Abstract
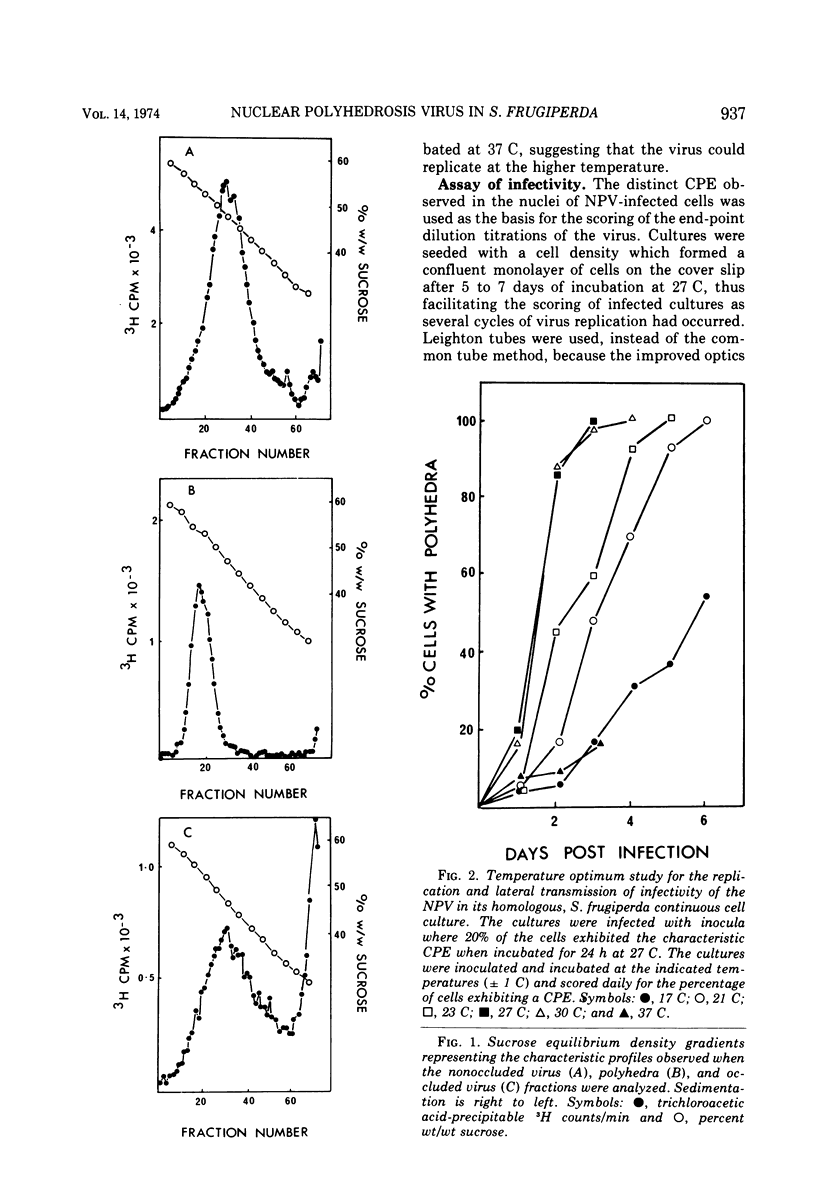
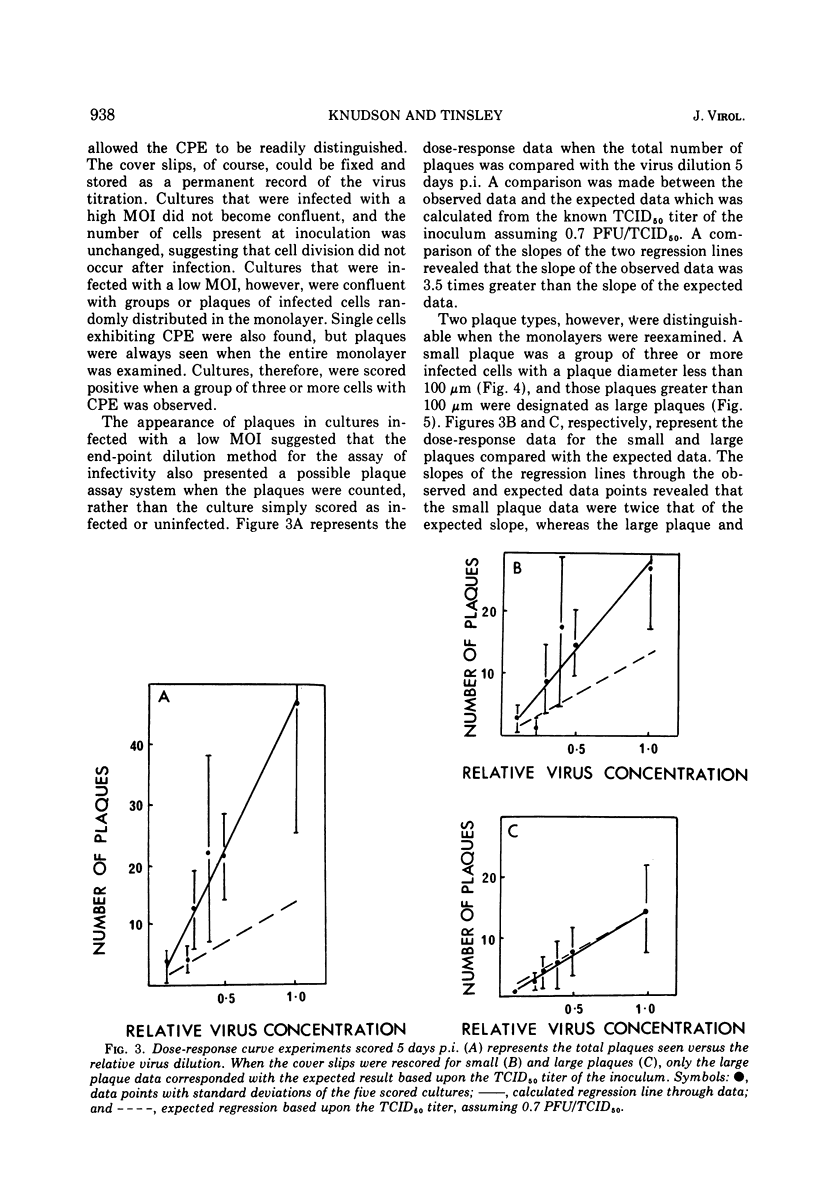
Nonoccluded virus, polyhedra, and occluded virus were purified from a continuous cell culture of Spodopera frugiperda infected with nuclear polyhedrosis virus. The optimal temperature for the replication and lateral transmission of infectivity for the nuclear polyhedrosis viruses (NPV) in cell culture was 27 C. End-point dilution and plaque assay procedures for the measurement of infectivity are described and compared. Dose-response data demonstrated that a single particle could initiate an infection, and the validity of the relationship of 0.7 PFU per mean tissue culture infective dose (TCID5 0) further substantiated the accuracy of these infectivity assays. Particle-infectious unit calculations gave a ratio of 62 to 310 nonoccluded virus particles TCID5 0. Growth cycle and lateral transmission experiments indicated that infectious material was released from cells 12 h postinfection (p.i.) and approached a maximal titer 4 days p.i. The number of polyhedra, nonoccluded virions, and TCID5 0 produced per cell was also presented. Typical yields of NPV produced per liter flask suggested that insect cell culture systems represent a feasible means by which the replication of these viruses could be investigated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Faulkner P., Henderson J. F. Serial passage of a nuclear polyhedrosis disease virus of the cabbage looper (Trichoplusia ni) in a continuous tissue culture cell line. Virology. 1972 Dec;50(3):920–924. doi: 10.1016/0042-6822(72)90448-5. [DOI] [PubMed] [Google Scholar]
- Goodwin R. H., Vaughn J. L., Adams J. R., Louloudes S. J. Replication of a nuclear polyhedrosis virus in an established insect cell line. J Invertebr Pathol. 1970 Sep;16(2):284–288. doi: 10.1016/0022-2011(70)90072-8. [DOI] [PubMed] [Google Scholar]
- Harrap K. A. The structure of nuclear polyhedrosis viruses. 3. Virus assembly. Virology. 1972 Oct;50(1):133–139. doi: 10.1016/0042-6822(72)90353-4. [DOI] [PubMed] [Google Scholar]
- Harrap K. A. The structure of nuclear polyhedrosis viruses. I. The inclusion body. Virology. 1972 Oct;50(1):114–123. doi: 10.1016/0042-6822(72)90351-0. [DOI] [PubMed] [Google Scholar]
- Harrap K. A. The structure of nuclear polyhedrosis viruses. II. The virus particle. Virology. 1972 Oct;50(1):124–132. doi: 10.1016/0042-6822(72)90352-2. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Faulkner P., MacKinnon E. A. Some biophysical properties of virus present in tissue cultures infected with the nuclear polyhedrosis virus of Trichoplusia ni. J Gen Virol. 1974 Jan;22(1):143–146. doi: 10.1099/0022-1317-22-1-143. [DOI] [PubMed] [Google Scholar]
- Hink W. F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970 May 2;226(5244):466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- Ignoffo C. M., Shapiro M., Hink W. F. Replication and serial passage of infectious Heliothis nucleopolyhedrosis virus in an established line of Heliothis zea cells. J Invertebr Pathol. 1971 Jul;18(1):131–134. doi: 10.1016/0022-2011(91)90021-h. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Characterization of deoxyribonucleic acid isolated from the granulosis viruses of the cabbage looper, Trichoplusia ni and the fall armyworm, Spodoptera frugiperda. Virology. 1972 Nov;50(2):459–471. doi: 10.1016/0042-6822(72)90397-2. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Characterization of nuclear polyhedrosis virus DNAs. J Virol. 1973 Dec;12(6):1336–1346. doi: 10.1128/jvi.12.6.1336-1346.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Granulosis virus deoxyribonucleic acid: a closed, double-stranded molecule. J Virol. 1972 Apr;9(4):710–713. doi: 10.1128/jvi.9.4.710-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]