Abstract
Background and purpose of the study
The objective of the study was to develop and characterize Diclofenac Diethylamine (DDEA) transdermal patch using Silicone and acrylic adhesives combination.
Methods
Modified solvent evaporation method was employed for casting of film over Fluoropolymer coated polyester release liner. Initial studies included solubilization of drug in the polymers using solubilizers. The formulations with combination of adhesives were attempted to combine the desirable features of both the adhesives. The effect of the permeation enhancers on the drug permeation were studied using pig ear skin. All the optimized patches were subjected to adhesion, dissolution and stability studies. A 7-day skin irritancy test on albino rabbits and an in vivo anti-inflammatory study on wistar rats by carrageenan induced paw edema method were also performed.
Results
The results indicated the high percent drug permeation (% CDP-23.582) and low solubility nature (1%) of Silicone adhesive and high solubility (20%) and low% CDP (10.72%) of acrylic adhesive. The combination of adhesives showed desirable characteristics for DDEA permeation with adequate % CDP and sufficient solubility. Release profiles were found to be dependent on proportion of polymer and type of permeation enhancer. The anti-inflammatory study revealed the sustaining effect and high percentage inhibition of edema of C4/OLA (99.68%). The acute skin irritancy studies advocated the non-irritant nature of the adhesives used.
Conclusion
It was concluded that an ideal of combination of adhesives would serve as the best choice, for fabrication of DDEA patches, for sustained effect of DDEA with better enhancement in permeation characteristics and robustness.
Keywords: Transdermal drug delivery system, Silicone adhesive, Acrylic adhesive, Permeation study, Dissolution, Skin irritancy and anti-inflammatory
Introduction
Drugs can be delivered across the skin to have an effect on the tissues adjacent to the site of application (topical delivery) or to have an effect after distribution through the circulatory system (systemic delivery). While there are many advantages for delivering drugs through the skin the barrier properties of the skin provide a significant challenge. By understanding the mechanisms by which compounds cross the skin it will be possible to devise means for improving drug delivery [1]. In the last decades, transdermal dosage forms have been introduced for providing a controlled delivery via the skin into the circulation system.
A transdermal patch or skin patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the blood stream. Drug-in-adhesive-type patches have been gaining increasing popularity as effective transdermal delivery systems during the last two decades [2] due to various advantages over other systems namely, they are easy to construct, less chances of dose dumping and patches with less thicknesses can be prepared.
Diclofenac is a well-established non-steroidal anti-inflammatory agent, widely used in musculoskeletal disorders, arthritis, toothache, dysmenorrhea, etc., for symptomatic relief of pain and inflammation [3]. Diethylammonium salt of Diclofenac (Diclofenac Diethylamine) is reportedly used for topical applications. Diclofenac Diethylamine (DDEA) gel (1.16%; Voltaren® Emulgel®, Novartis, Nyon, Switzerland) has been used extensively in Europe since 1985 to relieve the symptoms of OA of the knee, as well as other painful, inflammatory tendon, ligament, muscle, and joint conditions [4]. However, all NSAIDs include a boxed warning highlighting the potential for increased risk of cardiovascular events as well as serious potential life-threatening gastrointestinal bleeding. The drug undergoes substantial hepatic first-pass metabolism and thus only about 50% of the administered dose reaches systemic circulation [3,5]. This originates the need of an alternative route of administration, which can bypass the hepatic first-pass metabolism. Transdermal route is an alternative choice of route of administration for such drugs. The drug, Diclofenac Diethylamine also possesses the ideal characteristics, such as poor bioavailability (40 to 60%), short biological half-life (2 to 3 h), smaller dose (25 to 50 mg), etc., to be formulated into a transdermal patch. Transdermal patches offer added advantages, such as maintenance of constant and prolonged drug level, reduced frequency of dosing, minimization of inter and intra patient variability, self-administration and easy termination of medication, leading to patient compliance [6].
It has been postulated that Diclofenac transdermal exerts its pharmacological effects through localized accumulation at the site of application rather than from the systemic absorption. The bioavailability of Diclofenac transdermal is approximately 1% that of oral Diclofenac, with an elimination half-life of 12 h compared with 1.2 to 2 h with oral Diclofenac [7].
The present study aimed at developing TDDS drug-in-adhesive patches of DDEA using Silicone adhesives, Acrylic adhesives and blend of Silicone and Acrylic adhesives.
Material and methods
Materials
The Silicone polymers (S1 to S6) were purchased from Dow Corning Corporation, (midland, MIA, USA), Acrylic polymer (A) was purchased from National Starch and Chemical company (Bridge Water, NJ, USA). Fluoropolymer coated polyester release liner and Polyester Backing laminate was purchased from 3 M Scotchpak (st. paul, USA). The drug Diclofenac Diethylamine B.P (DDEA) was obtained from Sparsha Pharma International Pvt Ltd (Hyd, India). Methanol and Acetonnitrile were of HPLC grade and purchased from Sigma-Aldrich corporation, India. All other reagents used were of highest reagent grade available.
Preparation of patches containing silicone adhesives
Preparation of placebo silicone patches
Transdermal patches were prepared by modified solvent evaporation method. It is similar to conventional method except, that the drug-polymeric solution was spread over the release liner with the help of manual coater over release liner. Transdermal patches using different silicone polymers (Table 1) without drug were prepared. For preparing transdermal patches, an adequate amount of polymeric solution was taken and then spread over the release liner with the help of a manual coater. The polymeric solution coated liner was dried at 80°C in an oven for 10 min. The patches were then finally laminated with polyester backing membrane. The obtained sheets were punched using suitable dyes (3, 10 and 50 cm2) to get patches of appropriate sizes, packed in aluminum foil and stored in a desiccator for further studies. Patches were prepared using different grades of silicone adhesives.
Table 1.
Details of silicone and acrylic polymers used in the study
| Code | Functional group | Solvent | Solid content (%) | Viscosity (Mpa.s) |
|---|---|---|---|---|
|
Silicone Polymer |
|
|
|
|
| S1 |
Amine compatible |
Ethyl acetate |
60 |
350 |
| S2 |
Amine compatible |
Ethyl acetate |
60 |
800 |
| S3 |
Amine compatible |
Ethyl acetate |
60 |
1200 |
| S4 |
- |
Ethyl acetate |
60 |
650 |
| S5 |
- |
Ethyl acetate |
65 |
2500 |
| S6 |
- |
Ethyl acetate |
60 |
2600 |
|
Acrylic polymer |
|
|
|
|
| A, Polyacrylate | COOH | Ethylacetae and Hexane | 43.2 | 7000-19000 |
Physical evaluation of placebo patches
The tack of the patches – ball tack test
Tack is the ability of a pressure-sensitive adhesive to bond under conditions of light contact pressure and a short contact time. The tack of the skin contact adhesive was measured by the rolling ball tack test using primary adhesive tester (Labthink Instruments Co. Ltd., China). The patch with a size of 50 cm2 was fixed on a plate. Different diameter steel balls were released from the top of the inclined plate (angle 45°C). The number of the largest ball (0 – 9) which did not roll down was reported as the tack value [8].
Peel strength of patches
Peel strength measures the force required to peel away a pressure-sensitive adhesive once it has been attached to a surface. The test was performed with a Digital Peel tester with a load capacity up to 5 kg (Make: International Equipments, Model: CO). A piece of the patch which has a width of 10 mm and length of 25 mm was prepared, applied quickly to the end of the stainless steel plate and left the apparatus for 10 min.
The cello tape was affixed on the product. The free end of this tape was bending back 180° and it was attached firmly to the upper part of a peel testing machine with a clamp. The instrument was started with a speed of 300 mm/min and the values were recorded. Five patches form each batch were used measuring strength and their values were averaged [8].
Preparation of drug loaded silicone patches
Solubility of drug in adhesives
The solubility of drug in adhesive was tested in silicone adhesives (S3 and S6). Different concentrations of drug (5% w/w, 3% w/w, 2% w/w and 1% w/w of final patch formulation) were added to the adhesives under constant stirring with the help of magnetic stirrer. The stirring was continued for a period of 4 h in order to ensure complete mixing. The solution was kept aside overnight for visual observation. The solutions that showed turbidity were discarded and solutions that remained clear were coated over release liner and finally laminated with backing layer as described in the section 2.2.1.
Solubility enhancement techniques
Various solubility enhancements used to increase the solubility of Diclofenac Diethylamine in silicone adhesives include
Addition of solubilizers
Polyethylene glycol – 400 (PEG 400) and Propylene glycol (PG) in different concentrations (% w/w of final patch formulation) were used [9]. The adhesive polymeric solution, drug and solubilizer in required quantities were weighed and mixed with the aid of magnetic stirrer for a period of about 4–5 h. The solutions were monitored visually for appearance of turbidity/sedimentation. The formulations that showed clear solution after 24 h were coated over release liner and laminated with polyester backing laminate. The patches were packed in aluminum foil, kept aside for 10 days for appearence of crystals visually and microscopically. The patches which did not show crystals after 10 days were selected for further study.
Addition of oils
Four oils were slected based on preliminary study to improve the solubility of Diclofenac Diethylamine [10]. The oils, Oleic acid (OLA), Iso stearic acid (ISA), Pharamasolve (PS) and Iso propyl myristate (IPM) in different ratios of drug: solubilizer were mixed with polymeric solution and then monitored visually for turbidity. The clear solutions were used for preparation of patches which were kept aside for 10 days for appearance of crystals.
Ex vivo skin permeation studies
Preparation of skin barrier
Fresh full-thickness (75–80 mm) pig ear skin was used for the study. The experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals and approved by Animal Ethical Committee of Department of Genetics, Osmania University, Hyderabad, India (approval no.380/01/a/CPCSEA). Fresh pig ears were obtained from a local abattoir; to ensure integrity of the skin barrier, ears were removed post-sacrifice. The skin was dermatomed (Zimmer electric Dermatome Handset) to remove dermis [11,12]. The isolated epidermis (100 μm) was rapidly rinsed with hexane to remove surface lipids and then rinsed with water and used immediately.
The ex vivo skin permeation from the prepared drug polymeric patches across the porcine ear skin barrier was studied using Franz diffusion cell (Orchid Scientifics & Innovative India Pvt Ltd.), [13,14]. Twenty - five milliliters of phosphate buffer of pH 7.4 was used as an elution medium. The diameter of the donor compartment cell provided an effective constant area of 3.4 cm2. The dermatomed pig ear skin was mounted between the two compartments of Franz diffusion cell with stratum corneum facing towards the donor compartment. A 3 cm2 patch was used for the study. The release liner was removed. The patches to be studied were placed in between the donor and the receptor compartment in such a way that the drug releasing surface faced toward the receptor compartment. After securely clamping the donor and receptor compartments together, the elution medium was magnetically stirred for uniform drug distribution at a speed of 60 rpm. The temperature of the whole assembly was maintained at 32 ± 0.5°C by thermostatic arrangements. An aliquot of 0.5 mL was withdrawn at preset time intervals for a period of 24 h and an equivalent volume of fresh buffer was replaced. The samples removed were analysed by HPLC described below.
Preparation of patches containing acrylic adhesive
The formulations containing different concentrations of drug with acrylic adhesive were prepared by the method described under section 2.2. The patches prepared were monitered for appearance of crystals visually for 10 days. The properties of acrylic adhesive were mentioned in the Table 1. The patches which showed stability were subjected to peel test, ball test (described under 2.2) and permeation study (described in section 2.3).
Preparation of patches containing combination of silicone and arylic adhesives
Placebo patches containing combination of silicone and acrylate adhesives in different ratios and drug containing combinational patches were prepared by the following method:
In First step, required amount of drug (% w/w of final patch formulation) was made to dissolve completely in appropriate amount of acrylate adhesive by continuous stirring. Second step involves addition of silicone polymeric solution to clear solution formed in step 1 and then continuing mixing for a period of 12 h. The formulations that showed drug solubility after 24 h were laminated into patches. The patches which showed stability were subjected to peel test, ball test (described under 2.2) and permeation study (described in section 2.3).
Effect of permeation enhancers on drug loaded combinational patches
The incorporation of a permeation enhancer is indispensable for achieving the desired permeation rate for almost all drugs with the limited size of the patch. The permeation enhancers Oleic acid (OLA), Iso Stearic acid (ISA) and Isopropyl Myristate (IPM) at concentraions of 5% each were chosen to study their effect on permeation of Diclofenac Diethylamine across the skin. The solubilized combinational patches (C4 and C5) along with different permeation enhancers (5% concentration) were formulated and subjected for permeation study as described under 2.3.
Characterization of optimized patches
Various physicochemical tests employed for optimized transdermal patches were as shown
Thickness
Patch thickness was measured using digital micrometer screw gauge (Mitutoyo, Japan) at five different places. The average and standard deviation of five readings were calculated for each batch of the drug-loaded films.
Weight uniformity
Five different films from individual batches were weighed individuall, and the average weight was calculated the individual weight should not deviate significantly from the weight was calculated, the individual weight should not deviate significantly from the average weight, so the standard deviation was calculated [15].
Drug content
Assay of Diclofenac Diethylamine was done with the help of HPLC. All the solvents used were of HPLC grade [16].
Sample solution
For determination of drug content one patch of 50 cm2 was taken, dissolved in HPLC grade methanol and sonicated for 15 min. From above solution 1 mL was taken into a 50 mL volumetric flask, diluted up to the mark with methanol, filtered through Nylon membrane filters of 0.45 μ size (Pall Pharmalab Filtration Pvt. Ltd.) and injected (20 mL) into the HPLC column.
Standard solution
For preparing standard solution, 50 mg of Diclofenac Diethylamine was dissolved in 50 mL methanol (HPLC grade). From the above solution, 1 mL was taken and diluted to 50 mL with methanol which was finally filtered through a Nylon membrane filters of 0.45 μ size (Whatman GF/C) and injected (20 mL) into the HPLC column.
HPLC conditions
The HPLC system consisted of L-7110 pump (Shimadzu Corporation, Japan) with L-7420 variable-wavelength ultraviolet absorbance detector (Shimadzu Corporation, Japan) set at 274 nm. Analysis was performed on a reversed-phase column made of silica (150 mm × 4.6 mm i.d., 5 μm, Chemsil BDS C18, Beijing China), operated at 40°C. The mobile phase consisted of 45: 55 ratio of 0.5% Glacial acetic acid in water and Acetonitrile. HPLC grade water was used for the preparation of 0.5% Glacial acetic acid solution. The flow rate of mobile phase was set at 0.8 mL/min, was used. The injection volume is 20 μL.
In vitro release – dissolution studies
The release-rate determination is one of the most important studies to be conducted for all controlled release delivery systems. The dissolution studies of patches are very crucial, because one needs to maintain the drug concentration on the surface of stratum corneum consistently and substantially greater than the drug concentration in the body, to achieve a constant rate of drug permeation [17].
A Paddle over disc assembly (USP 23, Apparatus 5) was used for the assessment of release of DDEA. The TDDS patch was mounted on the disc and placed at the bottom of the dissolution vessel. The dissolution medium, 900 ml degassed distilled water at pH 7.0. The apparatus was equilibrated to 32 ± 0.5°C and operated at 50 rpm [18] during the entire study period (24 h). The dissolution medium was degassed by a combination of heating up to 45°C and vacuum filtration followed by vigorous stirring of media under vacuum.
Stability study
The optimized formulations were subjected to stability study by storing patches at 40 ± 2°C and 75% RH in stability chamber for three months. Two parameters namely, peel strength and drug content were analyzed.
Surface morphology
The surface morphology of formulated transdermal patches (both stable and unstable) were investigated by using Scanning electron microscope (model: SEM JSM-6610) at 15 kV under different magnifications (950x, 1000x and 1500x). In order to make the samples electrically conductive the samples were gold coated prior to the study.
Acute skin irritancy test
The study was conducted on the basis of the approval of institutional animal ethical committee. Albino rabbits of either sex, each weighing 1.5 to 2.0 kg, divided into two groups, were used in this study (n = 4 in each group) [14]. They were housed in cages in the animal house under controlled temperature and light conditions. They were fed a standard laboratory diet and had access to water ad libitum. The dorsal surface of the rabbits was cleared and the hair was removed by shaving. The skin was cleared with rectified spirit. The experimental patch (A1, group II), one patch per day, were applied to the shaved skin of rabbits and secured using USP adhesive tape (Johnson & Johnson limited, Mumbai). A 0.8% (v/v) aqueous solution of formaldehyde was applied as a standard irritant (group I). Its effect was compared with the test [19]. The animals were observed for any sign of erythema and edema for a period of 7 days and scored as reported by Draize et al. (1944) [20]. The Draize method of scoring was shown in Table 2.
Table 2.
Draize evaluation of dermal reaction
|
Scoring |
Reaction |
|
|---|---|---|
| Erythema | Edema | |
| 0 |
No erythema |
No edema |
| 1 |
Very slight erythema |
Very slight edema |
| 2 |
Well-defined erythema |
Slight edema |
| 3 |
Moderate to severe erythema |
Moderate edema |
| 4 | Severe erythema | Severe edema |
Anti-inflammatory study
The study was conducted in accordance with the Ethical Guide- lines for Investigations in Laboratory Animals and was approved by the Ethics Review Committee for Animal Experimentation of Osmania University. The anti-inflammatory activity and sustaining action of the drug-loaded drug in adhesive patches were evaluated using “carrageenan-induced hind paw edema” method developed by Winter et al. (1965) [21]. Wistar rats were used after being allowed to acclimatize for 1 week. Before the day of administration, rats were fasted overnight but were allowed access to water ad libitum. Eight rats weighing 180–220 g (6–8 weeks old) divided into two groups were used for the study. The backsides of rats were shaved 12 h before starting the experiments.
Group – I (Control group): Paw edema was induced by injecting 0.1 mL of a 1% w/v homogeneous suspension of carrageenan in double-distilled water [21] The volume of injected paw was measured immediately (0 h) and at 0.5, 0.75, 1, 2, 3, 4, 5, 6, 8, 10, 12, 16 and 24 h after injection using a IMCORP plethysmometer [22]. The amount of paw swelling with respect to initial volume was determined time to time. It is obtained by subtracting volume of injected paw at time ‘0’ from volume of injected paw at time ‘t’ divided by volume of injected paw at time ‘0’.
Group – II (Test): Treated similar to control group except that patches were applied half an hour before sub-plantar injection of carrageenan. Percent (%) inhibition of edema produced by each patch- treated group was calculated against the respective control group using the following formula
| (1) |
Results and discussion
Evaluation of placebo silicone patches
The placebo patches using six grades of silicone polymer were prepared. The patches were smooth, flexible and uniform. The S1 patches were ruled out because during preparation of patches after drying the polymer completely lost its adhesive property.
An ideal adhesive polymer for drug-in-adhesive system is one that exhibit greater adhesion value. The initial screening of the silicone adhesives was done by ball tack test and peel strength of patches. The peel test, ball test and adhesive mass test values of all placebo silicone patches of different thicknesses were as shown in Table 3. The ball test and peel test values for different formulations of thickness 200 μm were in the following order: S3 > S6 > S5 > S2 > S4. Hence, S6 and S3 polymers showed better peel adhesion and ball test values hence, selected for further study.
Table 3.
Adhesive mass values, ball test and peel test values for placebo silicone patches
|
Parameter and thickness |
Polymer type |
||||
|---|---|---|---|---|---|
| S2 | S3 | S4 | S5 | S6 | |
|
Ball tack testa |
4 |
8 |
0 |
5 |
8 |
| Peel test (Kg/cm)b | 0.384 | 0.646 | 0.0938 | 0.495 | 0.645 |
(S2, S3, S4, S5 and S6 represents different Silicone polymers used; a = 3 and b = 5).
Evaluation of drug loaded silicone patches
Solubility of drug in pure silicone adhesives
The solubility of Diclofenac Diethylamine in S3 and S6 was tested. The solutions that remained clear after 24 h were coated over the release liner. Results were shown in (Table 4). The Polymeric adhesive S3 only showed clear solution with 1% drug concentration. The results indicated low solubility of Diclofenac Diethylamine in Silicone adhesives and stresses on the need for the solubilizers for solubilization of drug.
Table 4.
Solubilization summary of drug loaded silicone polymers
| Formulation code | Drug concentration | Solubilizer concentration | Observation |
|---|---|---|---|
|
DS3 |
5% |
- |
Clear solution was not formed. |
|
DS3 |
3% |
- |
Clear solution was not formed. |
|
DS3 |
2% |
- |
Clear solution was not formed. |
|
DS3 |
1% |
- |
Clear solution indicating solubilization of drug |
|
DS3 |
0.5% |
- |
Clear solution indicating solubilization of drug. |
|
DS6 |
1% |
- |
Clear solution was not formed. |
|
DS3E1 |
5% |
5% PEG-400 |
Clear solution was not formed. |
|
DS3E2 |
3% |
5% PEG-400 |
Clear solution was not formed. |
|
DS3E3 |
2% |
3% PEG-400 |
Clear solution was formed. |
|
DS6E4 |
1% |
3% PEG-400 |
Clear solution was formed. |
|
DS6E5 |
2% |
3% PEG-400 |
Clear solution was not formed. |
|
DS3G1 |
5% |
5% PG |
Clear solution was not formed. |
|
DS3G2 |
2% |
5% PG |
Clear solution was formed. |
|
DS3G3 |
1% |
2% PG |
Clear solution was formed. |
|
DS3O1 |
3% |
2% OLA |
Clear solution was not formed |
|
DS3O2 |
2% |
2% OLA |
Clear solution was not formed |
|
DS3O3 |
1% |
2% OLA |
Clear solution was formed |
|
DS3I1 |
4% |
2% ISA |
Clear solution was not formed |
|
DS3I2 |
3% |
2% ISA |
Clear solution was not formed |
|
DS3I3 |
1% |
2% ISA |
Clear solution was formed |
|
DS3M1 |
1% |
2% IPM |
Clear solution was not formed |
|
DS3P1 |
1% |
2%Pharmasolve |
Clear solution was not formed |
| DS3O4I4 | 3% | 5% OLA & 5% ISA | Clear solution was formed. |
DS: drug with silicone polymer; S3: Silicone polymer grade, S3; S6: Silicone polymer grade S6; OLA: oleic acid; ISA: Iso stearic acid; IPM: Isopalmitic acid; PEG 400: Polyethylene glycol −400; PG: Propylene glycol.
Solubility enhancement techniques
Solubility of Drug in Silicone adhesives in the presence of solubilizers
Two solubilizers namely PEG - 400 and PG were tested to increase the solubility of Diclofenac Diethylamine in Silicone adhesives. Though PEG - 400 and PG increased Diclofenac Diethylamine solubility in water [9], their role to solubilize the drug in Silicone adhesive was abortive. Table 4 shows the drug concentration and solubilizer concentration used. Except few, all the solutions showed turbidity. In case of DS3E3 a clear solution was formed with 2% drug and 3% PEG - 400 while, in formulations containing S6 polymer (DS6E5) a clear solution was not formed with 2% drug and 3% PEG - 400. Similar to PEG - 400, PG showed slight improvement in solubility of drug in S3 polymer.
From solubility studies, it can be concluded that compared to S6, S3 polymer showed solubilization of DDEA to some extent. So, S3 polymer was chosen for further study.
Solubility of Drug in Silicone adhesives in the presence of oils
As formulations with PEG - 400 and PG showed little/no improvement in solubility, various oils namely oleic acid (OLA), IsoStearic acid (ISA), Pharmsolve® and Isopropyl Myristate (IPM) were tested for their ability to improve solubility of drug using the method described in experimental section. The formulations which remained clear after 24 h were coated over release liner. Among various oils tested, OLA and ISA were promising. However, only 1% drug was solubilized in both the cases (DS3O3 and DS3I3) While, IPM and Pharmsolve® did not even solubilize 1% drug. Combination of solubilizers was also tested but, only 3% drug solubilization was achieved in S3 polymeric adhesive at 10% solubilizer concentration (OLA and ISA, 5% each).
The prepared patches (DS3 with 1% drug, DS3E3, DS6E4, DS3G2, DS3G3, DS3O3, DS3I3 and DS3O4I4) were uniform. However, after 10 days patches with additives OLA and ISA (DS3O3, DS3I3 and DS3O4I4) ended up with formation of crystal growth. Figure 1 and Figure 2 shows crystallization in patches DS3O3 and DS3I3, respectively.
Figure 1.
Photograph of Silicone patch, DS3O1,showing crystal formation.
Figure 2.
Photograph of Silicone patch, DS3I3,showing crystal formation.
In case of formulations containing PEG - 400 and PG as additives, though the patches showed no crystallization, the solutions after 10 days took oil like consistency due to formation of oil globules in the solution resulting in loss of adhesion (Table 5).
Table 5.
Crystallization summary of drug loaded silicone patches with or without solubilizers
| Formulation code | Drug concentration | Solubilizers concentration | Patch Observation after 10 days | Solution observation after 10 days |
|---|---|---|---|---|
|
DS3 |
0.5% |
- |
No sign of crystallization |
Same as first day |
|
DS3 |
1% |
- |
No sign of crystallization |
Same as first day |
|
DS3E3 |
2% |
3% PEG-400 |
No sign of crystallization |
Oil globules were formed and the solution turned oily |
|
DS6E4 |
1% |
3% PEG-400 |
No sign of crystallization |
Oil globules were formed and the solution turned oily |
|
DS3G2 |
2% |
5% PG |
No sign of crystallization. |
Oil globules were formed and the solution turned oily |
|
DS3G3 |
1% |
2% PG |
No sign of crystallization. |
Oil globules were formed and the solution turned oily |
|
DS3O3 |
1% |
2% OLA |
Crystallization was seen. |
Same as first day |
|
DS3I3 |
1% |
2% ISA |
Crystallization was seen. |
Same as first day |
| DS3O4I4 | 3% | 5% OLA & 5% ISA | Crystallization was seen. | Same as first day |
The patches which did not contain any additives remained clear even after 10 days hence considered stable. Among various formulations prepared, DS3 containing 1% drug was chosen for further study.
Ex vivo skin permeation study
The DS3 patch containing 1% drug was chosen for conducting permeation study. The cumulative amount of drug permeated (CPD) at the end of 24 h was found to be 19.573 mcg/cm2 (Table 6). Though the amount of drug permeated was low, the percentage cumulative amount of drug permeated was 23.209%. The low CPD value might be due to less amount of drug (1%) in the patch.
Table 6.
Permeation study of formulation DS3 and DA
|
S. No |
TIME (h) |
DS3 |
DA |
||
|---|---|---|---|---|---|
| CDP (μg/cm2) | % CDP | CDP (μg/cm2) | % CDP | ||
| 1. |
0 |
0 |
0 |
0 |
0 |
| 2. |
2 |
1.185 |
1.405138 |
5.934 |
0.84051 |
| 3. |
4 |
2.6843 |
3.182964 |
12.847 |
1.819688 |
| 4. |
6 |
4.2813 |
5.07664 |
19.131 |
2.709773 |
| 5. |
8 |
5.9842 |
7.095889 |
25.819 |
3.657082 |
| 6. |
10 |
7.7583 |
9.199565 |
31.824 |
4.507649 |
| 7. |
12 |
9.3142 |
11.04451 |
38.119 |
5.399292 |
| 8. |
14 |
11.042 |
13.09328 |
45.248 |
6.409065 |
| 9. | 24 | 19.573 | 23.20909 | 75.692 | 10.72125 |
CDP: Cumulative drug permeated; % CDP: percent cumulative drug permeated; DA: drug with Acrylic polymer alone.
Evaluation of drug loaded acrylic patches
The extensive solubilization study conducted revealed that the silicone polymer is unsuitable for achieving very high concentrations of DDEA. Hence, solubility of drug in acrylic adhesive was tested by method described under experimental section I of IIIA. It was noticed that drug concentrations up to 20% was solubilized without use of any additives. The prepared patches were also stable after 10 days and did not showed crystal formation. While 25% of drug polymeric solution resulted in turbidity (Table 7). This might be because of drug loading greater than the saturation solubility of the drug in the adhesive used. However, the concentration of drug was fixed at 10% for further study since the formulation being studied is intended for topical use. The Ball test and peel strength values for DA were shown in Table 7.
Table 7.
Solubilization summary, peel test, ball test and adhesive mass value for acrylic adhesive patches
| Polymer | Drug concentration | Observation | |
|---|---|---|---|
| A |
10% |
Clear solution formed |
|
| A |
15% |
Clear solution formed |
|
| A |
20% |
Clear solution formed |
|
| A |
25% |
Clear solution was not formed |
|
|
Parameter evaluated |
DA |
|
|
| Peel test (Kg/cm) |
0.9306 |
|
|
| Ball test | 8 | ||
(n is equal to 5 and 3 for Peel test and Ball test respectively).
Ex vivo skin permeation study
Skin permeation of Diclofenac Diethylamine was studied using DA patch containing 10% drug. Study was conducted for 24 h without using permeation enhancer. The cumulative amount of drug permeated into the receptor compartment was 75.692 mcg/cm2 after 24 h (Table 6) that represents 10.72% of the total drug placed in the donor compartment.
Though the CDP of DA was significantly greater than CDP of DS3 the percent drug permeated was high in case of DS3 (23.209%). The CDP of DA was found to be significantly high because the drug concentration in DA was 10 times greater than that of DS3.
Evaluation of drug loaded combinational patches
Studies on Silicone adhesives revealed poor solubilization capacity and high percent cumulative drug permeation (%CDP) value whereas; acrylic polymers solubilized higher concentrations of drug but exhibited less%CDP. Hence an attempt was made to combine high%CDP property of Silicone and greater drug solubilization property of acrylic polymer by fabricating a drug formulation with combination of adhesives.
The placebo solutions containing different proportions of Silicone and Acrylic adhesives were prepared and used as reference for checking the solubility of drug in combination of adhesives. The combinations from C1 to C5 showed similar consistency as compared to respective placebo patches after addition of drug. Table 8 shows the Solubility data of different combinations of Silicone and acrylic with 10% drug. The results indicated that minimum 50% acrylic polymer is required in the formulation to achieve 10% drug solubility (As acrylic polymer alone can solubilize 20% drug without any solubilizer as mentioned earlier, it is evident that 50% acrylic polymer is sufficient to solubilize 10% drug). Hence, formulations C1, C2, C3, C4 and C5 were used for further study.
Table 8.
Solubility of drug in combinational patches
| Formulation code | Ratio of Silicone: Acrylic | Targeted drug concentration | Solubility observation |
|---|---|---|---|
| C1 |
10: 90 |
10% |
YES |
| C2 |
20: 80 |
10% |
YES |
| C3 |
30: 70 |
10% |
YES |
| C4 |
40: 60 |
10% |
YES |
| C5 |
50: 50 |
10% |
YES |
| C6 |
60: 40 |
10% |
NO |
| C7 |
70: 30 |
10% |
NO |
| C8 |
80: 20 |
10% |
NO |
| C9 | 90: 10 | 10% | NO |
C: combination patches with Acrylic and Silicone adhesives.
Ex vivo skin permeation experiment
In all the combinational patches, the CDP of combinational patches was higher than that DA patches which contains Acrylic polymer alone. Figure 3 shows the amount of drug permeated at the end of 24 h with and without combinational patches. The CDP of different combinational patches were in the following order:
Figure 3.
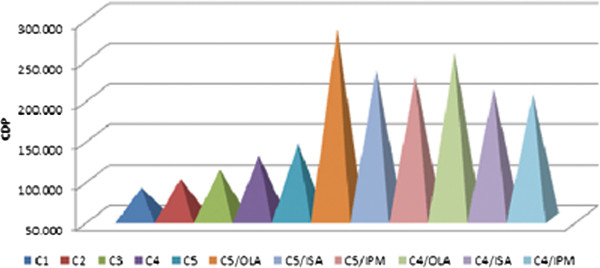
Graph showing cumulative amount of drug permeated (CDP) at the end of 24 h with and without permeation enhancers for combinational patches (C4/OLA, C4/ISA and C4/IPM reprsents C4patch with oleic acid (OLA), Isostearic acid and Isopalmitic Myristate as permeation enhancers, respectively. Similar in case of C5 combinational patches).
The above order once again reflected the previous results i.e. with increase in amount of Silicone polymer the amount of CDP increased. Among all five formulations C5 displayed high CDP value due to its high Silicone content (50% of the total polymer).
Figure 4 shows plot between flux and time for all the five combinations. The graph showed little/less variation in the flux between different time intervals for C4 and C5. Moreover, the CDP was found to be relatively high for these two formulations. Hence, the formulations C4 and C5 were chosen for further study.
Figure 4.
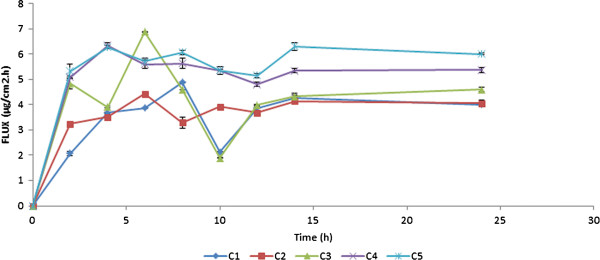
Graph showing the plot between Flux (μg/cm2.h) and time (h) for C1, C2, C3, C4and C5patches (data represented as mean ± S.D).
Effect of permeation enhancers on the permeation of DDEA
Three permeation enhancers namely, OLA, ISA and IPM were used at 5% concentration. The cumulative amount of drug permeated at the end of 24 h was represented in Figure 3. The permeation data revealed greater penetration enhancing capability of OLA than ISA and IPM. This is in line with the result reported where OLA increased the permeation of DDEA by 7–9 folds (Hussain Shah et al. 2012) [10]. Thus, it can be concluded that vehicles used here were predominantly influencing the partition of the drug into the skin. Hence, C4/OLA and C5/OLA which exhibited greater CDP among all were chosen as optimized formulations.
Figure 5 shows the plot between CDP and time for different formulations. From the graph, it can be predicted that C4 and C5 showed high CDP compared to DA indicating more drug permeation capacity compared to individual Acrylic formulations. OLA application as a permeation enhancer was well justified as significant increase in CDP value was observed compared to patches without enhancers.
Figure 5.
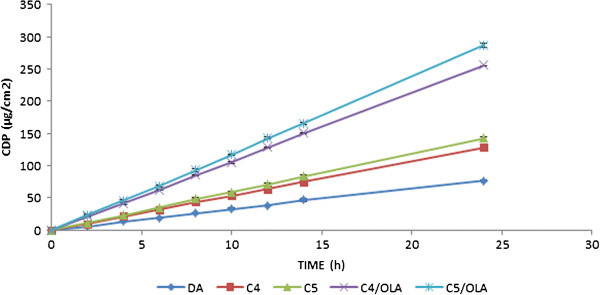
Graph showing the plot between cumulative amount of drug permeated (CDP, μg/cm2) and time (h) for DA, C4,C5, C5/OLA and C4/OLA (data represented as mean ± S.D).
Dissolution study of patches
In vitro release profile is an important tool that predicts in advance how the drug will behave in vivo. Thus, we can eliminate the risk of hazards during experimentation in living system. Five patches, DS3, DA, C4/OLA and C5/OLA were studied for drug release. The study was conducted for a period of 24 h. The percent drug release (Figure 6) was found to be in the following order:
Figure 6.
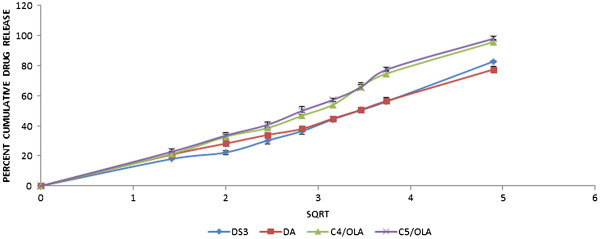
Graph showing the Higuchi plot for DS3, DA, C5/OLA and C4/OLA (data represented as mean ± S.D).
The dissolution values revealed that formulations containing OLA exhibited greater percent cumulative drug release (%CDR) than DS3 and DA. This might be due to increased solubility of poorly soluble drug, DDEA, in water due to OLA. Among C5/OLA and C4/OLA the formulation containing greater portion of silicone polymer, C5/OLA, showed greater%CDR.
Release kinetics
The dissolution data of C4/OLA and C5/OLA was put forth for release kinetic studies. Based on high R2 value it was shown that drug release from the formulations followed Higuchi pattern of drug release, with R2 value 0.978 for C4/OLA and 0.981 for C5/OLA, (Figure 6) where drug diffusion through the polymeric system was the main mechanism. The ‘n’ value from the korsemeyer-peppas plot revealed non-fickian/anomalous diffusion pattern (n>0.5).
Stability study
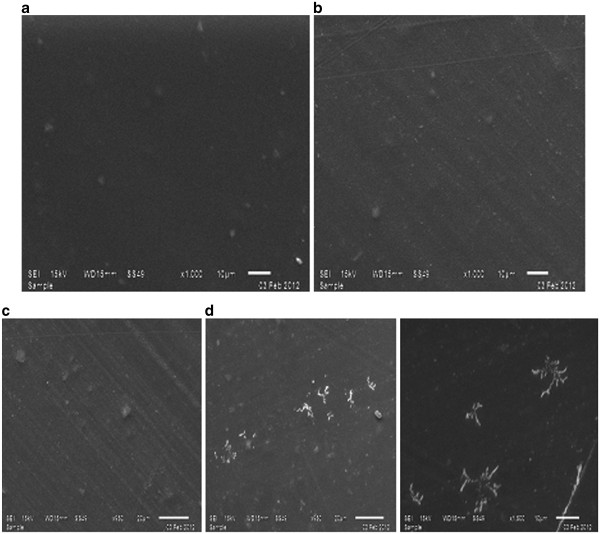
The formulations C5/OLA and C4/OLA were kept for 3 month stability study. During stability study in case of C5/OLA, crystallization (Figure 7) was observed which might be due to saturation of drug solubility which resulted in slow precipitation of drug. This is also reflected in its drug content shown in Table 9 where the percent drug content of the formulation kept on decreasing. Such a saturated matrix is unstable and the drug will recrystallize in such systems over time [23-25]. Recrystallization may however not be apparent immediately after manufacture because of the relatively low diffusion coefficients of drug in such highly viscous systems and the requirement of nucleation for the initiation of crystallization. Figure 8 shows the photograph of the C4/OLA after stability with no crystals and Figure 9 shows photograph of the C5/OLA after stability with crystal formation.
Figure 7.
SEM micrographs of optimized formulations before and after stability study: (a) and (c) represent C4/OLA and C5/OLA patches before stability, respectively; (b) and (d) represent C4/OLA and C5/OLA patches after stability, respectively.
Table 9.
Stability data for formulation C4/OLA and C5/OLA
| Tested parameters | 0 days | 45 days | 90 days |
|---|---|---|---|
|
C4/OLA | |||
| Peel Strength (Kg/cm) |
0.663 |
0.646 |
0.659 |
| Drug content (%) |
104.6 |
103.6 |
104.1 |
|
C5/OLA | |||
| Peel Strength (Kg/cm) |
0.654 |
0.649 |
0.652 |
| Drug content (%) | 101.64 | 98.86 | 94.38 |
(C4/OLA: C4 combination patch with oleic acid, OLA, as permeation enhancer; C5/OLA: C5combination patch with oleic acid, OLA, as permeation enhancer; n=3 for Drug content and n=5 for Peel strength).
Figure 8.

Photograph of the patches C4/OLA after stability.
Figure 9.
Photograph of the patches C5/OLA after stability.
The peel test of both the formulations showed no significant change during stability study indicating the sustainability of adhesive property of the polymeric combination.
However, in case of C4/OLA crystallization was not found and moreover the drug content remained stable representing robustness of the formulation during 3 month stability. Hence, the formulation C4/OLA was found to be the optimized formulation.
Physical evaluation of optimized patches
Figure 10(a) and 10(b) shows the original patch C4/OLA of sizes 10 cm2 and 3 cm2, respectively. The optimized formulation C4/OLA was tested for various physical parameters. The thickness (n = 5) of C4/OLA patches was found to be 181.63 ± 0.03 μm. Good weight uniformity among the batches was observed for all formulations and ranged from 214.33 – 216.35 mg. The results indicate that the process which was employed to prepare patches in this study was capable to produce patches with uniform drug content and minimal patch variability.
Figure 10.
Photograph of optimized C4/OLA patches both 10 cm2(a) and 3 cm2(b) patches.
Acute skin irritancy study
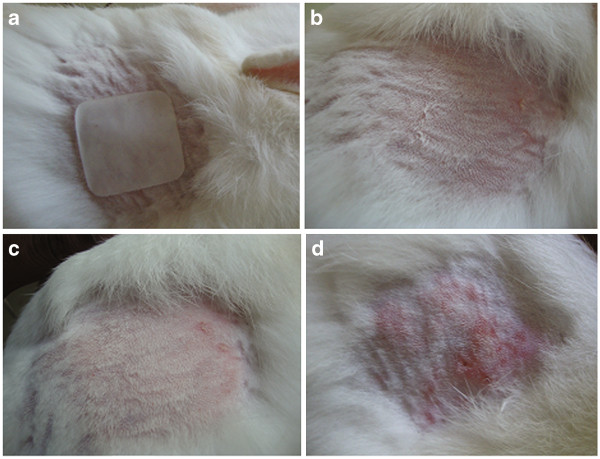
The 7 day skin irritancy study revealed that the test formulation showed a skin irritation score (erythema and edema) of less than 1 (Table 10 & Figure 11). From the Draize method of scoring, the control animals showed severe erythema and moderate to slight edema whereas the test animals showed only very slight erythema and no edema on the site of application. According to Draize et al. (1944) [20] compounds producing scores of 2 or less are considered non-irritant [14]. Hence from the study, we can conclude that formulations are non-irritable to skin and safer for therapeutic use.
Table 10.
Acute skin irritancy data for C5/OLA (n = 4)
|
Day |
Parameter |
Standard |
Test |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
|
Day 0 |
Erythema |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| |
Edema |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Day 7 |
Erythema |
4 |
4 |
3 |
4 |
0 |
1 |
1 |
1 |
| Edema | 3 | 2 | 2 | 3 | 0 | 0 | 0 | 0 | |
(C4/OLA: C4combination patch with oleic acid, OLA, as permeation enhancer).
Figure 11.
Images of skin irritancy study: (a) patch application to shaved area; (b) skin of rabbit at 0 day; (c) skin of test group rabbit after 7 days of patch application; (d) skin of standard group rabbit after 7 days.
In vivo anti-inflammatory studies
The result of carrageenan induced paw edema test was shown in the Table 11. The table shows the data for the percent increase in edema with respect to initial volume and percentage inhibition of edema with respect to control during 24 h study for the test formulation. As shown in the table in case of control group animals, the mean percent increase in edema with respect to initial volume (Group –I) was 114.3 ± 15.0 at the end of 24 h which is because of swelling nature of carrageenan. While in case of test group animals the value is 0.37 ± 0.54 at the end of 24 h indicating that test patches, C4/OLA, are effective in inhibiting carrageenan induced inflammation. Moreover, the test group animals showed 99.68% inhibition of edema with respect to control after 24 h indicating the efficacy of the formulation during the period. The initial percent increase in edema with respect to initial volume in case of test group half an hour after the carrageenan induction was 0.4 ± 0.54 as opposed to Control group (3.57 ±1.08) indicating that the test patch, C4/OLA showed action from the first hour without any appreciable lag time. Throughout the study the percent increase in edema value with respect to initial volume for test group remained well below than the control group indicating the sustaining effect of the drug against carrageenan challenge.
Table 11.
Paw edema data obtained on carrageenan induced rats half an hour after the patch application (data represented as mean ± S.D, n = 4)
|
Time (h) | ||||||||||||||
| |
0.5 |
0.75 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
10 |
12 |
16 |
24 |
|
% Edema with respect to initial volume | ||||||||||||||
|
Control |
3.57 ±1.08 |
14.28 ± 2.05 |
35.71 ± 7.06 |
42.8 ±9.64 |
50.0 ± 12.95 |
60.7 ± 20.82 |
71.5 ± 19.72 |
83.0 ± 21.85 |
92.8 ± 11.05 |
100.0 ± 15.1 |
111.1 ± 18.6 |
121.4 ± 11.76 |
121.4 ±15.9 |
114.3 ± 15.0 |
|
Test (C4/OLA) |
0.4 ± 0.54 |
5.6 ± 2.91 |
11.3 ± 5.83 |
18.5 ± 0.97 |
39.4 ± 4.16 |
54.1 ± 0.31 |
62.6 ± 1.44 |
52.7 ± 9.29 |
42.6 ± 0.39 |
36.2 ± 4.39 |
21.2 ± 2.68 |
15.6 ± 0.82 |
2.5 ± 2.49 |
0.37 ± 0.54 |
|
% inhibition of Edema with respect to control | ||||||||||||||
| Test (C4/OLA) | 88.80 | 60.78 | 68.36 | 56.78 | 21.20 | 10.87 | 12.45 | 36.51 | 54.09 | 63.80 | 80.92 | 87.15 | 97.94 | 99.68 |
Conclusion
The extensive solubilization study conducted on Silicone adhesive polymers revealed their unsuitability in fabrication of DDEA transdermal patches alone as not more than 1% drug was solubilized even with high concentration of solubilizer. On the other hand, Acrylic polymer showed high drug loading and greater control releasing capacity hence, alone can be used for fabricating transdermal patches of DDEA. However, use of Acrylic alone requires greater amount of drug incorporation due to its low value of percent cumulative drug permitted (10.72%). Hence, the combinations of adhesives were tested with the objective combining the greater permeation capacity of Silicone polymer and greater drug loading capacity of Acrylic polymer. The combinational patches incorporating the both the desired properties were successfully prepared. Among various permeation enhancers tested OLA proved to be a good permeation enhancer as compared to ISA and IPM for DDEA. C4/OLA was found to be optimized formulation displaying robustness in stability. The skin irritancy study revealed the non-irritant nature of the C4/OLA patches and sustaining action of the patches were confirmed by anti-inflammatory test by carrageenan induced paw edema model. Thus, it can be concluded that an ideal of combination of adhesives would serve as the best choice, for fabrication of DDEA patches, for sustained effect of DDEA with better enhancement in permeation characteristics and robustness.
Competing interests
The manuscript has no conflict of interest and there are no financial sources for many organizations and the work is purely part of student thesis work.
Authors’ contributions
The author DPM helped in conceptual design of entire work, the author SP was responsible for the entire practical work, TP contributed to the interpretation of data obtained at various steps, the author BD helped in carrying out the studies involving animals and AKA helped in the calculation part and in preparation and follow up of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Dandigi M Panchaxari, Email: pmdandigi@yahoo.co.in.
Sowjanya Pampana, Email: soujanya.pampana@gmail.com.
Tapas Pal, Email: dtp@sparsha.com.
Bhavana Devabhaktuni, Email: rd@sparsha.com.
Anil Kumar Aravapalli, Email: anil.avs219@gmail.com.
Acknowledgement
The authors are grateful to Sparsha Pharma International Pvt. Ltd., Hyderabad, India for extending their timely help and full co-operation for carrying out the entire research work under their guidance.
References
- Debjit B, Chiranjib, Chandira M, Jayakar B, Sampath KP. Recent advances in transdermal drug delivery system. Int. J Pharm Tech Res. 2010;2(1):68–77. [Google Scholar]
- Jain P, Banga AK. Inhibition of crystallization in drug-in-adheisve-type transdermal therapeutic patches. Int J Pharm. 2010;394:68–74. doi: 10.1016/j.ijpharm.2010.04.042. [DOI] [PubMed] [Google Scholar]
- John VA. The pharmacokinetics and metabolism of diclofenac sodium (Voltarol™) in animals and man. Rheumatol Rehabil. 1979;Suppl 2:22–37. [PubMed] [Google Scholar]
- Fritz UN, Morris SG, Gail SS, Jiun-min L, Markus U, Helmut HA, Francois E. Efficacy of topical diclofenac diethylamine gel in osteoarthritis of the knee. J Rheumatol. 2005;32:2384–2392. [PubMed] [Google Scholar]
- Keith AD. Polymer matrix consideration for transdermal devices. Drug Dev Ind Pharm. 1983;9:605–621. doi: 10.3109/03639048309044695. [DOI] [Google Scholar]
- Nauman Rahim K, Gul Majid K, Abdur Rahim K, Abdul W, Muhammad Junaid A, Muhammad A, Abid H. Formulation, physical, in vitro and ex vivo evaluation ofdiclofenac diethylamine matrix patches containing turpentine oil as penetration enhancer. Afr J Pharm Pharmaco. 2012;6(6):434–439. [Google Scholar]
- Haroutinaian S, Drennan DA, Lipman AG. Topical NSAID therapy for musculoskeletal pain. Pain Med. 2010;11(4):535–549. doi: 10.1111/j.1526-4637.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- Changshun R, Liang F, Lei L, Qiang W, Sihai L, LiGang Z, Zhonggui H. Design and evaluation of Indipamide transdermal patch. Int J Pharm. 2009;370(1–2):129–135. doi: 10.1016/j.ijpharm.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Khalil E, Najjar S, Sallam A. Aqueous solubility of diclofenac diethylamine in the presence of pharmaceutical additives: a comparative study with diclofenac sodium. Drug Dev Ind Pharm. 2000;26(4):375–381. doi: 10.1081/DDC-100101243. [DOI] [PubMed] [Google Scholar]
- Hussain Shah SN, Salman M, Ahmad M, Rabbani M, Badshah A. Effect of oleic acid on the permeation kinetics of Diclofenac Diethylamine. J Chem Soc Pak. 2012;34(1):1–8. [Google Scholar]
- Atrux-Tallau N, Pirot F, Falson F, Roberts MS, Maibach HI. Qualitative and quantitative comparison of heat separated epidermis and dermatomed skin in percutaneous absorption studies. Arch Dermatol Res. 2007;299(10):507–511. doi: 10.1007/s00403-007-0789-y. [DOI] [PubMed] [Google Scholar]
- Kaidi Z, Singh J. In vitro percutaneous absorption enhancement of propranolol hydrochloride through porcine epidermis by terpenes/ethanol. J Control Rel. 1999;62(3):359–366. doi: 10.1016/S0168-3659(99)00171-6. [DOI] [PubMed] [Google Scholar]
- Bonferoni M, Rossi S, Ferrari F, caramella C. A modified Franz diffusion cell for simulataneous assessment of drug release and washability of mucoadhesive gels. Pharm Dev Technol. 1999;4(1):45–53. doi: 10.1080/10837459908984223. [DOI] [PubMed] [Google Scholar]
- Mamatha T, venkateswara rao J, Mukkanti K, Ramesh G. Development of matrix type transdermal patches of lercanidipine hydrochloride: physicochemical and in-vitro characterization. DARU. 2010;18(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Verma PR, Iyer SS. Transdermal delivery of propranolol using mixed grades of Eudragit: design and in-vitro and in-vivo evaluation. Drug Dev Ind Pharm. 2000;26(4):471–476. doi: 10.1081/DDC-100101257. [DOI] [PubMed] [Google Scholar]
- Vijaya Bhanu P, Shanmugam V, Lakshmi PK. Development and evaluation of Diclofenac Emulgel for topical drug delivery. Pharmacie Globale IJCP. 2011;9(10):1–4. [Google Scholar]
- Sood A, Panchagnula R. Role of dissolution studies in controlled release drug delivery system. STP Pharma Sci. 1999;9:157–168. [Google Scholar]
- Mohamed A, Yamin S, Asgar A. Matrix type transdermal drug delivery systems of metoprolol tartrate: in vitro characterization. Acta Pharm. 2003;53(2):119–125. [PubMed] [Google Scholar]
- Shinde AJ, Shinde AL, More HN. Design and evaluation transdermal drug delivery system of gliclazide. Asian J Pharm. 2010;4(2):121–129. doi: 10.4103/0973-8398.68463. [DOI] [Google Scholar]
- Draize JH, Woodword G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;8:377–379. [Google Scholar]
- Winter CA. In: Nonsteroidal antiinflammatory drugs. Garattini S, Dukes MNG, editor. Amsterdam: Excerpta Medica Foundation; 1965. Antiinflammatory testing methods: Comparative evaluation of indomethacin and other agents; pp. 190–202. series no. 82. [Google Scholar]
- Priyanka A, Biswajit M. Design, development, physicochemical, and in vitro and In Vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J Pharm Sci. 2002;91(9):2076–2089. doi: 10.1002/jps.10200. [DOI] [PubMed] [Google Scholar]
- Hadgraft J. Passive enhancement strategies in topical and transdermal drug delivery. Int J Pharm. 1999;184(1):1–6. doi: 10.1016/S0378-5173(99)00095-2. [DOI] [PubMed] [Google Scholar]
- Latsch S, Selzer T, Fink L, Kreuter J. Determination of the physical state of norethindrone acetate containing transdermal drug delivery systems by isothermal microcalorimetry, X-ray diffraction, and optical microscopy. Eur J Pharm Biopharm. 2004;57(2):383–395. doi: 10.1016/S0939-6411(03)00158-9. [DOI] [PubMed] [Google Scholar]
- Cilurzo F, Minghetti P, Casiraghi A, Tosi L, Pagani S, Montanari L. Polymethacrylates as crystallization inhibitors in monolayer transdermal patches containing ibuprofen. Eur J Pharm Biopharm. 2005;60(1):61–66. doi: 10.1016/j.ejpb.2005.02.001. [DOI] [PubMed] [Google Scholar]