collection and handling, draft the manuscript and revising it. AH: study design, patient selection, Sample collection and handling, revising manuscript. MA: study design, sample analyzing via commercially available enzyme-linked immunosorbent assay kit, draft the manuscript and revising it. KK: study design, patient selection, Statistical analysis, draft the manuscript and revising it. SM: study design, patient selection, Sample collection and handling, draft the manuscript and revising it. AN: study design, patient selection, Sample collection and handling, draft the manuscript and revising it. MB: sample analyzing via commercially available enzyme-linked immunosorbent assay kit, draft the manuscript and revising it. HH: patient selection, Sample collection and handling, analysis and interpretation of data, draft the manuscript and revising it. LK: patient selection, Sample collection and handling, analysis and interpretation of data, draft the manuscript and revising it. MRJ: analysis and interpretation of data , draft the manuscript and revising it. MM: study design, patient selection, Sample collection and handling, sample analyzing via commercially available enzyme-linked immunosorbent assay kit, Statistical analysis, analysis and interpretation of data, draft the manuscript and revising it. All authors read and approved the final manuscript.
Contributor Information
Kaveh Eslami, Email: kaveheslami@yahoo.com.
Ata Mahmoodpoor, Email: amahmoodpoor@yahoo.com.
Arezoo Ahmadi, Email: aarezoo24@gmail.com.
Mohammad Abdollahi, Email: mohammad@tums.ac.ir.
Koorosh Kamali, Email: kkamali71@yahoo.com.
Sarah Mousavi, Email: Solarsara2000@yahoo.com.
Atabak Najafi, Email: atabaknajafi@hotmail.com.
Maryam Baeeri, Email: M_baery@yahoo.com.
Hadi Hamishehkar, Email: hamishehkar@gmail.com.
Leila Kouti, Email: layla21k@yahoo.com.
Mohammad Reza Javadi, Email: Javadirctex1351@yahoo.com.
Mojtaba Mojtahedzadeh, Email: mojtahed@tums.ac.ir.
Acknowledgement
We gratefully thankful from nurses and ICU staff of Sina and Shohada hospital. We also thanks Iranian ministry of health which financially supported the study.
References
- Moreno RP, Metnitz B, Adler L, Hoechtl A, Bauer P, Metnitz PGH. Sepsis mortality prediction based on predisposition, infection and response. Intensive Care Med. 2008;34:496–504. doi: 10.1007/s00134-007-0943-1. [DOI] [PubMed] [Google Scholar]
- Vesteinsdottir E, Karason S, Sigurdsson SE, Gottfredsson M, Sigurdsson GH. Severe sepsis and septic shock: a prospective population-based study in Icelandic intensive care units. Acta Anaesthesiol Scand. 2011;55:722–731. doi: 10.1111/j.1399-6576.2011.02437.x. [DOI] [PubMed] [Google Scholar]
- Caille V, Bossi P, Grimaldi D, Vieillard-Baro A. [Physiopathology of severe sepsis] Presse Med. 2004;33:256–261. doi: 10.1016/S0755-4982(04)98551-X. discussion 269. [DOI] [PubMed] [Google Scholar]
- Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Critical Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S, Mojtahedzadeh M, Abdollahi M. Place of iron chelators like desferrioxamine and deferasirox in management of hyperoxia-induced lung injury: A systematic review. Int J Pharmacol. 2010;6:326–337. [Google Scholar]
- Shiehmorteza M, Ahmadi A, Abdollahi M, Nayebpour M, Mohammadi M, Hamishehkar H, Najafi A, Pazoki M, Mojtahedzadeh M. Recombinant human erythropoietin reduces plasminogen activator inhibitor and ameliorates pro-inflammatory responses following trauma. DARU J Pharmaceut Sci. 2011;19:159–165. [PMC free article] [PubMed] [Google Scholar]
- Soltan-Sharifi MS, Mojtahedzadeh M, Najafi A, Khajavi MR, Rouini MR, Moradi M, Mohammadirad A, Abdollahi M. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Hum Exp Toxicol. 2007;26:697–703. doi: 10.1177/0960327107083452. [DOI] [PubMed] [Google Scholar]
- Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro-versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- Dimopoulou I, Orfanos S, Kotanidou A, Livaditi O, Giamarellos-Bourboulis E, Athanasiou C, Korovesi I, Sotiropoulou C, Kopterides P, Ilias I. Plasma pro-and anti-inflammatory cytokine levels and outcome prediction in unselected critically ill patients. Cytokine. 2008;41:263–267. doi: 10.1016/j.cyto.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Hamishehkar HB MT, Abdollahi M, Ahmadi A, Ahmadi A, Mahmoodpour, Mirjalili MR, Abrishami R, Khoshayand MR, Eslami K, Kanani M, Baeeri M, Mojtahedzadeh M. Identification of enhanced cytokine generation following sepsis. Dream of magic bullet for mortality prediction and therapeutic evaluation. DARU. 2010;18:155–162. [PMC free article] [PubMed] [Google Scholar]
- Keh D, Goodman S, Sprung CL. Corticosteroid therapy in patients with severe sepsis and septic shock. Semin Respir Crit Care Med. 2004;25:713–719. doi: 10.1055/s-2004-860985. [DOI] [PubMed] [Google Scholar]
- Minneci PC, Deans KJ, Natanson C. Corticosteroid therapy for severe sepsis and septic shock. JAMA. 2009;302:1643. doi: 10.1001/jama.2009.1480. author reply 1644–1645. [DOI] [PubMed] [Google Scholar]
- Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M. et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, Tellado JM, Salgado-Remigio A, Zimlichman R, Withington S. et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Harrell FE Jr, LaBrecque JF, Wagner DP, Pribble JP, Draper EA, Fisher CJ Jr, Soll L. Use of predicted risk of mortality to evaluate the efficacy of anticytokine therapy in sepsis. The rhIL-1ra Phase III Sepsis Syndrome Study Group. Crit Care Med. 1996;24:46–56. doi: 10.1097/00003246-199601000-00010. [DOI] [PubMed] [Google Scholar]
- Suputtamongkol Y, Intaranongpai S, Smith MD, Angus B, Chaowagul W, Permpikul C, Simpson JA, Leelarasamee A, Curtis L, White NJ. A double-blind placebo-controlled study of an infusion of lexipafant (Platelet-activating factor receptor antagonist) in patients with severe sepsis. Antimicrob Agents Chemother. 2000;44:693–696. doi: 10.1128/AAC.44.3.693-696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Spapen H, Bakker J, Webster NR, Curtis L. Phase II multicenter clinical study of the platelet-activating factor receptor antagonist BB-882 in the treatment of sepsis. Crit Care Med. 2000;28:638–642. doi: 10.1097/00003246-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strauss R, Meier-Hellmann A, Insel R, Radke J. et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118–126. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]
- Valenta J, Brodska H, Drabek T, Hendl J, Kazda A. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med. 2011;37:808–815. doi: 10.1007/s00134-011-2153-0. [DOI] [PubMed] [Google Scholar]
- Abrishami RA A, Abdollahi M, Moosivand A, Khalili H, Najafi A, Gholami K, Hamishehkar H, Yazdi AP, Mojtahedzadeh M. Comparison the inflammatory effects of early supplemental parenteral nutrition plus enteral nutrition versus enteral nutrition alone in critically ill patients. daru. 2010;18:103–106. [PMC free article] [PubMed] [Google Scholar]
- Bernard GR. Drotrecogin alfa (activated) (recombinant human activated protein C) for the treatment of severe sepsis. Crit Care Med. 2003;31:S85–93. doi: 10.1097/00003246-200301001-00012. [DOI] [PubMed] [Google Scholar]
- Hack CE, Ogilvie AC, Eisele B, Jansen PM, Wagstaff J, Thijs LG. Initial studies on the administration of C1-esterase inhibitor to patients with septic shock or with a vascular leak syndrome induced by interleukin-2 therapy. Prog Clin Biol Res. 1994;388:335–357. [PubMed] [Google Scholar]
- Peng Z, Pai P, Han-Min W, Jun Z, Hong-Bao L, Rong L, Chen H. Evaluation of the effects of pulse high-volume hemofiltration in patients with severe sepsis: a preliminary study. Int J Artif Organs. 2010;33:505–511. doi: 10.1177/039139881003300801. [DOI] [PubMed] [Google Scholar]
- Patwardhan B, Gautam M. Botanical immunodrugs: scope and opportunities. Drug Discov Today. 2005;10:495–502. doi: 10.1016/S1359-6446(04)03357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paydary K, Emamzadeh-Fard S, Khorshid H, Kamali K, Seyed AS, Mohraz M. Safety and Efficacy of Setarud (IMOD (TM)) Among People Living with HIV/AIDS. A Review. Recent patents on anti-infective drug discovery. 2012;7:66–72. doi: 10.2174/157489112799829756. [DOI] [PubMed] [Google Scholar]
- Mohammadirad AK-KH, Gharibdoost F, Abdollahi M. etarud (IMODTM) as a multiherbal drug with promising benefits in animal and human studies: A comprehensive review of biochemical and cellular evidences. Asian J Anim Vet Adv. 2011;6:1185–1192. doi: 10.3923/ajava.2011.1185.1192. [DOI] [Google Scholar]
- Mohraz M, S B, F M, K K, c R. Evaluation of safety and efficacy of IMOD inb patients with HIV/AIDS. Iranian J Allergy Asthma Immunol. 2007;6:10. [Google Scholar]
- Mohraz M, Khairandish P, Kazerooni PA, Davarpanah MA, Shahhosseiny MH, Mahdavian B, Vaziry S, Shahriary S, Kamali K, Khorshid KHR. et al. A clinical trial on the efficacy of IMOD in AIDS patients. Daru-J Pharmaceut Sci. 2009;17:277–284. [Google Scholar]
- Keirandish PMM, khorram Khorshid H, Heshmat R, Gharibdust F. Aphase I clinical trial to determine the maximum tolerated dose and toxicity of IMOD in HIV infected asymptomatic patiets in Iran. Iranian J Allergy,Asthma Immunol. 2007;6:5. [Google Scholar]
- Mahmoodpoor A, Eslami K, Mojtahedzadeh M, Najafi A, Ahmadi A, Dehnadi-Moghadam A, Mohammadirad A, Baeeri M, Abdollahi M. Examination of Setarud (IMOD (TM)) in the management of patients with severe sepsis. Daru-Journal of Faculty of Pharmacy. 2010;18:23–28. [PMC free article] [PubMed] [Google Scholar]
- Hicks P, Cooper DJ. The Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Resusc. 2008;10:8. [PubMed] [Google Scholar]
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- Busund R, Koukline V, Utrobin U, Nedashkovsky E. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive care medicine. 2002;28:1434–1439. doi: 10.1007/s00134-002-1410-7. [DOI] [PubMed] [Google Scholar]
- Dhainaut JF, Laterre PF, LaRosa SP, Levy H, Garber GE, Heiselman D, Kinasewitz GT, Light RB, Morris P, Schein R. The clinical evaluation committee in a large multicenter phase 3 trial of drotrecogin alfa (activated) in patients with severe sepsis (PROWESS): Role, methodology, and results. Critical care medicine. 2003;31:2291–2301. doi: 10.1097/01.CCM.0000085089.88077.AF. [DOI] [PubMed] [Google Scholar]
- Laterre PF, Levy H, Clermont G, Ball DE, Garg R, Nelson DR, Dhainaut JF, Angus DC. Hospital mortality and resource use in subgroups of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial*. Critical care medicine. 2004;32:2207. doi: 10.1097/01.ccm.0000145231.71605.d8. [DOI] [PubMed] [Google Scholar]
- Wiedermann C, Kaneider N. A meta-analysis of controlled trials of recombinant human activated protein C therapy in patients with sepsis. BMC emergency medicine. 2005;5:7. doi: 10.1186/1471-227X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnikov EA, Baranov GA, Chuvanov MV, Skalozub O, Reshetnikov EA, Baranov GA, Chuvanov MV, Skalozub OI. [Immunotherapy in complex treatment of surgical sepsis] Khirurgiia (Mosk) 2008;7:11–14. [PubMed] [Google Scholar]
- Li W, Ashok M, Li J, Yang H, Sama AE, Wang H. A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS One. 2007;2:e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Lahni PM, Hake PW, Denenberg AG, Wong HR, Snead C, Catravas JD, Zingarelli B. The green tea polyphenol epigallocatechin-3-gallate improves systemic hemodynamics and survival in rodent models of polymicrobial sepsis. Shock. 2007;28:353–359. doi: 10.1097/shk.0b013e3180485823. [DOI] [PubMed] [Google Scholar]
- Abedi-valugerdi MAN, Khorram Khorsid H, Tavasoti Kheiry M, Garibdust F. In vivo and ex vivo immune response on herbal drug IMOD in animal experiments. Iranian J Allergy Asthma Immunol. 2007;6:9. [Google Scholar]
- Rezvanfar MA, Ahmadi A, Baeeri M, Abdollahi M. Inhibition of tumor necrosis factor-alpha and nitrosative/oxidative stresses by Setarud (IMOD®); a molecular mechanism of protection against letrozole-induced polycystic ovary. Toxicology Letters. 2011;205:S246. [Google Scholar]
- Bauer M. [The physiopathology of sepsis. Current concepts] Anaesthesist. 1996;45:312–322. doi: 10.1007/s001010050266. [DOI] [PubMed] [Google Scholar]
- Cavazzoni SLZ, Dellinger RP. Hemodynamic optimization of sepsis-induced tissue hypoperfusion. Critical Care. 2006;10:S2. doi: 10.1186/cc4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. Mitochondrial dysfunction, bioenergenic impairment, and metabolic down-regulation in sepsis. shock. 2007;28:24–28. doi: 10.1097/01.shk.0000235089.30550.2d. [DOI] [PubMed] [Google Scholar]
- Khairandish P, Mohraz M, Farzamfar B, Abdollahi M, Shahhosseiny MH, Madani H, Sadeghi B, Heshmat R, Gharibdoust F, Khorram-Khorshid HR. Preclinical and phase 1 clinical safety of Setarud (IMOD (TM)), a novel immunomodulator. Daru-J Pharmaceut Sci. 2009;17:148–156. [Google Scholar]