Abstract
Background
The high cost and low level of cancer survival urge the finding of new drugs having better mechanisms. There is a high trend of patients to be “back to nature” and use natural products as an alternative way to cure cancer. The fact is that some of available anticancer drugs are originated from plants, such as taxane, vincristine, vinblastine, pacitaxel. Curcumin (diferuloylmethane), a dietary pigment present in Curcuma longa rizhome is reported to induce cell cycle arrest in some cell lines. Other study reported that genistein isolated from Glycine max seed inhibited phosphorylation of cdk1, gene involved during G2/M transition and thus could function as G2 checkpoint abrogator. The inhibition of cdk1 phosphorylation is one of alternative strategy which could selectively kill cancer cells and potentially be combined with DNA damaging agent such as curcumin.
Methods
T47D cell line was treated with different concentrations of curcumin and genistein, alone or in combination; added together or with interval time. Flow Cytometry and MTT assay were used to evaluate cell cycle distribution and viability, respectively. The presence of apoptotic cells was determined using acridine orange-ethidium bromide staining.
Results
In this study curcumin induced G2 arrest on p53 deficient T47D cells at the concentration of 10 μM. Increasing concentration up to 30 μM increased the number of cell death. Whilst genistein alone at low concentration (≤10 μM) induced cell proliferation, addition of genistein (20 μM) 16 h after curcumin resulted in more cell death (89%), 34% higher than that administered at the same time (56%). The combination treatment resulted in apoptotic cell death. Combining curcumin with high dose of genistein (50 μM) induced necrotic cells.
Conclusions
Genistein increased the death of curcumin treated T47D cells. Appropriate timing of administration and concentration of genistein determine the outcome of treatment and this method could potentially be developed as an alternative strategy for treatment of p53 defective cancer cells.
Keywords: Cell cycle, p53, Curcumin, Genistein, G2 arrest
Background
Most of chemotherapeutic drugs such as etoposide, cisplastin, doxorubicin and camptotechin induce DNA damage on cancer cells. These DNA (deoxy nucleid acid) damaging agents arrest or delay cell cycle progression, allowing time for DNA repair. This mechanism of repair functions to ensure that division only occur on cells carrying complete DNA, not mutated or damage. If the damage is beyond repair, the cells may permanently enter cellular senescence or undergo apoptosis [1,2]. The mechanism of cell cycle arrest is mediated by ATM (ataxia telangiectasia-mutated protein kinase) or ATR (ATM and Rad3-related protein kinase) through the activation of Ser/Thr kinases checkpoint kinase 1 (Chk1) and checkpoint kinase 2 (Chk2). The activation of Chk1 and Chk2 in turn modulates phosphorylation events such as phosphorylation of cdc25 phosphatases which normally activate cdk1 in G2/M boundary and result in cell cycle arrest at the G2/M or S phase [3]. Based on oncologic point of view, this mechanism of repair is beneficial to normal cells in that they have mechanism to reduce toxic effect of the chemotherapeutic agents. However, this system of protection limits the efficacy of chemotherapy on cancer cells.
In responds to DNA damaging agents, cell cycle arrest on G1 phase depends on tumor suppressor protein p53. Normal cells carrying wild type p53 are able to arrest at G1, S and G2 phase, whilst cells having defect on p53 gene, which occur on >50% of tumor, would progress through S phase and arrest at G2 phase [4-6]. Any agents which capable of abrogating cell cycle arrest at G2 phase would induce premature entry into mitosis, with cells still carry damaged DNA and resulting in apoptosis. Inducing mitotic catastrophe in G2 arrested cell can be used as a strategy to selectively kill tumor cell lacking functional p53, and at the same time provide opportunities for normal cells to survive.
Although conventional chemotherapy using DNA damaging agents resulting in tumor cell death, the deleterious side effects are well known. Furthermore, the high cost of treatment urges the finding of selective agents with more affordable price. Currently, there is trend of patients to use natural medicine as an alternative therapy against cancer. The fact that some of available anticancer drugs such as taxan, vincristine, vinblastine and pacitaxel are medicinal plant origin, provide great opportunities to effectively use natural compounds to treat cancer. A lot of studies have been conducted to examine the potential of natural compounds against cancer with one of example is curcumin.
Curcumin (diferuloylmethane) is dietary pigment and presence as major compound in Curcuma longa rizhome. This compound has a potential to be developed as anticancer agent and was in phase II clinical trials [7,8]. Curcumin was reported to be cytotoxic and inactive in normal and primary cells. In mouse embryonic fibroblast line C3H/10 T1/2, rat embryonic fibroblasts, and human foreskin fibroblast, curcumin did not induce cell death, whereas in cancer cells it stimulated cell death through mechanism of apoptosis [8-12]. Curcumin also induced cell cycle arrest in colorectal tumor line HCT116, medulloblastoma and human acute promyelocytic leukemia HL-60 [12-14]. The ability to induce cell death increased with addition of piperine, the major compound of Piper nigrum L) which reportedly increase the bioavailability of curcumin [14-17].
The activity of curcumin as anticancer agent can be increased by combination with compounds having effect as G2 checkpoint abrogator. Flavonoid is natural polyphenolic compounds potential to be developed as anticancer agents [18,19]. Isoflavonoid genistein was found to be active in pancreatic cells by modulating cell cycle and inhibition of angiogenesis [20,21]. Following administration of irinotecan, this compound inhibited phosphorylation of cdk1 mediated by wee1 kinase, a negative regulator of cdk1 kinase activity [22]. Inhibition of cdk1 phosphorylation could be a potential strategy to abrogate G2 checkpoint activation [23].
Methods
Materials
Genistein was obtained from Sigma, curcumin was kindly given by Dr. Hilda Ismail, 86% purity by HPLC (High Pressure Liquid Chromatography). They were dissolved in ethanol absolute, divided into aliquots, and stored frozen at −20°C.
Cell lines and culture conditions
T47D (Human ductal breast epithelial tumor cell line) was cultured in RPMI 1640 media supplemented with 10% Fetal Bovine Serum (Gibco) 2% Penicillin - Streptomycin (Gibco), dan 0.5% Fungizon (Gibco), 2% Sodium bicarbonate (Gibco) and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (Invitrogen). Cell lines were maintained at 37°C in a humidified incubator containing 5% CO2.
Cell cycle analysis
Treated cells were harvested using trypsine and washed three times with 1×PBS. Cell pellets were resuspended with 500 μl staining solution (40 μg/ml propidium iodide (Sigma) and 500μg/ml RNase A (Sigma), covered with aluminium foil and incubated at 37°C for 30 minutes. Cells were analysed using FACS (Fluorescence Activated Cell Sorting) Calibur (BD).
Viability assay
A hundred μl of media containing 5x103 cells was added to 96-well plate and incubated for 48 hours until 70% – 80% confluent. Curcumin was added alone or in combination with genistein, added together or in interval time. Cells were incubated at 37°C in CO2 incubator. Following the treatment, cells were gently washed with 1X PBS (Phosphate Buffer Saline), and 100 μl of MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) 0.5 mg/ml was added to the well. The cells were incubated for 4 hours at 37°C and the reaction was stopped by adding 100 μl SDS (sodium dodecyl sulfate) 10%. The plates were incubated overnight and read in microplate reader (Bio-Rad) at 595 nm.
Apoptotic assay
T47D cells were plated onto 6 well plate containing coverslip and treated with curcumin, genistein or in combination as indicated. Following the treatment, the cells on coverslip were treated with ethidium bromide-acridine orange and the cells were analysed under fluorescence microscope (Carl Zeiss).
Results
Curcumin induced G2 arrest in T47D cells
To study the mechanism of action of curcumin in vitro, we have tested the effect of increasing concentration of curcumin in inducing G2 arrest in T47D cells. The cells were treated at different concentration of curcumin and incubated overnight. As shown in Table 1 and Figure 1, curcumin at 10 μM induced G2 arrest in T47D cells. The cells showed a strong block in the G2/M phase of the cell cycle (reaching 44% compared with 25% in the untreated controls). Increasing the concentration up to 30 μM did not significantly increase G2/M population. Instead, it appeared a marked sub-G1 peak (from 2% in control cells to 12% at 20 μM and 14% at 30 μM of treated cells), because of the presence of dead cells. Adding curcumin at low as 5 μM did not induce cell cycle arrest (data not shown). These data demonstrate that curcumin at 10 μM arrest T47D cells at G2/M with little cytotoxicity.
Table 1.
Cell cycle distribution of T47D cells after treatment of different concentration of curcumin overnight
| Treatment | Sub G1 (%) | G1 (%) | S (%) | G2 (%) |
|---|---|---|---|---|
| Control |
2.32 |
37.23 |
27.88 |
25.34 |
| 10 μM curcumin |
1.26 |
22.74 |
19.30 |
44.13 |
| 20 μM curcumin |
12.78 |
22.13 |
13.79 |
37.43 |
| 30 μM curcumin | 14.28 | 28.38 | 11.39 | 39.57 |
Figure 1.

Cell cycle analysis of T47D cell using flowcytometry after treatment with different concentration of curcumin. T47D cells were plated onto 6 well plate and treated overnight with (A) control untreated cells, (B) 10 μM curcumin, (C), 20 μM curcumin and (D) 30 μM curcumin. Arrow indicated the presence of sub G1 population.
Genistein abrogated G2 arrest induced by curcumin
In an attempt to discover natural compounds that disrupt G2 checkpoint in cancer cells, we used genistein, an inhibitor of cdk1 phosphorylation [22]. We examine the ability of different concentration of genistein in modulating G2 arrest induced by curcumin. Firstly, we activated the G2 checkpoint by adding curcumin at the concentration of 10 μM overnight. Sixteen hours after DNA damage, the cells were treated with different concentration of genistein for another 24 hours. The cells were harvested and the cell cycle distribution was analysed. Indeed, as shown in Table 2 and Figure 2, genistein at concentration of 20 μM abrogated the G2/M block induced by curcumin, decreasing G2/M population from 28% in curcumin only treated cells to 6% in combination with 20 μM genistein. Increasing concentration of genistein up to 50 μM did not significantly reduce the shift of G2/M population. The G2/M population only decreased to 10% in combination with 50 μM genistein. Combination of curcumin with genistein increased the subG1 population which represents dead cells, from 2% to 44% (20 μM genistein) and 47% (50 μM genistein).
Table 2.
Cell cycle distribution of T47D cells after treatment of 10 μM curcumin overnight followed by 20 μM genistein or 50 μM genistein
| Treatment | Sub G1 (%) | G1 (%) | S (%) | G2 (%) |
|---|---|---|---|---|
| Control |
8.51 |
48.85 |
11.13 |
18.52 |
| 10 μM curcumin |
2.71 |
31.12 |
18.86 |
28.22 |
| 10 μM curcumin + 20 μM genistein |
44.25 |
39.66 |
5.72 |
6.44 |
| 10 μM curcumin + 50 μM genistein | 47.47 | 29.17 | 6.76 | 10.14 |
Figure 2.
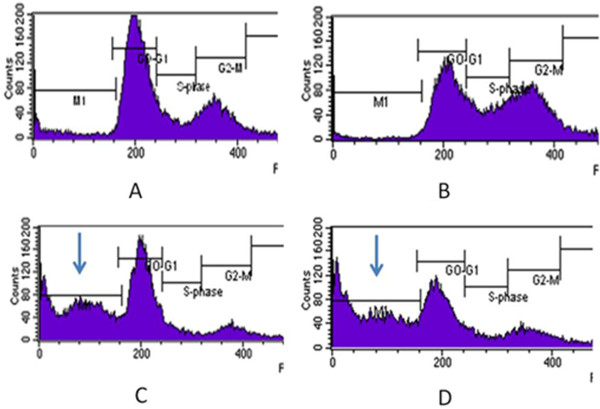
Cell cycle analysis of T47D cell using flowcytometry after treatment with curcumin and genistein. T47D cells were plated onto 6 well plate and treated with (A) control untreated cells, (B) 10 μM curcumin, (C), 10 μM curcumin overnight followed by 20 μM genistein and (D) 10 μM curcumin overnight followed by 50 μM genistein. Arrow indicated the presence of subG1 population.
Addition of genistein following G2 arrest induced by curcumin resulted in more cell death
To check the effect of combination of genistein with curcumin on cell viability, we treated the cells with curcumin, genistein and in combination with various interval times by means of MTT assay. The experiments were performed in three replicates. Previously we found that curcumin at concentration of 10 μM induced G2/M arrest with little toxicity. In this experiment we confirmed the finding that treatment curcumin at given concentration retained the viability of cells of 83.66%. An attempt to increase the sensitivity of this compound in inducing cell death is conducted by addition of genistein. Table 3 shows that genistein alone at low concentration of 5 and 10 μM induced cell proliferation as compared to control, reaching 191.80% and 171.22% respectively. Increasing concentration to 20 μM maintained the viability of 90.41%, whereas high concentration of 50 μM induced 61.28% cell death. In this study we found that genistein (20 μM) which was added 16 hours after 10 μM curcumin resulted in more cell death, from 83.66% viability in curcumin only treated cells to only 10.88% in combination. Adding higher concentration of genistein (50 μM) only slightly increased the percentage of cell death (8.48%). Interestingly, in this study we found that adding genistein following DNA damage induced by curcumin produced more cell death (89.12%) compared to adding at the same time (56.51%). The effect of inducing cell death by combination of curcumin and genistein were confirmed by observing the morphology of the cells after treatment with curcumin or in combination with genistein using ethidium bromide and acridine – orange double staining under fluoresence microscope. The presence of apoptotic cells were shown as orange to red population as compared to healthy green cell population [24]. As shown in Figure 3, only few dead cells were observed in curcumin only treated cells. However, combining curcumin with genistein resulted in high accumulation of apoptotic cells. Combination with high concentration of genistein induced the presence of necrotic cells, as appear as a form of debris (Figure 3).
Table 3.
Persentage of cell viability following the administration of curcumin, genistein and combination (n =3)
| No | Treatment | % viability ± SE |
|---|---|---|
| 1 |
Curcumin 10 μM |
83.66 ± 8.24 |
| 2 |
Genistein 5 μM |
191.80 ± 8.94 |
| 3 |
Genistein 10 μM |
171.22 ± 6.63 |
| 4 |
Genistein 20 μM |
90.41 ± 2.13 |
| 5 |
Genistein 50 μM |
38.72 ±3.99 |
| 6 |
Curcumin 10 μM + Genistein 20 μM* |
10.88 ± 1.85 |
| 7 |
Curcumin 10 μM + Genistein 50 μM* |
2.40 ± 0.59 |
| 8 |
Curcumin 10 μM + Genistein 20 μM** |
43.49 ± 4.90 |
| 9 | Curcumin 10 μM + Genistein 50 μM** | 15.83 ± 0.88 |
** Curcumin and genistein were added at the same time. * Genistein was added 16 hours after the addition of curcumin. These data represent minimal two independent experiments.
Figure 3.
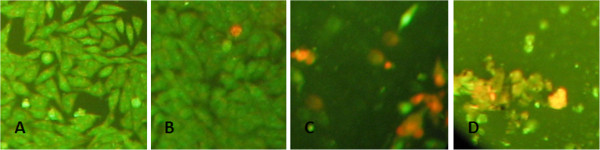
Morphology of cells treated with curcumin, genistein or in combination. T47D cells were plated onto 6 well plate and treated with (A) control cells, (B) 10 μM curcumine only, (C) curcumin (10 μM) was added overnight followed by treatment with genistein (20 μM), (D) curcumin (10 μM) was added overnight followed by treatment with genistein (50 μM).
Discussion
Conventional chemotherapeutics have been widely applied in treatment against various type of cancer. The effectiveness of these therapies depends on their ability to kill proliferating cancer cells by damaging their DNA and inducing apoptosis. The cell responds to DNA damage to induce cell cycle arrest at any stage, ensuring the damage is repaired prior to enter subsequent phase of cell cycle [25]. This mechanism of control is function to maintain genetic integrity, with the failure of repair resulted in mutations and eventually cell death. However, these agents convey some drawbacks in which they developed resistance to DNA damage-induced cell killing and they are also toxic to actively proliferating normal cells.
One of the characteristics of tumor growth is uncontrolled cell proliferation as a result of loss in normal cell cycle control. Currently there is an increasing of interest to target cell cycle in effort to find targeted anticancer therapies [26-29]. In fact >50% of human cancer have loss in the tumor suppressor gene p53. This gene is an essential component for apoptosis induction in response to DNA damage and also a component of cell cycle checkpoint. The loss of p53 resulted in cell reliance on G2 phase checkpoint in response to DNA damage inducing agent which easily can be bypassed by adding G2 phase checkpoint inhibitors to induce aberrant mitosis and eventually increased cell death [30,31].
In Indonesia, there is a high trend of using herbal medicine in promoting health with some of the herbal materials commonly used are curcuma rizhome (Curcuma longa L) and soy bean (Glycin max) which contain bioactive compound of curcumin and genistein, respectively. The bioactivities of these natural products have been widely reported [13,15,21,32]. In this study we tested the combined effects of genistein with curcumin in inhibiting the growth of cancer cells. As was seen in T47D cells, curcumin induces G2 phase arrest, similar as reported by others [12,13] and genistein abrogated the G2 checkpoint controls induced by curcumin. The combination of the two agents reduced cell viability up to seven fold, in comparison of adding the agent alone. Since many of cancers have defective of checkpoint control due to the loss of p53 gene, further deregulation of this mechanism of control can result in increased apoptotic cell death.
In this study we demonstrated the success of applying strategies to deliver a more cancer-specific cytotoxic treatment by synthetic lethality targeting p53 defective cancer cells. These synthetic lethality strategies are currently being used to search new drugs or targets within specific tumor types such as inhibition of Chk1 in p53 mutant cancers to achieve better outcomes [33,34]. Using this strategy, reduced side effects and better outcomes could be achieved since doses of chemotherapeutic agents can be reduced at least twofold [35-37]. In this study we showed the selectivity of synthetic lethality strategy by targeting the loss of function of p53 and application of DNA damage inducing agent. In T47D, p53 deficient cells, survival of the cells following DNA damage are dependent on activation of the Chk1 pathway to induce G2 cell cycle arrest and DNA repair. Disruption of this Chk1-dependent pathway by genistein can selectively sensitize cells to exit G2 phase before the damage are repaired and eventually the cells undergo apoptosis. In this study we demonstrated that adding combination of curcumin followed by genistein on T47D cells showed greater cell death, indicating the importance of appropriate timing of administration in inducing cell death. Similar study was reported by Tse et al. [38], when combining topoisomerase I posion and checkpoint inhibitor 7-hydroxystaurosporine in human colon cancer. Sequential treatment of SN-38 followed by UC-01, a Chk1 inhibitor, resulted in enhanced apoptosis in p53 mutant cells, and this was proven to be effective in p53 mutant but to lesser degree in p53 intact cells.
Collectively, these studies provide a new insight in using naturally derived agents to treat cancer with selective methods to achieve better outcomes. This approach is based on understanding that in the synthetic lethal strategy targeting p53 defective cells, the loss of stress response mechanism can reduce cell viability. This response of mechanisms, when combined with the loss of compensatory systems can generate complete loss of cell viability [33,39].
Conclusions
In this study we demonstrated that genistein increased the death of curcumin treated T47D cells. We showed that the inhibition of checkpoint pathway is generated by genistein and the selectivity is achieved by the loss of p53 function in T47D cells and application of curcumin. Since the main active compounds used in this study are natural product origin, it is expected that this study could support the rational of using traditional medicines for maintaining health. Appropriate use of these natural products could help cancer patients in using alternative therapies with scientific evidence based medicines.
Competing interest
The authors declare that there is no conflict of interest related to this publication.
Authors’ contributions
PA contributes to the concept, design, data acquisition, analysis and interpretation; preparation of the manuscript and approval of final article. EDU contributes to data acquisition and analysis, preparation of the manuscript and approval of final article. Part of the data was collected for her master thesis. AWN participated in data acquisition and analysis and helped to draft the manuscript. SS participated in the design and coordination, preparation of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Puji Astuti, Email: p.astuti@gmail.com.
Esti D Utami, Email: estifarma@yahoo.com.
Arsa W Nugrahani, Email: arsa_wahyu@yahoo.com.
Sismindari Sudjadi, Email: sismindari@yahoo.com.
Acknowledgements
This work was performed with Hibah Berkualitas Prima Faculty of Pharmacy UGM 2011. We are grateful to Dr. Hilda Ismail for the donation of curcumin used in these experiments.
References
- Kastan BM, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Eastman A. Cell cycle checkpoints and their impact on anticancer therapeutic strategies. J Cell Biochem. 2004;91:223–231. doi: 10.1002/jcb.10699. [DOI] [PubMed] [Google Scholar]
- Lukas J, Lukas C, Bartek J. Mammalian cell cycle checkpoints: signaling pathways and their organization in space and time. DNA Repair (Amst) 2004;3:997–1007. doi: 10.1016/j.dnarep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Booher RN, Kraker A, Lawrence T, Leopold WR, Sun Y. Radiosensitization of p53 Mutant Cells by PD0166285, a Novel G2 Checkpoint Abrogator. Cancer Res. 2001;61:8211–8217. [PubMed] [Google Scholar]
- Eastman A, Kohn EA, Brown MK, Rathman J, Livingstone M, Blank DH, Gribble DW. A Novel Indolocarbazole, ICP-1, Abrogates DNA Damage induced Cell Cycle Arrest and Enhances Cytotoxicity: Similarities and Differences to the Cell Cycle Checkpoint Abrogator UCN-011. Mol Cancer Ther. 2002;1:1067–1078. [PubMed] [Google Scholar]
- Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm. 2002;8:1695–1706. doi: 10.2174/1381612023394016. [DOI] [PubMed] [Google Scholar]
- Jiang MC, Yang-Yen HF, Yen JJ, Lin JK. Curcumin induces apoptosis in immortalized NIH 3 T3 and malignant cancer cell lines. Nutr Cancer. 1996;26:111–120. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]
- Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:117–129. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- Song G, Mao YB, Cai QF, Yao LM, Ouyang GL, Bao SD. Curcumin induces human HT-29 colon adenocarcinoma cell apoptosis by activating p53 and regulating apoptosis-related protein expression. Braz J Med Biol Res. 2005;38:1791–1798. doi: 10.1590/S0100-879X2005001200007. [DOI] [PubMed] [Google Scholar]
- Tan TW, Tsai HR, Lu HF, Lin HL, Tsou MF, Lin YT, Tsai HY, Chen YF, Chung JG. Curcumin-induced cell cycle arrest and apoptosis in human acute promyelocytic leukemia HL-60 cells via MMP changes and caspase-3 activation. Anticancer Res. 2006;26(6B):4361–4371. [PubMed] [Google Scholar]
- Jiang Z, Jin S, Yalowich JC, Brown KD, Rajasekaran B. The Mismatch Repair System Modulates Curcumin Sensitivity through Induction of DNA Strand Breaks and Activation of G2-M Checkpoint. Mol Cancer Ther. 2010;9(3):558–568. doi: 10.1158/1535-7163.MCT-09-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin MH, Shinwari Z, Hendrayani SF, Al-Hindi H, Al-Shail E, Khafaga Y, Al-Kofide A, Aboussekhra A. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog. 2010;49(3):302–314. doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of Curcumin: Problems and Promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76(11):1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122(3):777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Fan J, Bertino JR. Selective sensitization of retinoblastoma protein-deficient sarcoma cells to doxorubicin by flavopiridol-mediated inhibition of cyclin-dependent kinase 2 kinase activity. Cancer Res. 2001;61(6):2579–2582. [PubMed] [Google Scholar]
- Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65(15):6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- Buchler P, Reber HA, Buchler MW, Friess H, Lavey RS, Hines OJ. Antiangiogenic activity of genistein in pancreatic carcinoma cells is mediated by the inhibition of hypoxia-inducible factor-1 and the down-regulation of VEGF gene expression. Cancer. 2004;100(1):201–210. doi: 10.1002/cncr.11873. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65(19):9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- Papazisis KT, Kalemi TG, Zambouli D, Geromichalos GD, Lambropoulos AF, Kotsis A, Boutis LL, Kortsaris AH. Synergistic effects of protein tyrosine kinase inhibitor genistein with camptothecins against three cell lines in vitro. Cancer Lett. 2006;233(2):255–264. doi: 10.1016/j.canlet.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Leijen S, Beijnen JH, Schellens JH. Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents. Curr Clin Pharmacol. 2010;5(3):186–191. doi: 10.2174/157488410791498824. [DOI] [PubMed] [Google Scholar]
- Baskić D, Popović S, Ristić P, Arsenijević NN. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30(11):924–932. doi: 10.1016/j.cellbi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Medema RH, Macůrek L. Checkpoint control and cancer. Oncogene. 2012;31(21):2601–2613. doi: 10.1038/onc.2011.451. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yan Z, Painter CL, Zhang Q, Chen E, Arango ME, Kuszpit K, Zasadny K, Hallin M, Hallin J, Wong A, Buckman D, Sun G, Qiu M, Anderes K, Christensen JG. PF-00477736 mediates checkpoint kinase 1 signaling pathway and potentiates docetaxel-induced efficacy in xenografts. Clin Cancer Res. 2009;15:4630–4640. doi: 10.1158/1078-0432.CCR-08-3272. [DOI] [PubMed] [Google Scholar]
- Hagimori K, Fukuda T, Hasegawa Y, Omura S, Tomoda H. Fungal malformins inhibit bleomycin-induced G2 checkpoint in Jurkat cells. Biol Pharm Bull. 2007;30(8):1379–1383. doi: 10.1248/bpb.30.1379. [DOI] [PubMed] [Google Scholar]
- Warrener R, Beamish H, Burgess A, Waterhouse NJ, Giles N, Fairlie D, Gabrielli B. Tumor cell-selective cytotoxicity by targeting cell cycle checkpoints. FASEB J. 2003;17:1550–1552. doi: 10.1096/fj.02-1003fje. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay UK, Senderowicz AM, Ferbeyre G. RNA silencing of checkpoint regulators sensitizes p53-defective prostate cancer cells to chemotherapy while sparing normal cells. Cancer Res. 2005;65:2872–2881. doi: 10.1158/0008-5472.CAN-04-2502. [DOI] [PubMed] [Google Scholar]
- Ma CX, Janetka JW, Piwnica- Worms H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med. 2011;17:88–96. doi: 10.1016/j.molmed.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AH, Ziegler RG, Nomura AM, West DW, Kolonel LN, Horn-Ross PL, Hoover RN, Pike MC. Soy intake and risk of breast cancer in Asians and Asian Americans. Am J Clin Nutr. 1998;68(Suppl 6):1437S–1443S. doi: 10.1093/ajcn/68.6.1437S. [DOI] [PubMed] [Google Scholar]
- Gabrielli B, Brooks K, Pavey S. Defective cell cycle checkpoints as targets for anti-cancer therapies. Front Pharmacol. 2012;3(9):1–6. doi: 10.3389/fphar.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken HJ, Beijersbergen RL. Exploration of synthetic lethal interactions as cancer drug targets. Future Oncol. 2010;6:1789–1802. doi: 10.2217/fon.10.131. [DOI] [PubMed] [Google Scholar]
- Blasina A, Hallin J, Chen E, Arango ME, Kraynov E, Register J, Grant S, Ninkovic S, Chen P, Nichols T, O’Connor P, Anderes K. Breaching the DNA damage checkpoint via PF-00477736, a novel small-molecule inhibitor of checkpoint kinase1. Mol Cancer Ther. 2008;7:2394–2404. doi: 10.1158/1535-7163.MCT-07-2391. [DOI] [PubMed] [Google Scholar]
- Tse AN, Rendahl KG, Sheikh T, Cheema H, Aardalen K, Embry M, Ma S, Moler EJ, Ni ZJ, Lopes De Menezes DE, Hibner B, Gesner TG, Schwartz GK. CHIR-124, a novel potent inhibitor of Chk1, potentiates the cytotoxicity of topoisomerase I poisons invitro and invivo. Clin Cancer Res. 2007;13:591–602. doi: 10.1158/1078-0432.CCR-06-1424. [DOI] [PubMed] [Google Scholar]
- Zabludoff SD, Deng C, Grondine MR, Sheehy AM, Ashwell S, Caleb BL, Green S, Haye HR, Horn CL, Janetka JW, Liu D, Mouchet E, Ready S, Rosenthal JL, Queva C, Schwartz GK, Taylor KJ, Tse AN, Walker GE, White AM. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol Cancer Ther. 2008;7:2955–2966. doi: 10.1158/1535-7163.MCT-08-0492. [DOI] [PubMed] [Google Scholar]
- Tse A, Schwartz G. Potentiation of cytotoxicity of topoisomerase I poison in human colon carcinoma cells by concurrent and sequential treatment with the checkpoint inhibitor 7-hydroxystaurosporine involves disparate mechanisms resulting in different pharmacological endpoints. Cancer Res. 2004;64:6635–6644. doi: 10.1158/0008-5472.CAN-04-0841. [DOI] [PubMed] [Google Scholar]
- Kaelin WG Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]


