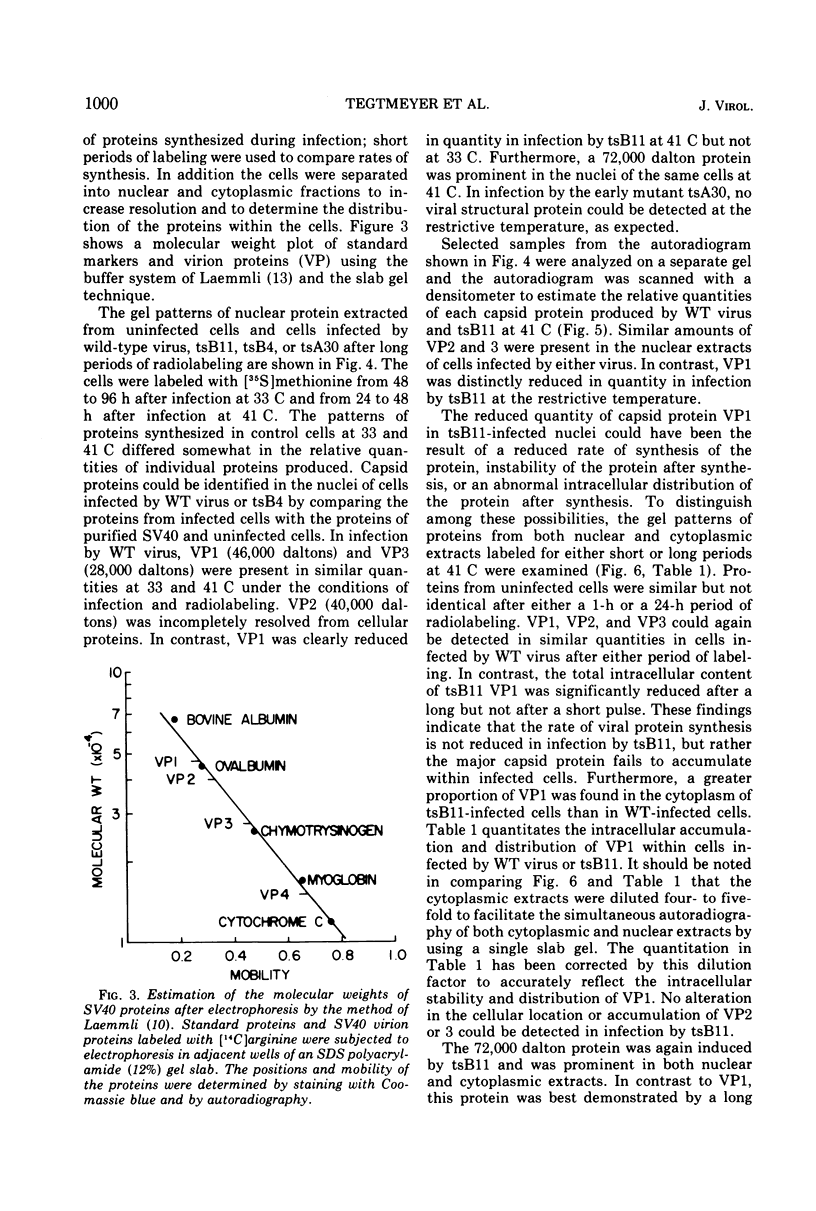
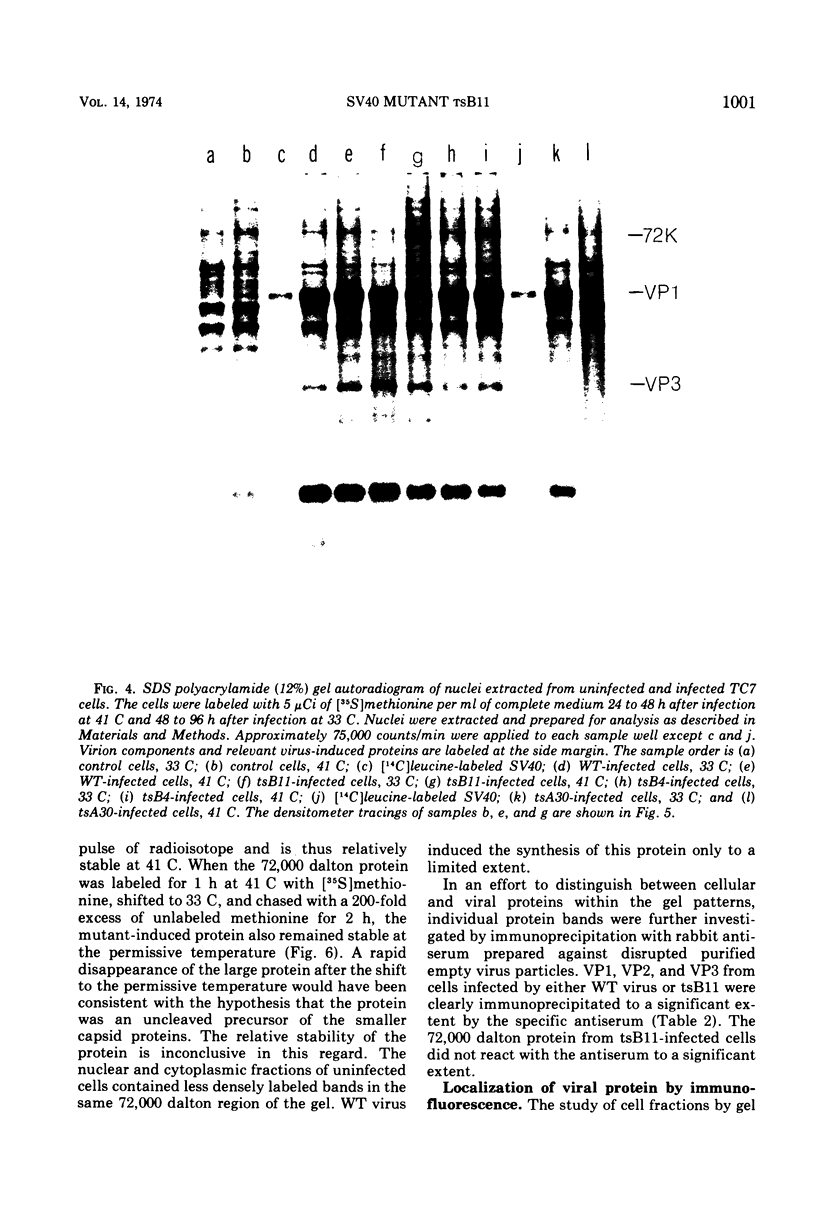
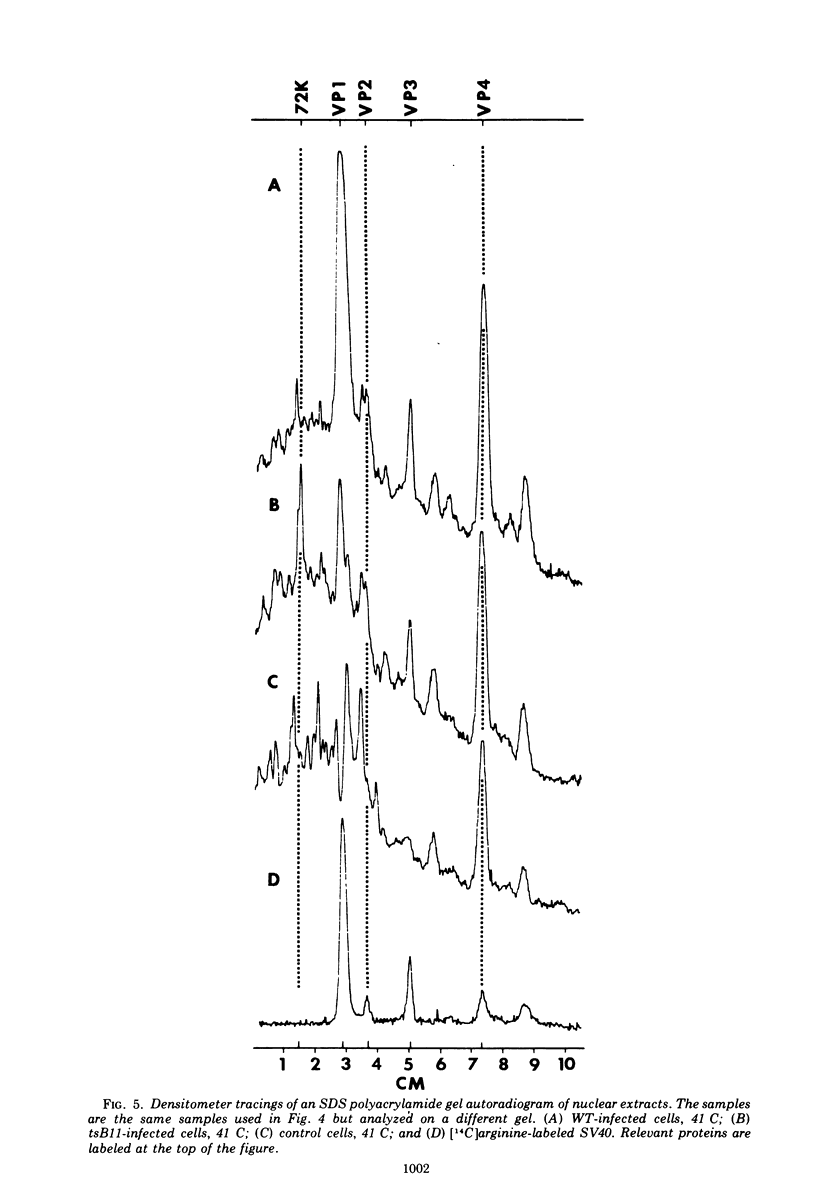
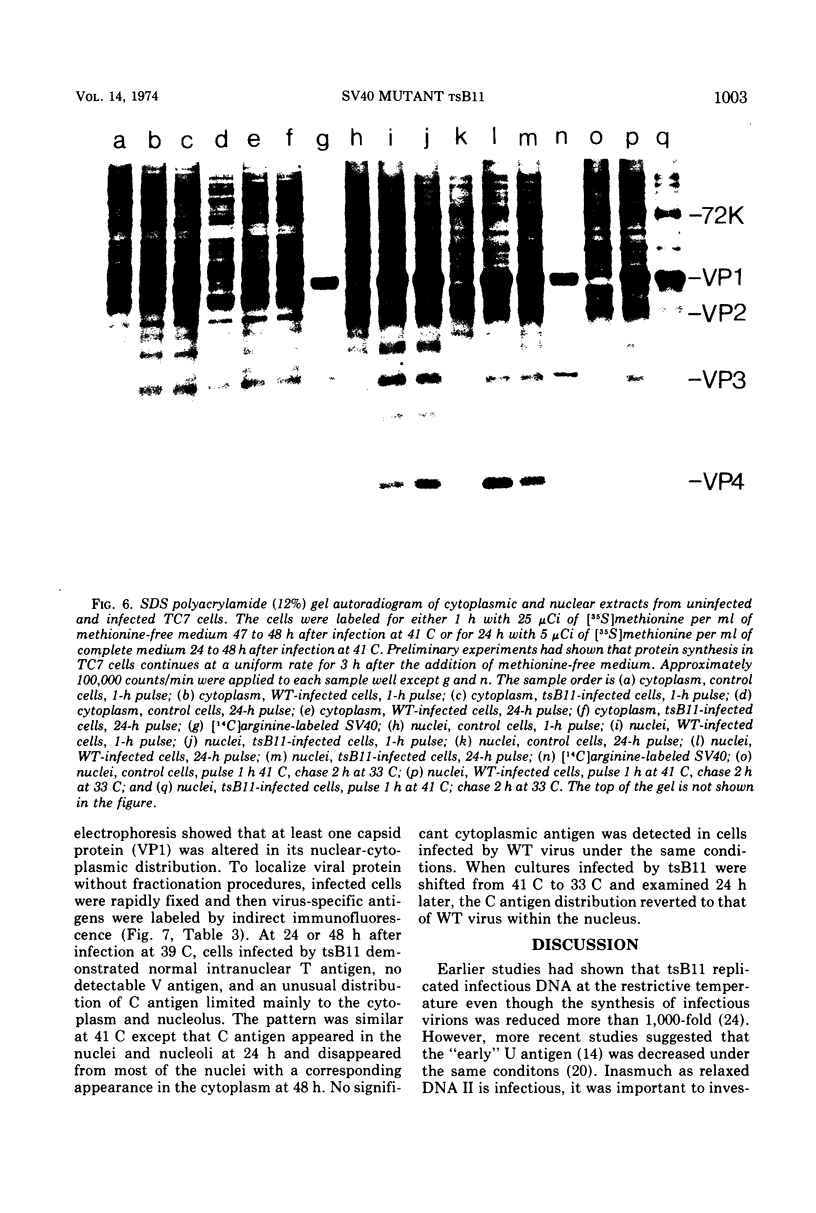
Abstract
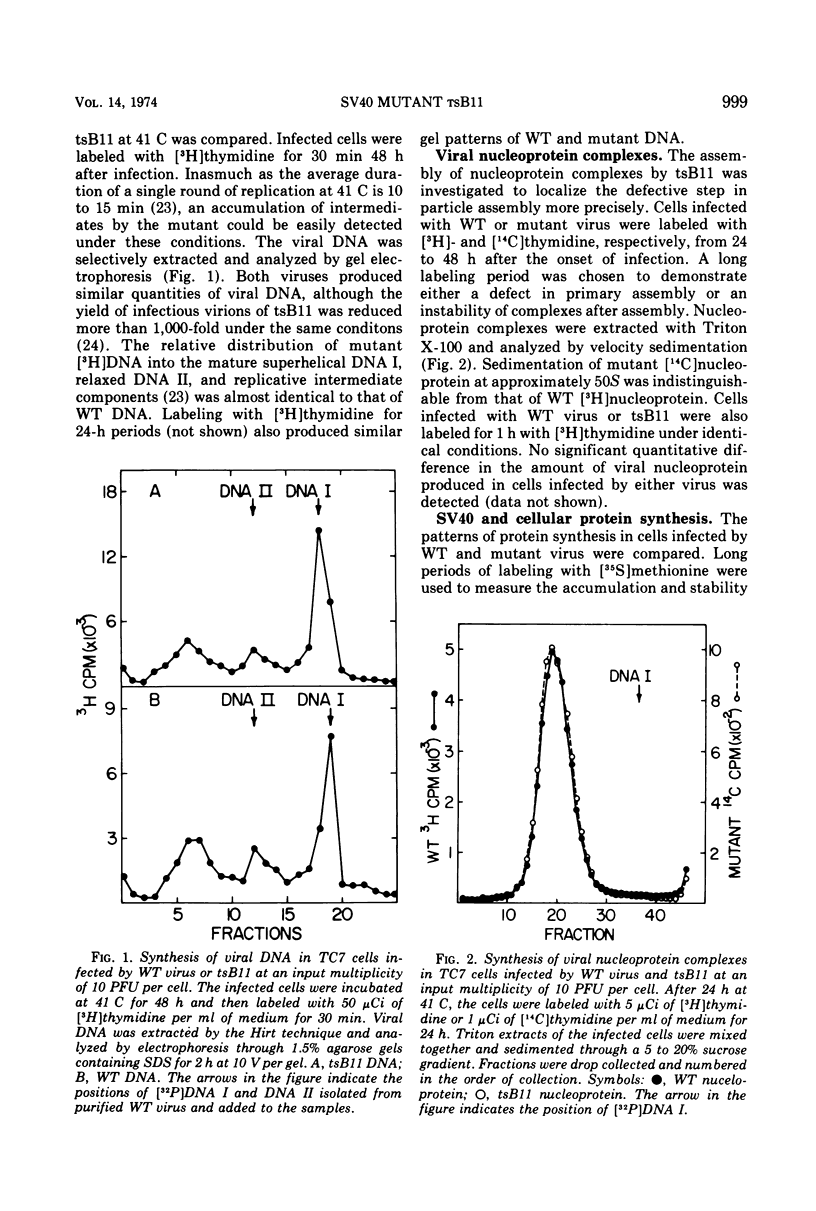
The DNA of the temperature-sensitive mutant tsB11 is replicated at the same rate as the DNA of wild-type virus in infection at the restrictive temperature. The progeny mutant DNA cannot be distinguished from wild-type DNA by gel electrophoresis and is assembled into a nucleoprotein complex with the same velocity sedimentation characteristics as the wild-type complex. Analysis of in vivo protein synthesis by sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoprecipitation techniques demonstrated that the capsid components VP1, VP2, and VP3 of the mutant and wild-type virus are synthesized at a similar rate, but VP1 fails to accumulate within cells infected by tsB11. Furthermore, VP1 is located predominantly in the cytoplasmic rather than in the nuclear fraction of extracts from cells infected by the mutant. Immunofluorescent studies localized virion antigen within the nucleolus as well as the cytoplasm. The altered intracellular distribution and stability of VP1 suggest that it may be the mutant protein of tsB11. The synthesis of a 72,000 dalton protein is consistently induced in significant quantity in cells infected by tsB11 at the restrictive temperature. A protein of the same apparent molecular weight is present in smaller quantities in uninfected cells and is only slightly increased in quantity in cells infected by wild-type virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Gesteland R. F. Pattern of protein synthesis in monkey cells infected by simian virus 40. J Virol. 1972 May;9(5):758–765. doi: 10.1128/jvi.9.5.758-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barban S., Goor R. S. Structural proteins of simian virus 40. J Virol. 1971 Feb;7(2):198–203. doi: 10.1128/jvi.7.2.198-203.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Villano B. C., Defendi V. Characterization of the SV40 T antigen. Virology. 1973 Jan;51(1):34–46. doi: 10.1016/0042-6822(73)90363-2. [DOI] [PubMed] [Google Scholar]
- Dubbs D. R., Rachmeler M., Kit S. Recombination between temperature-sensitive mutants of simian virus 40. Virology. 1974 Jan;57(1):161–174. doi: 10.1016/0042-6822(74)90117-2. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fischer H., Sauer G. Identification of virus-induced proteins in cells productively infected with simian virus 40. J Virol. 1972 Jan;9(1):1–9. doi: 10.1128/jvi.9.1.1-9.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Marty L., Suarez F. Capsid proteins of Simian virus 40. Biochem Biophys Res Commun. 1970 Jul 13;40(1):97–102. doi: 10.1016/0006-291x(70)91051-x. [DOI] [PubMed] [Google Scholar]
- Green M. H., Miller H. I., Hendler S. Isolation of a polyoma-nucleoprotein complex from infected mouse-cell cultures. Proc Natl Acad Sci U S A. 1971 May;68(5):1032–1036. doi: 10.1073/pnas.68.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D. Protein metabolism in SV40-infected cells. Virology. 1973 Nov;56(1):313–333. doi: 10.1016/0042-6822(73)90309-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P. Studies on nondefective adenovirus-simian virus 40 hybrid viruses. I. A newly characterized simian virus 40 antigen induced by the Ad2+ND 1 virus. J Virol. 1971 Feb;7(2):189–197. doi: 10.1128/jvi.7.2.189-197.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L. Synthesis and assembly of simian virus 40. I. Differential synthesis of intact virions and empty shells. J Virol. 1972 Jan;9(1):41–51. doi: 10.1128/jvi.9.1.41-51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Tegtmeyer P. Synthesis and assembly of simian virus 40. II. Synthesis of the major capsid protein and its incorporation into viral particles. J Virol. 1972 Jan;9(1):52–60. doi: 10.1128/jvi.9.1.52-60.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. 3. Characterization of a temperature-sensitive mutant blocked at an early stage of productive infection in monkey cells. J Virol. 1972 Jun;9(6):956–968. doi: 10.1128/jvi.9.6.956-968.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Smith H. S., Scher C. D. Genetic analysis of simian virus 40. IV. Inhibited transformation of Balb-3T3 cells by a temperature-sensitive early mutant. J Virol. 1972 Jun;9(6):969–972. doi: 10.1128/jvi.9.6.969-972.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Tegtmeyer P., Ishikawa A., Ozer H. L. Antigenic phenotypes and complementation groups of temperature-sensitive mutants of simian virus 40. J Virol. 1974 Mar;13(3):662–665. doi: 10.1128/jvi.13.3.662-665.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Macasaet F. Simian virus 40 deoxyribonucleic acid synthesis: analysis by gel electrophoresis. J Virol. 1972 Oct;10(4):599–604. doi: 10.1128/jvi.10.4.599-604.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Roblin R., Dulbecco R. Protein synthesis in Simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1972 Apr;69(4):921–924. doi: 10.1073/pnas.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M., Eason R. Nucleoprotein complexes in simian virus 40-infected cells. J Virol. 1971 Oct;8(4):363–371. doi: 10.1128/jvi.8.4.363-371.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]