Abstract
Herein, we present a sensitive, selective, and facile label-free DNA functionalized single-walled carbon nanotube (SWNT)-based chemiresistive biosensor for the detection of Hg2+. SWNTs were functionalized with Hg2+ binding 15-bases long polyT oligonucleotide through covalent attachment using a bilinker molecule. The polyT was further hybridized with polyA to form a polyT-polyA duplex. When exposed to Hg2+ the polyT-polyA duplex was dehybridized combined with switching of polyT structure, leading to change in resistance/conductance of the SWNT chemiresistor device. The device provided a significant response within 100 to 1000 nM of Hg2+ concentration with a 6.72 × 10−3 nM−1 sensitivity.
Mercury (Hg) is a worldwide concern in aquatic environments due to its toxicity and biomagnification in food webs. Elevated exposure to mercury can affect the cardiovascular system, gastro-intestinal system, liver, kidneys, and neurological system of the human body.1 Industrial wastewater from chlor-alkali and mineral industries, burning of fossil fuels, and incineration of municipal solid waste are the major sources of mercury contamination in the environment. The total global mercury emission from all sources has been estimated at 7500 tons per year.2 The United States Environmental Protection Agency (EPA) has mandated a drinking water upper limit of 2 ppb (10 nM) for mercury (II) ion concentration.3
Protein based Hg2+ biosensor using electrochemical4, 5 and optical6 techniques has been demonstrated. However, poor stability of proteins at ambient condition restricts its use for real application. These stability issues could be overcome by using DNA molecules that have excellent stability at ambient condition and do not require stringent storage conditions. Highly sensitive and selective detection of Hg2+ ion based on thymine-Hg2+-thymine (T-Hg2+-T) structure-switching DNA using UV absorption, fluorescence, surface-enhanced Raman spectroscopy, resonance scattering, and electrochemical methods has been demonstrated.7, 8, 9, 10, 11, 12 However, these approaches require labels and/or sophisticated instruments.13
One-dimensional (1D) nanostructure has been studied extensively as a transducer element in biosensors for their high surface to volume ratio, which results in the surface phenomena predomination over the chemistry and physics that happen in the bulk.14, 15 Amongst different 1D materials, single-walled carbon nanotubes (SWNTs) have emerged as a promising material for the development of label-free biosensor because of its extreme sensitivity towards resistance/conductance changes upon adsorption/perturbation of analyte molecule on the SWNT surface and facile surface modification possibilities.16, 17 Further, the high electrical mobility of SWNTs enables developing low power microelectronics whereas the 1D nanostructure of SWNTs facilitates development of high-density sensor arrays within a limited space. In this work, we propose a label-free, chemiresistive biosensor for Hg2+ ion detection based on polyT-polyA duplex functionalized SWNT, which upon exposure to Hg2+ results in the dehybridization of polyT-polyA duplex and switching of polyT structure, leading to change in resistance/conductance of the SWNT chemiresistor device.
Initially, we investigated sensing of Hg2+ using a polyT-based SWNTs chemiresistive biosensor fabricated through non-covalent functionalization of SWNTs (Scheme I). Figures S1 and S2, respectively, show the schematic of the fabrication/sensing steps and the current vs. voltage (I–V) responses corresponding to each step.18 As shown in Figure S2, the resistance of the SWNTs network increased upon non-covalent immobilization of polyT (5′-TTT TTT TTT TTT TTT-3′) attributed to π-π stacking interaction and the negative charge density provided by the phosphate groups on a polyT-SWNTs hybrid on the p-type SWNTs.18, 19 Upon incubation of the final sensor device (after blocking of Hg2+ binding with the gold electrodes by a self-assembled monolayer of 6-mercapto-1-hexanol (MCH))20 with 1 μM Hg2+, the device resistance decreased (Figure S2). The resistance decrease or conductance increase is a result of removal/release of polyT from the SWNTs surface due to the formation of T-Hg2+-T complex,21 a hairpin like structure,22 causing a decrease of negative charge on SWNTs and/or thermodynamically favourable reduction of Hg2+ on SWNTs23 providing a hole carrier injection on SWNTs. While the selectivity of the Scheme I biosensor was very good (Figure S3)18 the sensitivity for Hg2+ was low and the relationship of response with the Hg2+ concentration was not linear (data not shown).
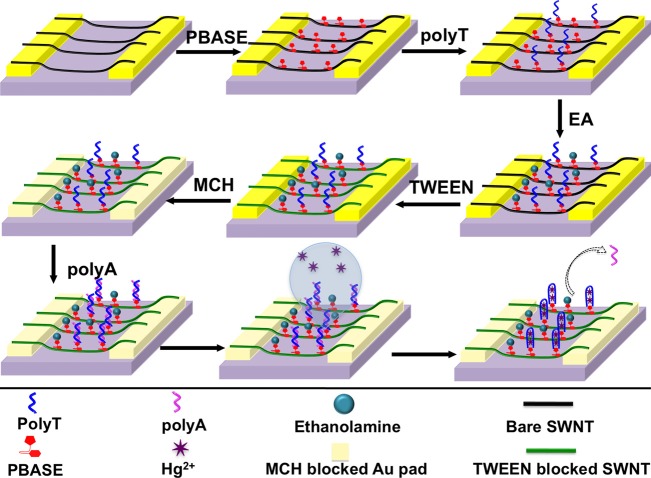
In order to improve the device performance in terms of sensitivity and selectivity, the fabrication protocol (Scheme II) was modified and the device was fabricated through covalent functionalization of SWNTs with amino-labelled polyT followed by hybridization with polyA (Figure 1) to form DNA duplex. Figure 2 shows current vs. voltage (I–V) recordings of the fabrication steps and Hg2+ sensing: non-covalent functionalization of SWNTs with 1-pyrenebutanoic acid succinimidyl ester (PBASE); covalent attachment of the amino-labelled capture oligonucleotide polyT (5′-/5AmMC6/TTT TTT TTT TTT TTT-3′) through the amide bond between the amine at 5′ end of the capture oligo and N-hydrosuccinimide ester (NHS) of PBASE; neutralization of unbound NHS with ethanolamine (EA) and blocking of unfunctionalized SWNTs with Tween 20; hybridization of amino-labelled polyT with polyA (5′-AAA AAA AAA AAA AAA-3′) to form polyT-polyA duplex; MCH blocking of gold pads of the electrode; and sensing of Hg2+ ion (please see supplementary material for details).18 An increase in resistance of the SWNTs was observed after PBASE modification. The resistance of PBASE modified SWNTs remains unchanged after covalent binding of amino-labelled polyT. Further, increase in resistance of the device was noticed for all successive steps of fabrication. The resistance increases were in accordance with the literature and a result of electron donation from these molecules to the nanotubes resulting in charge carrier reduction in SWNTs and/or scattering potential generated by the immobilization of the molecules and thereby decrease in the hole mobility.24 Upon incubation of the final biosensor with 250 nM Hg2+ solution for 30 min at room temperature, the source and drain current of the device increased, i.e., the resistance decreased due to the dehybridization of polyT:polyA duplex resulting the formation of T-Hg2+-T duplex and the release of polyA from SWNTs surface.25
Figure 1.
Schematic illustration of SWNTs chemiresistive label-free biosensor fabrication steps through covalent functionalization of SWNTs with amino-labeled polyT followed by hybridization with polyA.
Figure 2.
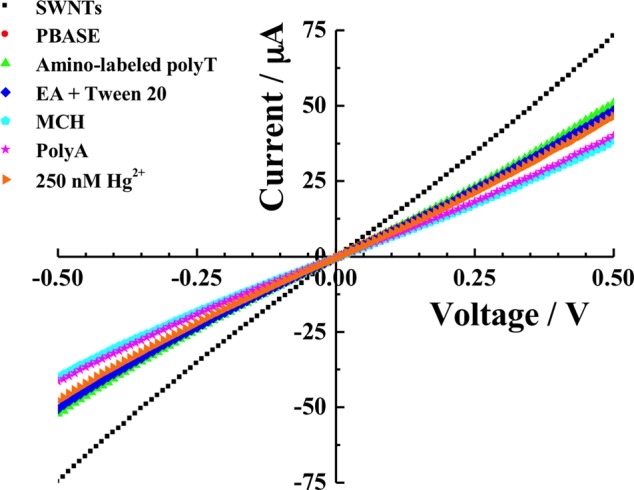
Current versus voltage (I–V) curves of SWNTs chemiresistive label-free biosensor at different stages of fabrication and upon exposure of 250 nM of Hg2+.
Negative controls, i.e., SWNTs devices functionalized with only the capture oligo and blocked with ethanolamine, Tween 20 and MCH, i.e., without the polyA, showed no response to Hg2+ (data not shown). These results confirmed the effectiveness of the proposed sensing modality for Hg2+ by a nanostructure-based chemiresistor biosensor.
Figure 3 shows the calibration curve, relationship between the SWNTs chemiresistive biosensor response [(R−RO)/RO], where R is the resistance after exposure to Hg2+ ion and RO is the resistance of SWNTs after hybridization with polyA and MCH blocking. The resistance was calculated as the inverse of the slope of the I–V curve between −0.1 and + 0.1 V (linear range). As shown in the figure, the sensor response was linear over the Hg2+ concentration ranging from 100 nM to 1 μM with a 6.72 × 10−3 nM−1 sensitivity. Also more than ten order of enhancement in sensor response was observed as compared to the device fabricated through Scheme I. This enhancement in sensitivity is attributed to the extremely high sensitivity of the chemiresistive transduction combined with the displacement principle.26
Figure 3.
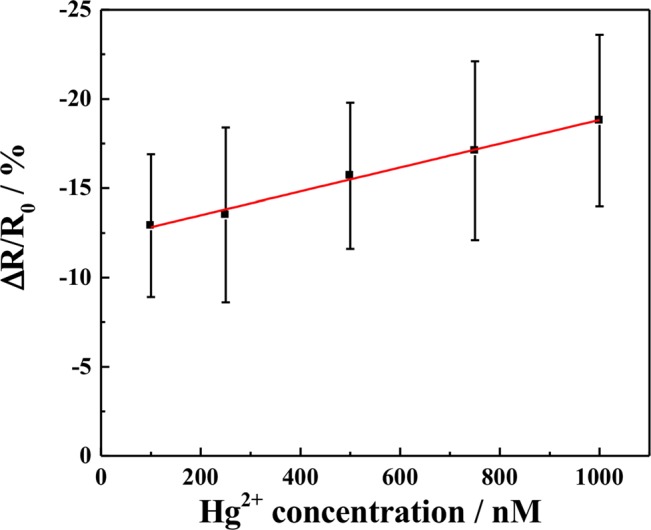
SWNTs chemiresistive label-free biosensor calibration for Hg2+. Each data point is an average of the measurements from 4 independent sensors prepared at different time and error bars represent ±1 standard deviation.
To examine the selectivity of the biosensors, SWNT devices were incubated with different metal ion solutions of the same concentration. The responses for Hg2+, Ca2+, Mg2+, and Mn2+ ion were found out to be −18.80 ± 4.8%, −7.31 ± 2.01%, −4.28 ± 0.60%, and −5.13 ± 3.49%, respectively (Figure 4), thus demonstrating that SWNT based chemirersistive biosensors were highly selective for Hg2+ ion detection.
Figure 4.
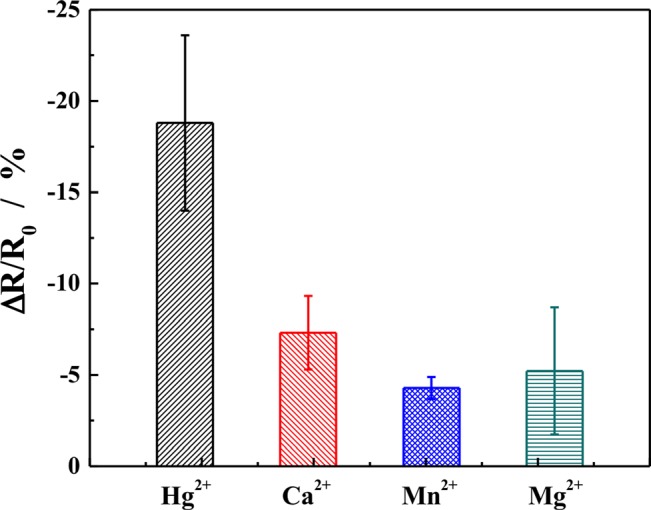
Responses of SWNTs chemiresistive label-free biosensor for different metal ions. The concentrations of the metal ions were 1 μM and incubation period was 30 min. Each data point is an average of measurements from four independent sensors at different point of time and the error bars represent ±1 standard deviation.
In conclusion, we have fabricated a SWNTs chemiresitive biosensor for Hg2+ ion detection based on structure-switching DNA. This biosensor exhibited good selectivity for Hg2+ ion and showed a linear response over the range of concentrations between 100 nM to 1 μM Hg2+ ion. Furthermore, compared with those reported in literature based on polyT,27, 28 the SWNTs nano-biosensor is a truly label-free system, requiring the aid of no labels attached to the polyA or the capture oligo.
Acknowledgments
We acknowledge the support of grants from NIH (U01ES016026) and NCET of China (NCET-09-0328). T.S. is grateful to Government of India for his financial assistance.
References
- Bridges C. C. and Zalups R. K., Toxicol. Appl. Pharmacol. 204, 274 (2005). 10.1016/j.taap.2004.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh Y. D. and Komarneni S., Environ. Sci. Technol. 45, 6954 (2011). 10.1021/es200712r [DOI] [PubMed] [Google Scholar]
- Guo L.-Q., Yin N., Nie D.-D., Gan J.-R., Li M.-J., Fu F.-F., and Chen G.-N., Analyst 136, 1632 (2011). 10.1039/c0an00880j [DOI] [PubMed] [Google Scholar]
- Bontidean I., Ahlqvist J., Mulchandani A., Chen W., Bae W., Mehra R. K., Mortari A., and Csöregi E., Biosens. Bioelectron. 18, 547 (2003). 10.1016/S0956-5663(03)00026-5 [DOI] [PubMed] [Google Scholar]
- Chen C., Xie Q., Wang L., Qin C., Xie F., Yao S., and Chen J., Anal. Chem. 83, 2660 (2011). 10.1021/ac1031435 [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Hamaguchi K., Osaka I., and Arakawa R., Adv. Funct. Mater. 21, 3508 (2011). 10.1002/adfm.201100886 [DOI] [Google Scholar]
- Tanaka Y., Oda S., Yamaguchi H., Kondo Y., Kojima C., and Ono A., J. Am. Chem. Soc. 129, 244 (2007). 10.1021/ja065552h [DOI] [PubMed] [Google Scholar]
- Li T., Dong S., and Wang E., Anal. Chem. 81, 2144 (2009). 10.1021/ac900188y [DOI] [PubMed] [Google Scholar]
- Zhang L., Li T., Li B., Li J., and Wang E., Chem. Commun. 46, 1476 (2010). 10.1039/b921191h [DOI] [PubMed] [Google Scholar]
- Kang T., Yoo S. M., Yoon I., Lee S., Choo J., Lee S. Y., and Kim B., Chem.-Eur. J. 17, 2211 (2011). 10.1002/chem.201001663 [DOI] [PubMed] [Google Scholar]
- Jiang Z., Fan Y., Chen M., Liang A., Liao X., Wen G., Shen X., He X., Pan H., and Jiang H., Anal. Chem. 81, 5439 (2009). 10.1021/ac900590g [DOI] [PubMed] [Google Scholar]
- Liu S.-J., Nie H.-G., Jiang J.-H., Shen G.-L., and Yu R.-Q., Anal. Chem. 81, 5724 (2009). 10.1021/ac900527f [DOI] [PubMed] [Google Scholar]
- Liu Z. D., Li Y. F., Ling J., and Huang C. Z., Environ. Sci. Technol. 43, 5022 (2009). 10.1021/es9001983 [DOI] [PubMed] [Google Scholar]
- Stern E., Klemic J. F., Routenberg D. A., Wyrembak P. N., Turner-Evans D. B., Hamilton A. D., Lavan D. A., Fahmy T. M., and Reed M. A., Nature 445, 519 (2007). 10.1038/nature05498 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen K.-S., Meyer N. L., Yuan J., Hirst L. S., Chase P. B., and Xiong P., Biosens. Bioelectron. 26, 4538 (2011). 10.1016/j.bios.2011.05.019 [DOI] [PubMed] [Google Scholar]
- Collins P. G., Bradley K., Ishigami M., and Zettl A., Science 287, 1801 (2000). 10.1126/science.287.5459.1801 [DOI] [PubMed] [Google Scholar]
- Snow E. S., Perkins F. K., Houser E. J., Badescu S. C., and Reinecke T. L., Science 307, 1942 (2005). 10.1126/science.1109128 [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4773569 for SWNTs device fabrication, SWNTs functionalization protocol, and sensing response of noncovalant functinalized SWNTs with polyT towards Hg2+.
- Zheng M., Jagota A., Semke E. D., Diner B. A., Mclean R. S., Lustig S. R., Richardson R. E., and Tassi N. G., Nature Mater. 2, 338 (2003). 10.1038/nmat877 [DOI] [PubMed] [Google Scholar]
- Tang X., Bansaruntip S., Nakayama N., Yenilmez E., Chang Y.-L., and Wang Q., Nano Lett. 6, 1632 (2006). 10.1021/nl060613v [DOI] [PubMed] [Google Scholar]
- Liu X., Tang Y., Wang L., Zhang J., Song S., Fan C., and Wang S., Adv. Mater. 19, 1471 (2007). 10.1002/adma.200602578 [DOI] [Google Scholar]
- Ono A. and Togashi H., Angew. Chem., Int. Ed. 43, 4300 (2004). 10.1002/anie.200454172 [DOI] [PubMed] [Google Scholar]
- Kim T. H., Lee J., and Hong S., J. Phys. Chem. C 113, 19393 (2009). 10.1021/jp908902k [DOI] [Google Scholar]
- Gruner G., Anal. Bioanal. Chem. 384, 322 (2005). 10.1007/s00216-005-3400-4 [DOI] [PubMed] [Google Scholar]
- Oh B. N., Park S., Ren J., Jang Y. J., Kim S. K., and Kim J., Dalton Trans. 40, 6494 (2011). 10.1039/c1dt10083a [DOI] [PubMed] [Google Scholar]
- Cella L. N., Sanchez P., Zhong W., Myung N. V., Chen W., and Mulchandani A., Anal. Chem. 82, 2042 (2010). 10.1021/ac902791q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. R., Huang W. T., Xie W. Y., Wen T., Luo H. Q., and Li N. B., Analyst 137, 3300 (2012). 10.1039/c2an35528k [DOI] [PubMed] [Google Scholar]
- Wang G., Huang H., Zhang X., and Wang L., Biosens. Bioelectron. 35, 108 (2012). 10.1016/j.bios.2012.02.029 [DOI] [PubMed] [Google Scholar]