SUMMARY
Subtypes of GABAergic interneurons (INs) are crucial for cortical function, yet their specific roles are largely unknown. In contrast to supra- and infra-granular layers, where most somatostatin-expressing (SOM) INs are layer 1-targeting Martinotti cells, the axons of SOM INs in layer 4 somatosensory cortex largely remain within layer 4. Moreover, we found that whereas layers 2/3 SOM INs target mainly pyramidal cells (PCs), layer 4 SOM INs target mainly fast-spiking (FS) INs. Accordingly, optogenetic inhibition of SOM INs in an active cortical network increases the firing of layers 2/3 PCs whereas decreases the firing of layer 4 principal neurons (PNs). This unexpected effect of SOM INs on layer 4 PNs occurs via their inhibition of local FS INs. These results reveal a novel disinhibitory microcircuit in the thalamorecipient layer through interactions among subtypes of GABAergic INs, and suggest that SOM IN-mediated disinhibition represents an important circuit mechanism for cortical processing.
INTRODUCTION
The complex functions of the cerebral cortex rely on neuronal networks of highly interconnected glutamatergic principal neurons (PNs) and GABAergic interneurons (INs). Although GABAergic INs represent a minority of all cortical neurons (10-20% in rodents) their dense axonal arborization allows them to control the entire cortical network. There is a large diversity of cortical GABAergic INs based on differences in morphology, intrinsic membrane properties, connectivity, the efficacy, kinetics and dynamics of their input and output synapses, and the expression of specific molecular markers (Ascoli et al., 2008; Fishell and Rudy, 2011; Freund and Buzsaki, 1996; Kawaguchi and Kubota, 1997; Rudy et al., 2011; Somogyi and Klausberger, 2005). This diversity critically contributes to the ability of the cerebral cortex to perform complex operations (Isaacson and Scanziani, 2011; Klausberger and Somogyi, 2008; McBain and Fisahn, 2001; Somogyi and Klausberger, 2005). Determining the specific roles of different subtypes of GABAergic INs is therefore fundamental to understand cortical function. To date, however, the specific roles played by different GABAergic interneuron subtypes are poorly understood.
In sensory cortex, layer 4 is the primary recipient of sensory input from the thalamus and relays sensory information to other neocortical layers for further processing. The activity of layer 4 neurons is largely determined by the interplay of synaptic excitation and synaptic inhibition (Miller et al., 2001). Synaptic excitation can be initiated by relatively sparse but synchronous thalamocortical inputs and amplified by highly recurrent intracortical synapses interconnecting PNs (Bruno and Sakmann, 2006; Douglas et al., 1995; MacLean et al., 2005). Synaptic inhibition in this layer is mediated by two main subtypes of GABAergic INs, i.e., parvalbumin-expressing (PV) fast-spiking (FS) INs, which constitute 60% of GABAergic neurons in layer 4, and somatostatin-expressing (SOM) INs, which constitute 20-30% of GABAergic neurons in this layer (Lee et al., 2010; Rudy et al., 2011).
PV-expressing FS INs make powerful synapses onto the somatic and perisomatic compartments of PNs (Kawaguchi and Kubota, 1997), receive strong excitatory input from the thalamus and mediate disynaptic feedforward inhibition of PNs, thus controlling spike timing of the output neurons (Cruikshank et al., 2007; Gabernet et al., 2005; Higley and Contreras, 2006; Miller et al., 2001; Pinto et al., 1996). In contrast, SOM INs receive facilitating excitatory input from local PNs but do not receive strong input from thalamus (Beierlein et al., 2003; Cruikshank et al., 2010) and their function in regulating layer 4 activity is not known.
In this study we combined electrophysiological and optogenetic approaches in mouse somatosensory cortex to characterize the properties and connectivity of SOM INs and investigated their function in the cortical network. We found that layer 4 SOM INs differ in morphology, intrinsic electrophysiological properties and output connectivity from layers 2/3 SOM INs. We observed a great deal of specificity of the inhibitory connections made by SOM INs in terms of their preferred targets in different layers. Importantly, this connection specificity is associated with functional consequences in an active cortical network: in layers 2/3 SOM INs suppress pyramidal cell (PC) activity; in layer 4 SOM INs suppress FS INs and hence relieve FS INs-mediated inhibition of PNs and consequently enhance PNs output. The disinhibitory circuit revealed in this study represents a novel circuit mechanism by which SOM INs regulate cortical information processing in the thalamorecipient cortical layer 4.
RESULTS
Layer 4 SOM INs Mainly Target Layer 4
In neocortex SOM INs have traditionally been identified as Martinotti cells, neurons that have an ascending axon that usually gives rise to an axonal arborization in layer 1 and makes synaptic contacts with the distal apical dendrites of pyramidal cells (PCs). Analysis of a transgenic mouse line known as X94 suggested that at least some of the SOM INs in layer 4 may differ from Martinotti cells (Ma et al., 2006). In the X94 line a subset of SOM INs express GFP. The labeled neurons are concentrated in layer 4 and instead of targeting layer 1 they profusely innervate layer 4 (Ma et al., 2006). However, less than half of all SOM INs in layer 4 express GFP in the X94 line (Ma et al., 2006) (see also Figure S1). In the present study, we first set out to characterize the non-X94 SOM INs in this layer.
To identify non-X94 SOM INs we used SOM-Cre mice, in which Cre recombination occurs in nearly all cortical SOM INs (Taniguchi et al., 2011), crossed to an RFP reporter line to label SOM INs with red fluorescent protein (Luche et al., 2007) (Figure 1A). These mice were then crossed to X94 mice to generate the SOM-Cre/RFP/X94 mouse line, in which non-X94 SOM INs were labeled with red fluorescent protein, while X94 cells were labeled with both red and green fluorescent proteins (Figure 1A). This strategy allowed us to successfully visualize and differentiate X94 and non-X94 SOM INs in brain slices. To optimize the columnarity of sections in order to optimize preservation of ascending axons, brains were tilted of approximately 30° dorsally from the standard coronal plane such that the vibratome blade cut perpendicularly to the pial surface at the level of the barrel field (Markram et al., 1997).
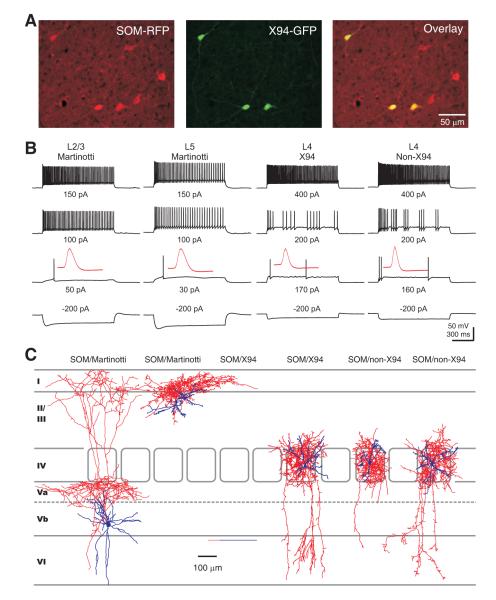
Figure 1. Layer 4 SOM INs in mouse somatosensory cortex are a single population with axons that mainly target layer 4.
(A) Confocal image of RFP-expressing neurons (left), X94 neurons (middle) and their overlay (right) in layer 4 barrel cortex of an adult SOM-Cre/RFP/X94 mouse. Note that 3 out 6 SOM-RFP neurons in the image are X94 cells. (B) Representative membrane potential responses of subgroups of SOM INs to the indicated current injections. Note that the non-X94 SOM interneuron responded to current injections in a manner identical to the X94 cell. The traces in the third row show the responses at threshold current injection, and the insets illustrate the first action potential from the corresponding traces, shown at the same vertical scale and at a 200-fold expanded horizontal scale. Note the differences in spike width between layer 4 and layers 2/3 or 5 SOM INs. (C) Representative morphologies of reconstructed SOM INs. The SOM-expressing Martinotti cells (2 leftmost) from either infragranular or supragranular layers send their axons to layer 1 and branched extensively in this layer, however layer 4 SOM-expressing X94 cells (2 middle) and non-X94 cells (2 rightmost) confined their axons largely to layer 4. Soma and dendrites are shown in blue, axons in red. See also Figure S1.
Whole cell patch clamp recordings showed that non-X94 SOM INs responded to incremental step current injections in a manner strikingly different from Martinotti cells but similar to X94 neurons (Figure 1B). Non-X94 SOM INs and X94 SOM INs exhibited indistinguishable membrane properties including resting membrane potential, input resistance, membrane time constant, rheobase and spike width (Table 1). However, compared to layers 2/3 or layer 5 Martinotti cells, layer 4 SOM INs (either X94 or non-X94) exhibited significantly hyperpolarized resting membrane potential, smaller input resistance, faster membrane time constant, larger rheobase and narrower spike (Figure 1B, Table 1). Moreover, layer 4 SOM INs were capable of firing at much higher frequency than SOM INs in layers 2/3 and 5, both initially (Fmax, initial) and at steady state (Fmax, steady state) (Figure 1B, Table 1).
Table 1.
Electrophysiological parameters of subgroups of SOM INs in somatosensory cortex. Note that layer 4 non-X94 SOM INs are indistinguishable from layer 4 X94 INs in these parameters but are significantly different from layers 2/3 or layer 5 Martinotti cells.
| L4 X94 (n = 23) |
L4 non-X94 (n = 30) |
L2/3 Martinotti (n = 24) |
L5 Martinotti (n = 15) |
|
|---|---|---|---|---|
| Vrest (mV) | −64.6 ± 0.6 | −64.9 ± 0.5 | −59.5 ± 0.7**** | −59.6 ± 0.9**** |
| Rin (MΩ) | 132 ± 9 | 122 ± 6 | 251 ± 21*** | 265 ± 19**** |
| τm (ms) | 8.4 ± 0.4 | 8.2 ± 0.3 | 17.7 ± 0.9**** | 21.6 ± 1.4**** |
| Rheobase (pA) | 174 ± 8 | 196 ± 10 | 59 ± 7**** | 53 ± 8**** |
| Threshold (mV) | −43.1 ± 0.9 | −41.2 ± 0.7 | −42.3 ± 0.8 | −43.0 ± 1.7 |
| Spike width (ms) | 0.41 ± 0.01 | 0.41 ± 0.02 | 0.50 ± 0.01**** | 0.55 ± 0.02**** |
| Fmax, steady-state (Hz) | 110 ± 9 | 101 ± 5 | 77 ± 8** | 61 ± 8*** |
| Fmax, initial (Hz) | 272 ± 15 | 270 ± 14 ` | 191 ± 12*** | 202 ± 14** |
| Spike adaptation ratio | 0.40 ± 0.03 | 0.38 ± 0.03 | 0.40 ± 0.02 | 0.31 ± 0.03* |
For an explanation of the parameters, see Experimental Procedures. Values shown are expressed as mean ± SEM. Significant differences between layer 4 non-X94 SOM INs and other subgroups of SOM INs are expressed as:
p < 0.05
p < 0.01
p < 0.001
p < 0.0001. Two sample t test.
Post hoc morphological reconstructions of a portion of the recorded cells (26 out of 108 SOM INs: 6 L4 X94 SOM INs; 10 L4 non-X94 SOM INs; 8 L2/3 Martinotti INs; 2 L5 Martinotti INs) showed that non-X94 SOM INs, like X94 SOM INs, restricted their axonal arbors largely to layer 4, a feature that distinguished them from Martinotti INs (Figure 1C). Taken together, both the electrophysiological and the morphological evidence suggest that, although only half of the SOM INs in layer 4 of somatosensory cortex express GFP in the X94 mouse, most, if not all, are X94-cell like and their axonal projections preferentially target layer 4.
Layer 4 SOM INs Preferentially Inhibit Layer 4 FS INs
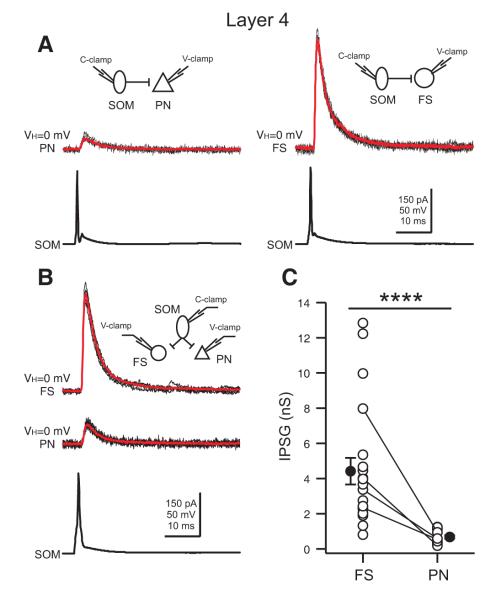
The functional connectivity of a neuron is fundamental to its role in cortical information processing. Given that the axons of layer 4 SOM INs mainly target layer 4, we next performed dual recordings in acute brain slices of somatosensory cortex to examine their functional connections with excitatory principal neurons (PNs) which are the dominant neuronal population in layer 4, and PV-expressing FS INs which are the largest population of inhibitory neurons in the layer. SOM INs made functional contacts with both PNs and FS INs (Figure 2A). The postsynaptic currents (IPSCs) elicited by SOM INs in both PNs and FS INs were fully blocked by 10 μM GABAzine (a GABAaR antagonist, Figure S2A) and had a reversal potential (EIPSC = −76.1 ± 0.6 mV, n = 6) close to theoretical Cl− equilibrium potential (ECl− = −77 mV) (Figure S2B-D). However, surprisingly, the amplitude of the unitary IPSC in FS cells was much larger than that in PNs (Figure 2A). To confirm this target cell type differential inhibition by SOM INs, we performed triple recordings between a SOM neuron and both a PN and a FS neuron. As shown in Figure 2B, the same SOM neuron produced a much larger unitary IPSC in the FS cell than in the PN. When data from dual and triple recordings were pooled, we found that the unitary inhibitory postsynaptic conductance (IPSG) in FS INs was almost 7 times larger than that in PNs (FS: 4.42 ± 0.75 nS, n = 21; PN: 0.68 ± 0.09 nS, n = 17; two sample t test, p < 0.0001, Figure 2C).
Figure 2. Layer 4 SOM INs preferentially inhibit FS INs.
(A) Representative paired recordings between a layer 4 SOM interneuron and a PN (left) or a FS interneuron (right). Single action potentials in SOM INs (bottom) elicited IPSCs in the PN (top left; red, average of 10) or the FS interneuron (top right; red, average of 10). Note that the amplitude of the unitary IPSC in the FS interneuron is much larger than the one in the PN. (B) Representative triple recordings between a SOM interneuron and a PN and a FS interneuron. Single action potentials in the SOM interneuron (bottom) elicited IPSCs in both the PN (middle; red, average of 10) and the FS interneuron (top; red, average of 10). Note that the same SOM interneuron produced a unitary IPSC of larger amplitude in the FS cell. (C) Population results showing that SOM INs produced a significantly larger unitary inhibitory postsynaptic conductance (IPSG) in FS INs than in PNs. Data from triple recordings are connected by a line. Open symbols represent IPSGs of individual neurons, and filled symbols represent the mean IPSG. Error bars indicate s.e.m. The difference in IPSG between FS INs and PNs was highly significant (**** p < 0.0001, two sample t test). Schematics in A and B illustrate recording configurations.
Since FS INs and PNs have a different input resistance in slices (FS: 77.5 ± 12.4 MΩ, n = 15; PN: 187.2 ± 29.9 MΩ, n = 15; two sample t test, p < 0.001), we also examined the unitary inhibitory postsynaptic potentials (IPSPs) and found that SOM INs produced unitary IPSPs in FS cells that were significantly larger than those in PNs (FS: 1.54 ± 0.29 mV, n = 16; PN: 0.64 ± 0.11 mV, n = 14; two sample t test, p < 0.01). Although the difference in unitary IPSP amplitude between FS cells and PNs is still large, it is less than the difference in IPSCs, likely because of the difference in input resistance of the two cell types in slices. However, since in vivo the PNs have a much lower input resistance (Gentet et al., 2010), the differences in unitary IPSPs between the two cell types in vivo might be larger and approach the difference seen for IPSCs. Furthermore, in addition to the large difference in synaptic strength, the connection probability of SOM INs with FS INs was much higher than their connection probability with PNs (FS: 62%, 37 connections out of 60 pairs; PN: 35%, 31 connections out of 89 pairs, Fisher’s exact test, p < 0.01).
The functional impact of this connectivity patterns will also depend on the short term dynamics of the synapses made by SOM INs. For example, if the synapse with FS cells was depressing but the synapse with PNs was facilitating, SOM INs could have a larger effect on PNs during repetitive activity in spite of the smaller unitary conductance and lower connection probability. However, we found that the amplitude of unitary IPSCs evoked by SOM INs (5 pulses at 10 Hz) was moderately depressing in both FS and PNs, and the degree of depression was similar (Figure S3A, B). Therefore, layer 4 SOM INs preferentially inhibit layer 4 FS INs during both sparse and repetitive activity.
Target Cell Type Differential Inhibition by SOM INs Is Layer Specific
To determine whether the target cell type differential inhibition by layer 4 SOM INs was a general property of SOM INs, we next performed dual and triple recordings between SOM INs and PCs or FS INs in layers 2/3. Interestingly, contrary to what was observed in layer 4, in layers 2/3, SOM INs produced larger unitary IPSC in PCs than in FS INs (Figure 3A, B). When data from dual and triple recordings were pooled we found that the unitary IPSG in PCs was significantly larger than that in FS INs (FS: 0.79 ± 0.10 nS, n = 9; PC: 1.31 ± 0.14 nS, n = 35; two sample t test, p < 0.01, Figure 3C). In addition, the connection probability from SOM INs to PCs was two times higher than their connection probability with FS INs (FS: 29%, 9 connections out of 31 pairs; PC: 63%, 35 connections out of 56 pairs, Fisher’s exact test, p < 0.01).
Figure 3. Layers 2/3 SOM INs preferentially inhibit PCs.
(A) Representative paired recordings between a SOM interneuron and a PC (left) or a SOM interneuron and a FS cell (right). Single action potentials in the SOM interneuron elicited IPSCs in the PC (top left; red, average of 10) or the FS interneuron (top right; red, average of 10). Note that the amplitude of the unitary IPSC in the PC is larger than the IPSC in the FS interneuron. (B) Representative triple recordings between a SOM interneuron and a PC and a FS interneuron. Single action potentials in the SOM neuron induced IPSCs in both the PC (middle; red, average of 10) and the FS neuron (top; red, average of 10). Note that the same SOM interneuron produced a unitary IPSC of larger amplitude in the PC. (C) Population results showing that SOM INs produced a significantly larger IPSG in PCs than in FS INs. Data from triple recordings are connected by a line. Open symbols represent IPSGs of individual neurons, and filled symbols represent the mean IPSGs. Error bars indicate s.e.m. The difference of the IPSG in FS INs and PCs was significant (** p < 0.01, two sample t test). Schematics in A and B illustrate recording configurations. See also Figure S4.
As discussed earlier, the functional impact of the observed differences in connectivity could be affected by differences in the short term dynamics of the synapses of layers 2/3 SOM INs with PCs or FS INs. However, we found that the synapses of the SOM INs with both types of postsynaptic cells depressed. Furthermore, while they depressed more than the synapses made by SOM INs in layer 4, the degree of depression of the synapses made by layers 2/3 SOM INs with PCs or FS INs was similar (Figure S3).
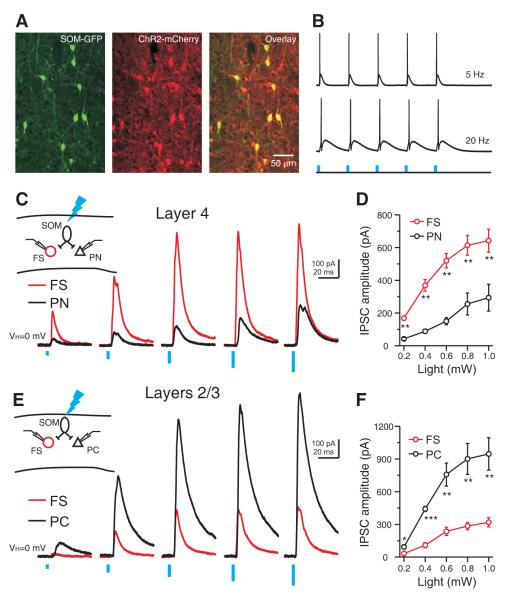
The layer and target cell type differential inhibition by SOM INs revealed using electrophysiological recordings of connected pairs was further confirmed using an optogenetic approach. We used viral injection to conditionally express mCherry-tagged channelrhodopsin (ChR2-mCherry) in GFP-labeled SOM INs in somatosensory cortex (Sousa et al., 2009; Taniguchi et al., 2011) (Figure 4). Immunohistochemical analysis revealed that there was a near complete overlap between mCherry and GFP expression in virus injected animals (98% of GFP neurons expressed ChR2-mCherry, n = 100; 97% of the ChR2-mCherry-expressing neurons also expressed GFP, n = 100) (Figure 4A), indicating efficient and selective expression of ChR2 in SOM INs.
Figure 4. Layer and target cell type differential inhibition by SOM INs confirmed by optogenetic approach.
(A) Confocal image of SOM-GFP neurons (left), ChR2- mCherry expression (middle) and the overlay (right) in barrel cortex of an adult SOM-Cre/RCE mice, showing that ChR2 expression was confined to SOM INs. (B) Brief photo stimulation reliably evoked action potentials in a ChR2-mCherry expressing SOM interneuron. Blue vertical bars represent photo stimulation (470 nm, 2 ms, 0.2 mW). (C) In layer 4, photostimulation (470 nm, 2 ms) of incremental intensity (0.2 to 1.0 mW at 0.2 mW steps) produced IPSCs of increasing amplitude in simultaneously recorded PN and FS cell, but the IPSC amplitude was larger in the FS interneuron at all light intensities. (E) In layer 2/3, photostimulation (470 nm, 2 ms) of incremental intensity (0.2 to 1.0 mW at 0.2 mW steps) produced IPSCs of increasing amplitude in the simultaneously recorded PC and FS neuron, but the IPSC amplitude was larger in the PC. (D, F) Population results showing layer and target-cell-type differential IPSC amplitude produced by photostimulation of ChR2-expressing SOM INs: in layer 4 IPSCs were significantly larger in FS INs (n = 7 pairs); in layers 2/3 IPSCs were significantly larger in PCs (n = 6 pairs). Open symbols represent the mean IPSCs. Error bars indicate s.e.m. * p < 0.05, ** p < 0.01, *** p < 0.001, paired t test. Schematics in C and E illustrate recording configurations. The spatial extent of efficient neuronal recruitment by the maximum photostimulation (470 nm, 2 ms, 1 mW) was about 200 μm in diameter under our experimental conditions (See also Figure S6).
Photo stimulation (470 nm) readily evoked spikes in ChR2-mCherry infected SOM INs in slices (Figure 4B). Consistent with the unitary connection studies, photo stimulation evoked IPSCs of larger amplitude in layer 4 FS INs than in PNs over a large range of light intensities (0.2 to 1.0 mW) (Figure 4C, D). In contrast, in layers 2/3, photo stimulation consistently evoked significantly larger amplitude IPSCs in PCs than in FS INs (Figure 4E, F). In conclusion, SOM INs produce a layer specific target cell type differential inhibition: in layers 2/3 SOM INs preferentially inhibit PCs; in layer 4 SOM INs preferentially inhibit FS INs. These results show that the inhibitory synapses made by SOM INs exhibit remarkable specificity in their connection probability and strength with specific targets in different layers.
Given that layer 4 SOM INs differ from those in layers 2/3 in anatomy and output connectivity, we also investigated the dynamics of their excitatory inputs. SOM-expressing Martinotti cells in supra- and infra-granular layers (Kapfer et al., 2007; Silberberg and Markram, 2007) are known to receive strong facilitating excitatory inputs from local PNs. We found that layer 4 PNs made frequent functional contacts with SOM INs (connection probability: 40%; 12 connections out of 30 pairs) yet their unitary EPSPs had small amplitude (0.32 ± 0.05 mV, n = 12) (Figure S5C). However, in response to brief trains of action potentials in the PNs, the EPSPs of the SOM INs displayed prominent short-term facilitation (Figure S5C, D). Thus with regard to this important property of Martinotti cells, layer 4 SOM INs behave in a similar fashion.
Suppression of SOM IN Activity in an Active Cortical Network
FS INs produce strong perisomatic inhibition of PNs and efficiently regulate their output (Cruikshank et al., 2007; Gabernet et al., 2005; Higley and Contreras, 2006; Miller et al., 2001). Given that SOM INs strongly inhibited FS INs in layer 4, we hypothesized that activity of SOM INs could prevent action potential generation in FS INs and therefore relieve FS INs-mediated inhibition of PNs. We set out to test this hypothesis in an active cortical network (the UP state) that was achieved by perfusing mouse somatosensory thalamocortical slices with a solution more closely mimicking the ionic composition of natural CSF (1 mM Ca2+, 1 mM Mg2+, and 3.5 mM K+) as previously described (Sanchez-Vives and McCormick, 2000; Shu et al., 2003). Spontaneously occurring UP states in a given neuron always appeared in other neighboring neurons, independent of cell type, in a temporally synchronous manner (Figure S7A), indicating that neocortical UP states involved the simultaneous participation of all neuronal subtypes within a local circuit. In agreement with previous studies (MacLean et al., 2005; Shu et al., 2003; Wester and Contreras, 2012), neocortical UP states could be initiated reliably through a single electrical stimulation to the ventrobasal nucleus of the thalamus (Figure S7B). As with spontaneous UP states, thalamically-triggered UP states occurred simultaneously in all local neuronal subtypes and they were typically accompanied by firing of action potentials (Figure S7B).
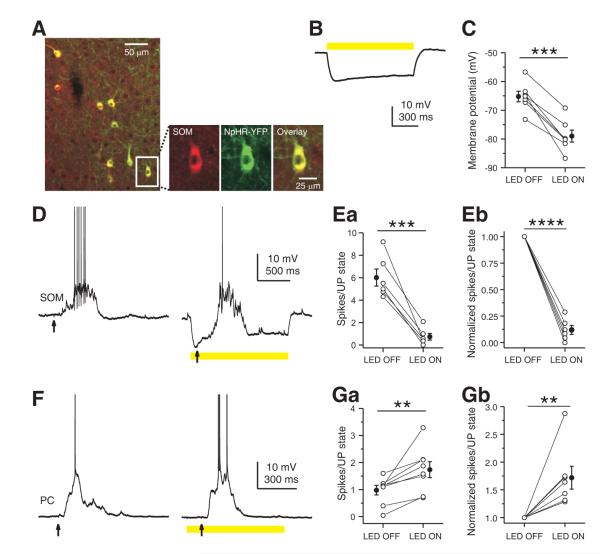
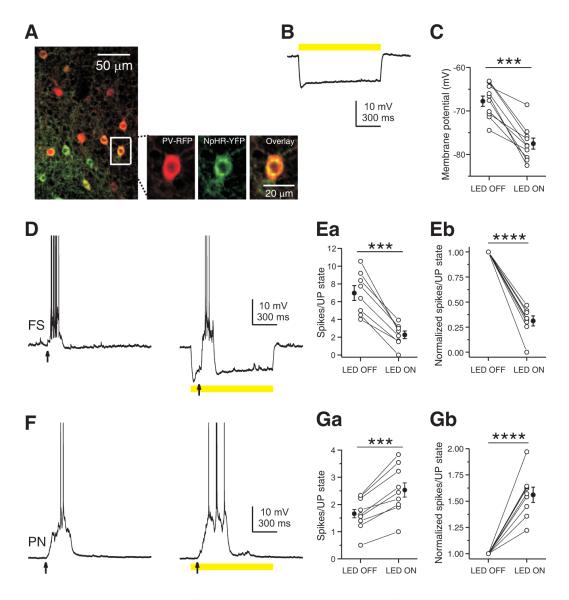
To assess the role of SOM INs in network activity, we employed a loss-of-function strategy to suppress the activity of SOM INs during UP states and examined the effect of this manipulation on PNs. We conditionally expressed the light-sensitive chloride pump halorhodopsin (NpHR-YFP) in somatosensory cortex using viral injection into SOM-Cre mice. Immunohistochemical analysis showed that NpHR expression (as detected by YFP-expression) extensively overlapped with SOM INs (93% of SOM immunopositive neurons expressed NpHR-YFP, n = 100; 92% of NpHR-YFP expressing neurons were SOM immunopositive, n = 100), indicating efficient and selective expression of NpHR in SOM INs (Figure 5A and Figure 7A).
Figure 5. Inhibition of SOM IN activity decreases firing of layer 4 PNs in an active network.
(A) Confocal images showing NpHR-YFP expression (green) was confined to SOM INs (red) in layer 4 barrel cortex of an adult SOM-Cre mouse. (B) Yellow light induced a large amplitude hyperpolarization in a layer 4 NpHR-YFP-expressing SOM interneuron. (C) Summary plot of the membrane potential of layer 4 NpHR-YFP-expressing SOM INs measured in the absence or presence of yellow light. (D) Yellow light reduced the spiking of a layer 4 NpHR-YFP-expressing SOM interneuron during a thalamus-triggered UP state due to the hyperpolarization induced by photostimulation. (E) Population results showing that yellow light nearly completely suppressed spiking activity of layer 4 NpHR-YFP-expressing SOM INs during UP states. (F) Yellow light increased spiking of a layer 4 FS interneuron during a thalamus-triggered UP state. (G) Population results showing that yellow light significantly increased spiking of FS INs during UP states. (H) Yellow light decreased spikes of a layer 4 PN during a thalamus-triggered UP state. (I) Population results showing that yellow light significantly decreased spiking of PNs during UP states. In B, D, F, and H, yellow bars represent photostimulation (591 nm, 1 s, 5 mW) and arrows represent thalamic stimulation. In C, E, G and I, data from the same cell are connected by a line. Open symbols represent the mean values for individual cells, and filled symbols represent mean values across cells. Error bars indicate s.e.m. ** p < 0.01, *** p < 0.001, paired t test.
Figure 7. Inhibition of SOM INs increases firing of layers 2/3 PCs in an active network.
(A) Merged confocal image of NpHR-YFP-expressing neurons (green) and SOM-immunopositive neurons (red) in layers 2/3 barrel cortex of an adult SOM-Cre mouse, indicating NpHR expression was confined to SOM INs. Boxed region is expanded to show the overlay (yellow, right) of NpHR-YFP expression (green, middle) and SOM immunostaining (red, left). (B) Yellow light induced a large amplitude hyperpolarization in a layers 2/3 NpHR-YFP-expressing SOM interneuron. (C) Summary plot of membrane potential of layers 2/3 NpHR-YFP expressing SOM INs measured in the absence or presence of yellow light. (D) Yellow light reduced spiking of a layers 2/3 NpHR-YFP-expressing SOM interneuron during thalamus-triggered UP state due to the large hyperpolarization induced by photostimulation. (E) Population results showing that yellow light nearly completely suppressed the spiking activity of layers 2/3 NpHR-YFP-expressing SOM INs during UP states. (F) Yellow light increased spikes of a layer 2/3 PC during a thalamus-triggered UP state. (G) Population results showing that yellow light significantly increased spiking of layers 2/3 PCs during UP states. In B, D, and F, yellow bars represent photostimulation (591 nm, 1 s, 5 mW) and arrows indicate thalamic stimulation. In C, E, and G, data from the same cell are connected by a line. Open symbols represent the mean values for individual cells, and filled symbols represent the mean values across cells. Error bars indicate s.e.m. ** p < 0.01, *** p < 0.001, paired t test.
We next examined the efficiency of NpHR in suppressing the activity of SOM INs in the active cortical network. Under our experimental conditions, photostimulation (591 nm, 200 ms, 5 mW) efficiently hyperpolarized infected SOM INs within a spatial extent of about 200 μm in diameter (Figure S6C, D). UP states were triggered by thalamic stimulation and spiking activity was compared in the presence and absence of photo stimulation. As shown in Figure 5B, photo stimulation (591 nm, 5 mW) induced a large hyperpolarization (-10.1 ± 1.4 mV, n = 11, Figure 5B, C) in NpHR-YFP expressing SOM INs in layer 4 and almost completely suppressed their spiking activity during UP states (LED OFF: 5.08 ± 0.94 spikes/UP state; LED ON: 0.73 ± 0.26 spikes/UP state; n = 7, p < 0.001, paired t test, Figure 5D, E) decreasing the mean firing rate by 86.8%. Thus, the optogenetic approach efficiently suppressed activity of SOM INs and allowed us to investigate the influence of SOM INs on the active cortical network.
Inhibition of SOM INs Decreases Firing of Layer 4 PNs
Next, we examined the effect of suppressing SOM INs on the activity of FS INs and PNs in layer 4 during UP states triggered by thalamic stimulation. Optogenetic inhibition of SOM INs did not affect the duration of UP states in either FS INs (LED OFF: 444.3 ± 91.9 ms; LED ON: 464.7 ± 102.7 ms; n = 12, p = 0.36, paired t test) or PNs (LED OFF: 495.8 ± 91.3 ms; LED ON: 518.6 ± 104.5 ms; n = 12, p = 0.47, paired t test). However, when SOM INs activity was reduced by photo stimulation, layer 4 FS INs fired 32.8% more action potentials (LED OFF: 6.86 ± 1.04 spikes/UP state; LED ON: 9.04 ± 1.11 spikes/UP state; n = 9, p < 0.001, paired t test, Figure 5F, G) increasing their mean firing rate by 32.3%, and in 6 out of 9 FS INs the increase was individually significant (two sample t test, p < 0.05). In contrast, PNs fired 27.2% less action potentials (LED OFF: 1.91 ± 0.22 spikes/UP state; LED ON: 1.39 ± 0.17 spikes/UP state; n = 12, p < 0.01, paired t test, Figure 5H, I) decreasing their mean firing rate by 28.5%, and in 7 out of 12 PNs the decrease was individually significant (two sample t test, p < 0.05).
Since SOM INs also inhibit PNs (Figure 2), suppressing SOM INs should produce a direct increase in their firing. Therefore, the observed decreased firing of PNs was most likely mediated by an indirect effect through FS INs which increased their firing after inhibition of SOM INs (Figure 5F, G). To obtain evidence that FS INs can regulate PNs activity in the active network studied above, we targeted NpHR expression to FS INs using PV-Cre mice and examined the effect of inhibiting FS INs activity on PNs. NpHR expression in the PV-Cre mouse was efficient and selective for PV-expressing FS INs (Figure 6A) and photo stimulation (591 nm, 5 mW) induced a large hyperpolarizing potential in NpHR-YFP expressing FS INs (-9.74 ± 1.28 mV, n = 10, Figure 6B, C) and significantly reduced their spiking during UP states (LED OFF: 6.97 ± 0.84 spikes/UP state; LED ON: 2.26 ± 0.43 spikes/UP state; n = 8, p < 0.0001, paired t test, Figure 6D, E) decreasing their mean firing rate by 68.8%. As anticipated, when FS INs were inhibited by photo stimulation, PNs fired 52.4% more action potentials during UP states (LED OFF: 1.66 ± 0.15 spikes/UP state; LED ON: 2.53 ± 0.26 spikes/UP state; n = 9, p < 0.001, paired t test, Figure 6F, G) increasing their mean firing rate by 56%, and in 7 out 9 PNs the increase was individually significant (two sample t test, p < 0.05).
Figure 6. Inhibition of PV INs increases firing of layer 4 PNs in an active network.
(A) Merged confocal image of NpHR-YFP-expressing neurons (green) and RFP-immunopositive neurons (red) in layer 4 barrel cortex of an adult PV-Cre-RFP reporter mouse. Note that NpHR-YFP expression occurs only in RFP-expressing PV INs. Boxed region is expanded to illustrate the overlay (yellow, right) of NpHR-YFP expression (green, middle) and RFP immunostaining (red, left). (B) Yellow light induced a large amplitude hyperpolarization in a layer 4 NpHR-YFP-expressing PV interneuron. (C) Summary plot of membrane potential of layer 4 NpHR-YFP-expressing PV INs measured in the absence or presence of yellow light. (D) Yellow light reduced spiking of a layer 4 NpHR-YFP-expressing PV interneuron during a thalamus-triggered UP state due to the large hyperpolarization induced by photostimulation. (E) Population results showing that yellow light potently inhibited spiking activity of layer 4 NpHR-YFP-expressing PV INs during UP states. (F) Yellow light increased spiking of a layer 4 PN during a thalamus-triggered UP state. (G) Population results showing that yellow light significantly increased spiking of layer 4 PNs during UP states. In B, D, and F, yellow bars represent photostimulation (591 nm, 1 s, 5 mW) and arrows indicate thalamic stimulation. In C, E, and G, data from the same cell are connected by a line. Open symbols represent the mean values for individual cells, and filled symbols represent the mean values across cells. Error bars indicate s.e.m. *** p < 0.001, paired t test.
Together, these data demonstrate that activity of SOM INs reduces the firing of FS INs and hence relieves the inhibition produced by FS INs onto PNs, and consequently enhances PNs output. Silencing SOM INs suppressed this disinhibitory circuit and as a result PNs became less responsive.
Inhibition of SOM INs Increases the Firing of Layers 2/3 PCs
Given the inhibition by SOM INs is layer specific, we also examined the effect of suppressing SOM INs activity in layers 2/3. As in layer 4, photo stimulation (591 nm, 5 mW) in layers 2/3 induced a large hyperpolarizing potential (-13.7 ± 1.7 mV, n = 7, Figure 7B, C) in NpHR-YFP expressing SOM INs and almost completely suppressed their spiking during UP states (LED OFF: 6.01 ± 0.76 spikes/UP state; LED ON: 0.74 ± 0.30 spikes/UP state; n = 6, p < 0.001, paired t test, Figure 7D, E) decreasing their mean firing rate by 88.1%. However, in contrast to what we observed in layer 4 PNs, when SOM INs were inhibited by photostimulation in layers 2/3, PCs fired 77.6% more action potentials during UP states (LED OFF: 0.98 ± 0.18 spikes/UP state; LED ON: 1.74 ± 0.29 spikes/UP state; n = 8, p < 0.01, paired t test, Figure 7F, G) increasing their mean firing rate by 71.8%, and in 5 of 8 PCs the increase was individually significant (two sample t test, p < 0.05). This effect is in agreement with the notion that SOM INs mediate strong disynaptic inhibition between PCs in supra- and infra-granular layers (Kapfer et al., 2007; Silberberg and Markram, 2007), and can be explained by our observation that in layers 2/3 SOM INs preferentially inhibit PCs (Figure 3, 4).
DISCUSSION
The cerebral cortex is the largest structure in the mammalian brain and is involved in functions ranging from sensory perception to learning and memory. The spatial and temporal properties of the computations required for these and other cortical functions are thought to rely on the existence of a diversity of GABAergic interneurons (INs) differentially controlling the inputs and outputs of PNs depending on their differential electroresponsiveness and synaptic dynamics and by targeting specific domains of the PNs and other INs. It is widely accepted that understanding INs diversity and its functional consequences is critical to understanding information processing within the cerebral cortex.
Diversity of SOM INs in Neocortex
In rodent neocortex around 30% of GABAergic INs express the neuropeptide SOM, thus representing the second largest population of GABAergic INs in neocortex (Lee et al., 2010; Rudy et al., 2011). SOM INs have typically been associated with Martinotti cells, neurons which send axons to supragranular layers and ramify in layer 1 spreading horizontally to neighboring columns and making synapses on the dendritic tufts of PCs (Silberberg and Markram, 2007; Wang et al., 2004). Martinotti cells can be recruited by repetitive firing in a single PC as a result of strong facilitating excitatory inputs, and hence mediate a strong feedback inhibition on neighboring PCs (Berger et al., 2010; Kapfer et al., 2007; Silberberg and Markram, 2007). By virtue of their unique anatomic and synaptic features, Martinotti cells are well suited to control dendritic integration of synaptic inputs onto PCs. Indeed, the dendritic inhibition mediated by Martinotti cells has been experimentally shown to regulate the activity of PCs both in vitro and in vivo (Berger et al., 2010; Gentet et al., 2012; Murayama et al., 2009).
A different subtype of cortical SOM INs was discovered in a mouse line (X94) exhibiting expression of GFP in a subset of SOM INs that differed from Martinotti cells in a number of intrinsic electrophysiological properties and axonal distribution (Ma et al., 2006). In the mouse somatosensory cortex, X94 cells were found to be enriched in layer 4, where they accounted for about half of all SOM INs in this layer (Ma et al., 2006) (see also Figure S1). In this study, we demonstrate that most, if not all, SOM INs in layer 4 of mouse somatosensory cortex are X94-cell like (Figure 1). In comparison with Martinotti cells, layer 4 SOM INs have axonal projections that instead of ascending and targeting layer 1, innervate profusely layer 4 (Figure 1C), they have a much lower input resistance and membrane time constant and are capable of firing at higher frequency than Martinotti cells (Figure 1B, Table 1). Moreover, we found that layer 4 SOM INs also differ significantly from layers 2/3 SOM INs (most of which are Martinotti cells (Xu et al., 2006)) in their output connectivity onto neighboring FS and PNs. Layer 4 SOM INs make much more frequent and stronger connections with PV-expressing FS INs than with PNs, while layers 2/3 SOM INs make much more frequent and stronger contacts with PCs (Figures 2-4). Layers 2/3 SOM Martinotti cells have an ascending axon that makes synapses with the distal dendrites and dendritic tufts of PCs in layer 1, whereas layer 4 SOM INs innervate within layer 4. Consistent with this anatomical difference, SOM IN-mediated IPSCs in layers 2/3 PCs had significantly slower kinetics than those in layer 4 PNs (rise time, PC: 1.19 ± 0.09 ms, n = 15; PN: 0.74 ± 0.07 ms, n = 16, two sample t test, p < 0.001, and decay time constant, PC: 11.1 ± 0.9 ms, n = 15; PN: 5.5 ± 0.3 ms, n = 16, two sample t test, p < 0.001). Martinotti cells in layers 2/3 can also contact PCs through their extensive local axonal collaterals within layers 2/3 (Wang et al., 2004) (Figure 1 and Figure S4). Since there are no PV cells in layer 1, these cells can only be innervated by the local collaterals of the SOM INs. This axonal anatomy likely contributes to the observation that layers 2/3 SOM INs produce larger responses in PCs than PV cells.
The striking differences in morphology, intrinsic electrophysiological properties and synaptic connectivity between layer 4 SOM INs and Martinotti cells strongly suggest that these subtypes of SOM INs have different functions in the cortical network. This hypothesis is supported by our observation that inhibition of SOM INs in an active cortical network increases the firing of PCs in layers 2/3 whereas it decreases the firing of PNs in layer 4 (Figures 5 and 7). We showed this unexpected effect of SOM INs on layer 4 PNs occurs via their inhibition of layer 4 FS INs (Figures 5-6). Together, our results reveal a novel disinhibitory microcircuit for tuning the output of layer 4 PNs through interactions among subtypes of GABAergic INs, and suggest that SOM INs-mediated disinhibition could be an important circuit mechanism for cortical information processing.
Specificity of the Synaptic Contacts of Neocortical SOM INs
The patterns of neuronal connectivity are fundamental to information processing in the brain. One central issue of cortical neuronal connectivity is the specificity of neuronal connections in the cortex. The specificity of excitatory connectivity serves to form vertical functional columns (Douglas and Martin, 2004). Even within columns, subnetworks of excitatory neurons are wired with a fine level of specificity (Yoshimura et al., 2005). Specificity of connection to PNs has also been found for cannabinoid type 1 receptor-expressing GABAergic basket cells in entorhinal cortex (Varga et al., 2010). In striking contrast, recent mapping studies using two-photon glutamate uncaging showed a lack of fine level connectivity of PV or SOM INs with PCs in upper layers of mouse frontal cortex (Fino and Yuste, 2011; Packer and Yuste, 2011). Consistent with these findings, our dual and triple recordings in mouse somatosensory cortex revealed a fairly high connection probability (63%) from SOM INs to PCs in layers 2/3, leaving little room for SOM INs to connect to specific PCs. However, we do find a great specificity of SOM INs in terms of their target cell types in different layers (Figures 2-3). And importantly, the connection specificity revealed in the present study is associated with functional consequences in an active cortical network, namely in layers 2/3 SOM INs suppress PC activity whereas in layer 4 SOM INs enhance the activity of PNs (Figures 5 and 7).
Interactions among Inhibitory Neurons in Neocortex
In neocortex, studies on inhibitory neuronal circuits have been mostly focused on the interactions between GABAergic neurons and glutamatergic PNs. GABAergic neurons are recruited by PNs and inhibit PNs, and this connectivity pattern forms the basis for ubiquitous feedforward and feedback inhibitory circuits in neocortex (Agmon and Connors, 1991; Cruikshank et al., 2007; Gabernet et al., 2005; Kapfer et al., 2007; Kruglikov and Rudy, 2008; Silberberg and Markram, 2007). Consequently, it is well accepted that GABAergic neurons produce an inhibitory effect on PNs and are therefore usually believed to suppress brain activity.
In addition to contacting PNs, GABAergic neurons also make inhibitory synapses onto the same type and/or different types of GABAergic neurons (Galarreta and Hestrin, 2002; Gibson et al., 1999; Hu et al., 2011; Tamas et al., 1998) (Figure 2 and Figure 3), yet we know little about the functional consequences of this connectivity. Theoretical studies suggest that inhibitory coupling between inhibitory neurons promotes synchronous firing (Van Vreeswijk et al., 1994) which has been confirmed experimentally by electrophysiological recordings (Hu et al., 2011). Another potential implication of interactions between GABAergic INs is to mediate disinhibition. For example, in the auditory cortex, a disinhibitory microcircuit consisting of layer 1 GABAergic INs inhibiting layers 2/3 FS INs was recently suggested to mediate associative fear learning (Letzkus et al., 2011). In hippocampus, a recent report shows PV INs provide large amplitude GABAergic input to dendrite-targeting bistratified SOM INs and hence disinhibit the dendritic compartment of CA1 PCs during CA3 Schaffer collateral input (Lovett-Barron et al., 2012).
The present study reveals a previously unappreciated role of SOM INs as a source of disinhibition of PNs, and specifically of the PNs in the thalamorecipient or input layer of the mouse somatosensory cortex. This novel form of disinhibition is implemented through interactions between SOM INs and FS INs based on three lines of evidence. First, SOM INs strongly and preferentially inhibit FS INs in layer 4 (Figures 2 and 4). Second, reducing the activity of SOM INs significantly increases the firing of FS INs in layer 4 (Figure 5F, G). Third, FS INs suppress PNs firing in an active cortical network (Figure 6).
Functional Implications of SOM IN-mediated Disinhibition
Although there is only scant data and no consensus regarding the activity of SOM INs in intact brain (Gentet et al., 2012; Kerlin et al., 2010; Ma et al., 2010), our finding suggests the activity of layer 4 SOM INs reduces the firing of FS INs and hence relieves the strong inhibition produced by FS INs onto PNs and consequently disinhibits PNs in this layer.
In layer 4 of sensory cortex, FS cells mediate feedforwad inhibition of thalamocortical inputs that has been shown to be important in determining the timing of firing of PNs in layer 4 and their receptive field properties (Cruikshank et al., 2007; Gabernet et al., 2005; Higley and Contreras, 2006; Miller et al., 2001). The SOM INs-mediated disinhibitory circuit via FS INs in layer 4 revealed by the present study may therefore contribute to the processing of sensory information. When may this disinhibitory circuit be activated? In principle, layer 4 SOM INs could be activated by thalamic inputs since this layer is the primary recipient of sensory input from the thalamus. If this input was facilitating, it could replace the thalamic activation of FS cells, which is strongly depressing. However, previous reports have indicated that in contrast to FS cells and PNs, layer 4 SOM neurons receive very weak, depressing thalamic input (Beierlein et al., 2003; Cruikshank et al., 2010). Our own experiments confirmed this conclusion. We recorded simultaneously from a SOM IN and either a PN or a FS IN in thalamocortical slices. Postsynaptic potentials (EPSPs) were evoked by stimulating thalamic afferents with an extracellular stimulating electrode. The amplitude of the maximum EPSP evoked in SOM INs was strikingly smaller than that evoked in FS cells or PNs (Figure S5A, B).
One possible scenario when the layer 4 SOM IN-mediated disinhibitory circuit might be activated is suggested by the finding that layer 4 SOM INs, like SOM INs in layers 2/3 and 5 (Fanselow et al., 2008; Kawaguchi, 1997), are potently activated by acetylcholine (ACh) via a muscarinic receptor mediated mechanism (Figure S8A). The disinhibition of PNs produced in layer 4 as a result of this cholinergic activation of SOM cells may act in conjunction with the muscarinic inhibition of GABA release from FS cells (Kruglikov and Rudy, 2008) and the nicotinic enhancement of thalamocortical synapses on PNs (Gil et al., 1997) to facilitate the entry of sensory information into the neocortex during arousal and attention (Hasselmo, 1995) (Figure S8B).
EXPERIMENTAL PROCEDURES
All experimental procedures were conducted in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the NYU School of Medicine.
Brain Slice Preparation
Mice were anesthetized with intraperitoneal injection of pentobarbital (100 mg/kg body weight) and decapitated. The brain was quickly removed and immersed in ice-cold oxygenated artificial CSF (ACSF) containing the following (in mM): 87 NaCl, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1 CaCl2, 2 MgSO4 and 10 glucose. As previously described (Agmon and Connors, 1991; Kruglikov and Rudy, 2008), coronal slices (300 μm) or thalamocortical slices (400 μm) through primary somatosensory cortex were generated using Vibratome 1000 plus (Vibratome, St. Louis, MO) and incubated in a holding chamber at 32-35 °C for approximately 30 mi nutes followed by continued incubation at room temperature for at least 1 h before physiological recordings. A slice was then transferred to a recording chamber attached to the microscope stage and completely submerged in ACSF containing (mM) 124 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 2 MgSO4, and 10 glucose, pH 7.4 (when bubbled with 95% O2/5% CO2). ACSF was perfused in the recording chamber at 5 ml/min at 32 °C. To induce active cortical network activity (UP states), the slice solution was modified to contain 1 mM CaCl2, 1 mM MgSO4, and 3.5 mM KCl as previously reported (Sanchez-Vives and McCormick, 2000; Shu et al., 2003).
Electrophysiological Recordings
Whole-cell patch-clamp recordings were obtained from visually identified neurons. For current clamp, the internal pipette solution contained (in mM) 130 K-gluconate, 0.5 EGTA, 7 KCl, 10 HEPES, 4 Mg-ATP, 0.3 Na-GTP, 5 phosphocreatine, pH 7.2 with KOH. For voltage clamp, the pipette solution contained (in mM) 130 Cs-gluconate, 0.5 EGTA, 7 KCl, 10 HEPES, 4 Mg-ATP, 0.3 Na-GTP, 5 phosphocreatine, 5 QX-314, pH 7.2 with CsOH. Patch electrodes (4-8 M ) were pulled from borosilicate glass capillary (1.5 mm OD). Series resistances were usually 15-30 M upon break-in and were compensated by ~70%, and only cells with stable series resistance (<20% change throughout the recording) were used for analysis. Data were collected using an Axopatch 700B amplifier (Molecular Devices), low-pass filtered at 5 kHz and digitally sampled at 20 kHz on-line, and analyzed off-line with pClamp9 software (Molecular Devices). To characterize the intrinsic membrane properties of neurons, current-clamp recordings were made and hyperpolarizing and depolarizing current steps were injected at 0.1 Hz. For dual and triple recordings, whole-cell current-clamp recordings in SOM neurons were paired with voltage-clamp recordings (VHOLD = 0 mV) in excitatory neurons and/or FS INs. To activate thalamic afferents, extracellular stimuli were delivered to the ventrobasal nucleus through a concentric bipolar-stimulating electrode. Stimulation intensities were chosen to be just above the threshold for inducing UP states (range 10- 60 μA).
Electrophysiological Analysis
The following parameters were measured to characterize neuronal membrane properties: resting membrane potential (Vrest) was recorded immediately after the rupture of the neuronal membrane; input resistance (Rin) was determined by measuring the voltage change in response to a small hyperpolarizing current pulse (-50 pA, 1000 ms) at resting potential; membrane time constant (τm) was determined using a monoexponential fit to the voltage produced by a small hyperpolarizing current pulse at resting potential (-50 pA, 1000 ms); rheobase was defined as the smallest 1000 ms rectangular current injection that elicited a spike; spike threshold was defined as the membrane potential at the point at which dV/dt = 10 mV/ms; spike width was measured at half height between threshold and peak action potential; Fmax, steady state was calculated as the reciprocal of the average of the last five inter-spike intervals, measured at the maximal current step applied before spike inactivation became evident; Fmax, initial was calculated as the reciprocal of the average of the first five inter-spike intervals, measured at the maximal current step applied before spike inactivation became evident; spike adaptation ratio was defined as the ratio of Fmax, steady state to Fmax, initial. Population data are presented as mean ± SEM. To compare the results between different groups, statistical tests (two sample t test, paired t test) were performed using statistic software (Origin 7.5; Origin-Lab, Northampton, MA). In all cases, statistical significance was defined as p < 0.05 unless otherwise indicated.
Virus Injection and Optical Stimulation
SOM-Cre or PV-Cre mice, aged between postnatal days 10 and 15, were anesthetized with 2% isoflurane and placed in a stereotaxic frame (Kopf, Model 1900). The skull was exposed under antiseptic conditions and a small craniotomy was made with a thin drill over barrel cortex (typical coordinate: 0.80 mm posterior to Bregma; 3.6 mm lateral to the midline). Adeno-associated viruses carrying fusion genes for ChR2 (AAV2/1.EF1.dflox.hChR2(H134R)-mCherry.WPRE.hGH) or NpHR (AAV2/1.EF1a.DIO.eNpHR-EYFP.WPRE.hGH) were delivered using a glass micropipette (tip diameter ~20 μm) attached to a Nanoliter 2000 pressure injection apparatus (World Precision Instruments). Over a 10 min period, 0.5-1 μl of virus was injected at a depth of 200-500 μm from the cortical surface. Experiments were conducted at least 2 weeks after virus injection.
Photostimuli were produced by light emitting diodes (LED, Blue: 470 nm, Yellow: 591 nm) and delivered through a 40X water immersion objective. To ensure that SOM/PV neurons were hyperpolarized during the UP state, photo stimulation was timed to precede the electrical thalamic stimulation by a short time period (100 ms) and to outlast the full duration of the UP states (See Figures 5-7).
Immunohistochemistry
Mice were transcardially perfused with 20 ml phosphate-buffered saline (PBS) containing heparin (10 U/ml), followed by 50 ml 4% paraformaldehyde in 0.1 M PB (pH 7.4). Dissected brains were post-fixed in 4% paraformaldehyde in 0.1 M PB for 2 h at 4 °C and then placed in a 30% sucrose solution at 4 ° C until the brains sank. Using a microtome (Leica VT100), 40-μm-thick coronal sections were collected in PBS. Sections were washed in PBS two times (15 min each time) and then incubated with blocking solution (10% normal goat serum, 1% BSA, 0.2% cold fish gelatin, and 0.3% Triton X-100 in PBS) for 1 h at room temperature. Sections were then incubated with primary antibody in diluted (1:10) blocking solution overnight at 4 °C. The following primary antibodies were used: rat anti-RFP (1:500; Allele Biotechnology), rabbit anti-GFP (1:1000; Invitrogen), rabbit anti-DsRed (1:500; Clontech), rabbit anti-somatostatin (1:1000; Peninsula Laboratories LLC), rat anti-somatostatin (1:500; Millipore). After washing in diluted (1:10) blocking solution three times (15 min each time), sections were then incubated with species-specific fluorophore-conjugated secondary antibodies (Alexa 488 or 594; 1:1000; Invitrogen) in diluted (1:10) blocking solution for 1 h at room temperature. After washing in PBS three times (15 min each time), sections were mounted on glass slides with Vectashield (Vector Laboratories) and coverslipped. Using a Zeiss LSM510 microscope with a 10X or 20X objective, confocal images were acquired for cell counting. Scans from each channel were collected in multi-track mode to avoid cross-talk between channels.
Neuronal Reconstruction
0.3-0.5% biocytin was added to the internal recording solution. After recording, a picture at low magnification of the pipette position above the slice was taken to confirm the position of the recorded cell. Brain slices were then fixed in 4% PFA. Slices were then washed with phosphate buffer solution and incubated with streptavidin conjugated Alexa (1:500) and 0.3% Triton-100 overnight. Slices were then washed with phosphate buffer and mounted on microscope slides. Confocal image stacks were acquired using a Zeiss LSM510 microscope with a 40X objective. Stacks were then imported on the Neurolucida software for digital reconstruction. Layers and barrels boundaries were determined using DAPI staining.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Gord Fishell, Ariel Agmon, György Buzsáki and the members of the Rudy and Fishell labs for scientific discussions. We thank Ariel Agmon for the X94 mouse line, Z. Josh Huang for B13, G42 and SOM-Cre mice, Gord Fishell for RCE mice and Karl Deisseroth for ChR2-mCherry and NpHR-YFP plasmids. We thank Soohyun Lee for help with virus injection, Chiung-Yin Chung and Deborah Han for animal genotyping. This research was supported by NIH Grants NS30989 and NS045217 to B.R. and a postdoctoral fellowship from the International Rett Syndrome Foundation to H.X.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Berger TK, Silberberg G, Perin R, Markram H. Brief bursts self-inhibit and correlate the pyramidal network. PLoS Biol. 2010:8. doi: 10.1371/journal.pbio.1000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat. Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–245. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J. Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu. Rev. Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc. Natl. Acad. Sci. USA. 2002;99:12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav. Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J. Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Ma Y, Agmon A. Submillisecond firing synchrony between different subtypes of cortical interneurons connected chemically but not electrically. J. Neurosci. 2011;31:3351–3361. doi: 10.1523/JNEUROSCI.4881-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J. Neurophysiol. 1997;78:1743–1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat. Neurosci. 2012;15:423–430. S421–423. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in "knock-in" Cre-reporter mice ideally suited for lineage tracing studies. Eur. J. Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- Ma WP, Liu BH, Li YT, Huang ZJ, Zhang LI, Tao HW. Visual representations by cortical somatostatin inhibitory neurons--selective but with weak and delayed responses. J. Neurosci. 2010;30:14371–14379. doi: 10.1523/JNEUROSCI.3248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J. Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. 1997;500(Pt 2):409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Miller KD, Pinto DJ, Simons DJ. Processing in layer 4 of the neocortical circuit: new insights from visual and somatosensory cortex. Curr. Opin. Neurobiol. 2001;11:488–497. doi: 10.1016/s0959-4388(00)00239-7. [DOI] [PubMed] [Google Scholar]
- Murayama M, Perez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 2009;457:1137–1141. doi: 10.1038/nature07663. [DOI] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J. Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto DJ, Brumberg JC, Simons DJ, Ermentrout GB. A quantitative population model of whisker barrels: re-examining the Wilson-Cowan equations. J. Comput. Neurosci. 1996;3:247–264. doi: 10.1007/BF00161134. [DOI] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J. Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb. Cortex. 2009;19(Suppl 1):i1–10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J. Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vreeswijk C, Abbott LF, Ermentrout GB. When inhibition not excitation synchronizes neural firing. J. Comput. Neurosci. 1994;1:313–321. doi: 10.1007/BF00961879. [DOI] [PubMed] [Google Scholar]
- Varga C, Lee SY, Soltesz I. Target-selective GABAergic control of entorhinal cortex output. Nat. Neurosci. 2010;13:822–824. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester JC, Contreras D. Columnar interactions determine horizontal propagation of recurrent network activity in neocortex. J. Neurosci. 2012;32:5454–5471. doi: 10.1523/JNEUROSCI.5006-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J. Comp. Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.