Abstract
Inverse agonism at the benzodiazepine site of α5 subunit-containing GABAA receptors is an attractive approach for the development of putative cognition-enhancing compounds, which are still far from clinical application. Several ligands with binding and/or functional selectivity for α5 GABAA receptors have been synthesized and tested in a few animal models. PWZ-029 is an α5 GABAA selective inverse agonist whose memory enhancing effects were demonstrated in the passive avoidance task in rats and in Pavlovian fear conditioning in mice. In the present study we investigated the effects of PWZ-029 administration in novel object recognition test and Morris water maze, in normal and scopolamine-treated rats. All the three doses of PWZ-029 (2, 5 and 10 mg/kg) improved object recognition after the 24-h delay period, as shown by significant differences between the exploration times of the novel and old object, and the respective discrimination indices. PWZ-029 (2 mg/kg) also successfully reversed the 0.3 mg/kg scopolamine-induced deficit in recognition memory after the 1-h delay. In the Morris water maze test, PWZ-029 (5, 10 and 15 mg/kg) did not significantly influence swim patterns, either during five acquisition days or during the treatment-free probe trial. PWZ-029 (2, 5 and 10 mg/kg) also proved to be ineffective in the reversal of the 1 mg/kg scopolamine-induced memory impairment in the water maze. The present mixed results encourage use of a variety of tests and experimental conditions in order to increase the predictability of preclinical testing of selective α5 GABAA inverse agonists.
Keywords: GABA, inverse agonist, memory, object recognition, water maze
1. Introduction
Classical benzodiazepines are positive allosteric modulators at the benzodiazepine binding site of inhibitory GABAA receptors and have been widely used for half a century as anxiolytics, hypnotics, myorelaxant and anticonvulsant drugs. Soon after introduction into clinical practice, their propensity to disrupt memory formation was recognized and became the subject of thorough examination [1,2]. Many studies examining the nature and mechanisms of such memory impairment eventually led to a discovery that negative modulation at the same receptors could elicit an opposite effect [3]. Negative modulators (i.e. inverse agonists) of the benzodiazepine binding site decrease chloride flux through the GABAA receptor ion channel, resulting in a decrease of GABAergic inhibition and increase of neuronal excitability. These molecular and neuronal mechanisms of inverse agonism are reflected behaviorally in increased vigilance, but also anxiogenic and proconvulsant actions [4]. Hence, inverse agonists at the benzodiazepine binding site were shown to concomitantly improve certain forms of memory in animals [5,3] and in human volunteers [6] and elicit convulsant/proconvulsant and anxiogenic effects [7,8].
In the last two decades, genetic and pharmacological studies, by the means of generation of mutant mouse lines [9] and synthesis of novel, subtype-selective ligands, respectively, have helped in linking the particular behavioral response to a distinct GABAA receptor subtype [11,12,13]. Of the four GABAA receptor populations (α1-, α2-, α3- and α5-GABAA receptors) that are sensitive to benzodiazepine modulation, those receptors containing α1 and α5 subunits were shown to be substantially involved in mediating their memory effects [10,14,15,16,17]. While the reduced expression of α5 GABAA receptors has been selectively linked to facilitation of some aspects of cognition [14,15], inverse agonism at the α1 subunit was also connected with the emergence of proconvulsive and convulsive activity [18,19], and hence GABAA receptors containing the α5 subunit became an ultimate target for the development of putative cognition-enhancing compounds [20].
Over the years, a number of compounds with binding and/or functional selectivity for α5 GABAA receptors have been synthesized and tested in different animal models and species [21,22,23,24]. Most of them improved performance in memory tasks, whilst at the same time being devoid of CNS-mediated adverse effects [25,26,27,28,29]. In healthy humans, pre-treatment with an α5 efficacy-selective inverse agonist significantly reduced the amnesic effect of alcohol on learning a word list, thus providing a valuable proof of the concept that α5 GABAA receptors play a principal role in memory processes [30]. However, to date there are no GABAA receptor inverse agonists in the clinical practice, which indicates the need for further search for ligands with optimal efficacy/safety profile, that would translate into drugs with a clear-cut cognition enhancing potential in humans [31].
PWZ-029 is a partial inverse agonist that binds with preferential affinity to α5 GABAA receptors, while at α1- and α3-containing receptors exhibits weak agonistic activity. In a previous study, PWZ-029 improved passive but not active avoidance learning task in rats, showed no effect on anxiety or muscle tone, and had no convulsive activity in the tested dose range (2-20 mg/kg) [32]. In mice, PWZ-029 at a dose of 10 mg/kg was able to robustly attenuate scopolamine-induced impairment of Pavlovian fear conditioned contextual memory [33]. The aim of the present study was to test PWZ-029 in unimpaired animals as well as against a scopolamine-induced deficit in Morris water maze (MWM) and novel object recognition test (NORT), two paradigms widely used for behavioral screening of putative memory enhancing compounds [34,35].
2. Material and methods
2.1. Animals
Male Wistar rats, weighing 200–230 g, were supplied by Military Farm, Belgrade, Serbia. Rats were housed in groups of four and were maintained under standard laboratory conditions (21 ± 2°C, relative humidity 40-45%) with free access to pellet food and tap water. They were kept on 12:12 h light/dark cycle with lights on at 07.00 h. All handling and testing took place during the light phase of the diurnal cycle. Experiments were carried out in accordance with the EEC Directive 86/609 and were approved by the Ethical Committee on Animal Experimentation of the Faculty of Pharmacy in Belgrade. Each of four experiments was performed using separate sets of experimentally naïve animals.
2.2. Drugs
PWZ-029, an α5 selective inverse agonist, was synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin–Milwaukee, USA. The compound was suspended in a vehicle containing 85% distilled water, 14% propylene glycol and 1% Tween-80, and administered intraperitoneally in a volume of 1 ml/kg, 20 minutes before the testing. Scopolamine hydrobromide (Sigma-Aldrich, Germany) was dissolved in saline and administered intraperitoneally in a volume of 1 ml/kg, 30 minutes before the testing.
2. 3. Novel object recognition test
2.3.1. Apparatus and procedure
Novel object recognition test was performed as described elsewhere [36]. The apparatus consisted of a rectangular chamber (65 × 45 × 45 cm) placed in a room with a red dim illumination (~ 20 lux). Animals were habituated to the empty chamber for 10 min, 24 h before the beginning of experiment. Novel object recognition test was divided in two phases, familiarization and testing. In the familiarization phase two identical objects were placed 15 cm from the sides of the chamber and 25 cm apart. After receiving appropriate treatment, each rat was placed in the arena between the two objects facing the opposite wall and allowed to explore them for 5 minutes. Animal was then removed from the apparatus and returned to the cage (1 h) or to the colony (24 h) for the prescribed retention interval. To test for object recognition, one of the familiar sample objects was replaced with a novel object and animal was returned to the apparatus and allowed to explore for 3 minutes. Object exploration was recorded when the animal was in close proximity (2 cm or less) and oriented to the object, and if the animal sniffed or pawed the object. Object set consisted of a semi-transparent pyramid (base 8 cm x 8 cm, height 7 cm) and a nontransparent dome-shaped paperweight (base diameter 9 cm, height 10 cm), which couldn’t be displaced by rats. Each object was available in triplicate and all combinations and locations of objects were used in a balanced manner to reduce potential biases due to preferences for particular locations or objects [37]. After each trial, the apparatus and objects were cleaned with 70% ethanol to reduce olfactory cues.
2.3.2. Experiments
In the first experiment, four groups of rats (n=10) received vehicle or PWZ-029 (2, 5 or 10 mg/kg) 20 minutes before familiarization phase. After 24 h, object recognition memory was tested. In the second experiment we examined the influence of PWZ-029 (2, 5 or 10 mg/kg) on the scopolamine-induced memory impairment in NORT. The dose of scopolamine (0.3 mg/kg) was selected according to our preliminary testing (unpublished data). There were five group of rats (n=12-15) and each group received one of the following treatments before the familiarization phase: saline + vehicle, scopolamine (0.3 mg/kg) + vehicle and scopolamine in combination with PWZ-029 (2, 5 and 10 mg/kg). Retention interval was 1 h. Rats were monitored using the Any-Maze video-tracking software. The amount of time exploring familiar (T1) and novel (T2) objects was automatically scored, and then total time exploring (T1+T2) and discrimination index ((T2-T1)/(T1+T2)) were calculated.
2.4. Morris water maze
2.4.1. Apparatus and procedure
Experiments were performed in a 2 m diameter circular pool filled to a height of 30 cm with water at 23 °C. The escape platform (15 cm x 10 cm) of the same color as a pool was submerged 2 cm below the water surface in the northeastern quadrant (NE region) of the pool. An indirect illumination in the experimental room was provided by white neon tubes fixed on the walls and many distal cues were present (doors, pipes on the walls and the ceiling, cupboards, a camera suspended above the center of the maze). On each of the five consecutive days rats were given one swimming block, consisting of two or four trials, depending on the experimental condition. Each trial lasted a maximum time of 120 s, after which rat was manually guided to the platform and left there for 15 s. Between two consecutive trials, rats were returned to their cage and given a 60-second rest. Start positions varied pseudo-randomly among the four cardinal points (W, NW, S, SE). During the acquisition phase, treatments were applied once daily before the swimming block. On the sixth day, rats were given a treatment–free probe trial (60 s) without the platform [38].
2.4.2. Experiments
In the first experiment, we examined the influence of PWZ-029 administration on water maze performance in rats. Four groups of rats (n=7) received vehicle or PWZ-029 (5, 10 or 15 mg/kg). We used a two-trial per day protocol which is associated with diminished performance of control animals, thus allowing for potential enhancement effects to be revealed [39]. In the second experiment, a standard four-trial per day protocol was used and the influence of PWZ-029 on scopolamine-induced impairment of water maze performance was assessed. The dose of scopolamine (1 mg/kg) was selected based on our pilot data as the dose that completely disrupted acquisition in MWM. The animals were divided into five groups (n = 8) and received one of the following treatments: saline + vehicle, scopolamine (1 mg/kg) + vehicle, and combination of scopolamine (1 mg/kg) and PWZ-029 (2, 5 and 10 mg/kg).
Animals were monitored with the Any-Maze (Stoelting, Wood Dale, USA) video analysis system. The pool was virtually divided into four quadrants, three concentric annuli and a target region consisting of the intersection of the platform quadrant and the platform (middle) annulus, as represented in an earlier paper [17]. Dependent variables chosen for tracking during the acquisition trials were: escape latency (s), path efficiency (the ratio of the shortest possible path length to actual path length), total distance travelled (m), distance in the peripheral ring (%) and swim speed (m/s). The indices of memory, assessed during the probe trial, included time in the NE region (s), time in the target zone (s) and time in the peripheral ring (s).
2.5. Statistics
All numerical data presented in the figures are given as the mean ± S.E.M. The data from the acquisition days in the Morris water were averaged for each rat (total data/total number of trials per day) and analyzed using two-way ANOVA with repeated measures. If the interaction between the day and treatment was significant, then one-way ANOVA for the treatment effect for each day was performed. The data from the probe trial were assessed using one-way ANOVA. Post-hoc comparisons, where applicable, were performed using Student–Newman–Keuls (SNK) test. In NORT, total exploration times of different treatment groups were analyzed using one-way ANOVA with post-hoc SNK test. The differences between exploration times spent with old and novel objects for each group were evaluated using paired t-test. Discrimination index was assessed by one-sample t-test per treatment group. Statistical analysis was performed using SigmaPlot 11 (Systat Software Inc., Richmond, USA) software. Differences were considered to be statistically significant when p was less than 0.05.
3. Results
3.1. Novel object recognition test
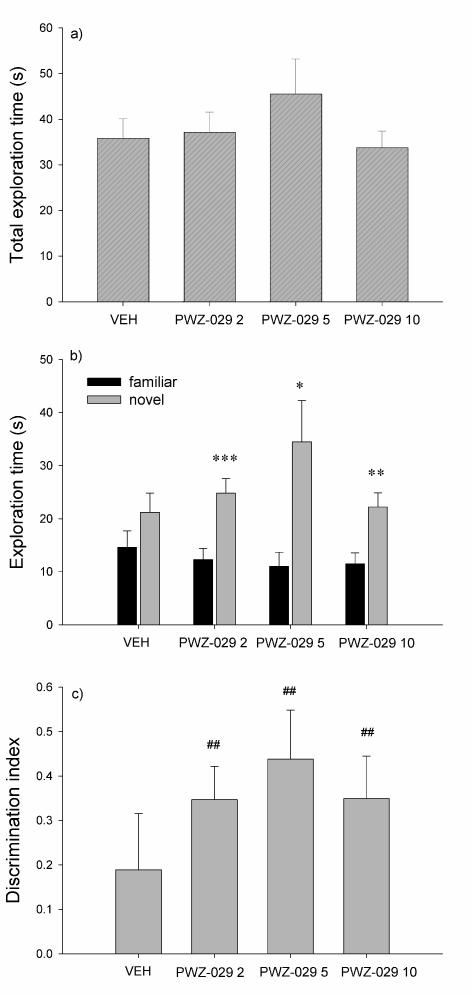
The effects of PWZ-029 administration on the rats’ behavior in the novel object recognition test are depicted in Fig. 1. One-way ANOVA revealed no differences between total exploration times among different treatment groups (F(3,36)=0.973, p=0.416) (Fig. 1a). However, rats receiving 2, 5 and 10 mg/kg PWZ-029 spent significantly more time exploring the new object than the familiar one (t= −5.884, p<0.001; t= −2.712, p=0.024; t= −3.526, p=0.006, respectively), whereas rats treated with vehicle showed no significant difference (t= −1.264, p=0.238) in the exploration time of the familiar object compared with that of the novel one (Fig. 1b). Discrimination indices (Fig. 1c) were significantly different from zero (i.e. chance level) for the rats treated with 2 mg/kg PWZ-029 (t=4.625; p=0.001), 5 mg/kg PWZ-029 (t=3.974; p=0.003) and 10 mg/kg PWZ-029 (t=3.670; p=0.005), but not for the control group (t=1.489; p=0.171).
Figure 1.
The effects of PWZ-029 (2, 5 and 10 mg/kg) on (a) total exploration time, (b) time exploring familiar and novel objects and (c) discrimination indices in the novel object recognition test using a 24-h delay (mean + SEM). Significant differences from control (VEH) are indicated with asterisks (paired-samples t-test, *p < 0.05, **p < 0.01). A significant difference from zero is indicated with hashes (one sample t-test, ##p < 0.01). Number of animals per each treatment group was 10. VEH = vehicle.
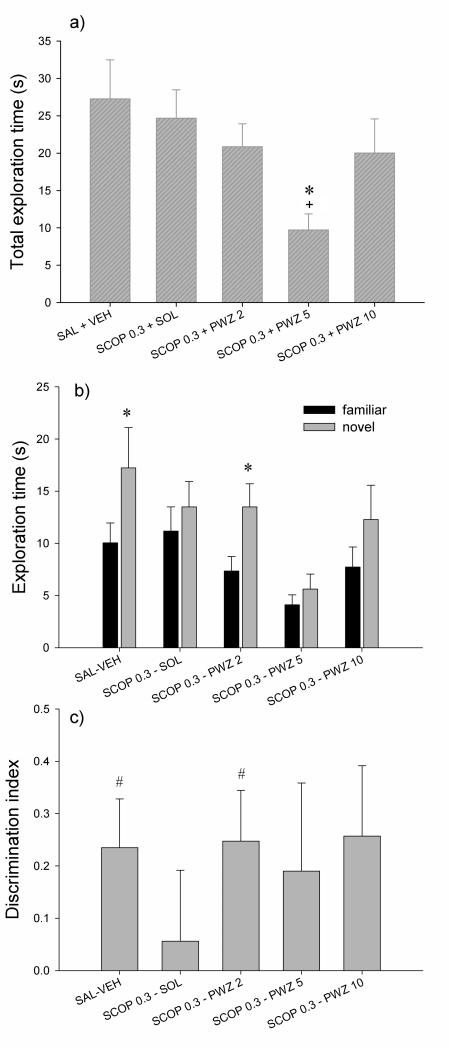
Figure 2 shows effects of PWZ-029 administration on scopolamine-pretreated rats in NORT. Analysis of variance showed significant effect of treatment on the total exploration time (F(4,65)=3.167, p=0.019); post hoc SNK test revealed that rats injected with combination of 0.3 mg/kg scopolamine and 5 mg/kg PWZ-029 spent less time (p=0.017) in exploring behavior compared to the control group as well as to group that received 0.3 mg/kg scopolamine + vehicle (p=0.03) (Fig. 2a). Comparison of the times spent in exploring familiar and novel object showed that only control group and group treated with combination of 0.3 mg/kg scopolamine and 2 mg/kg PWZ-029 spent significantly more time in exploring new object (t= −2.276, p=0.044; t= −3.106, p= 0.008, respectively) (Fig. 2b). Similarly, discrimination indices (Fig. 2c) were significantly different from zero for the control group (t= 2.522; p=0.028) and group treated with combination of 0.3 mg/kg scopolamine and 2 mg/kg PWZ-029 (t=2.552; p=0.023).
Figure 2.
The effects of 0.3 mg/kg scopolamine (SCOP 0.3) and combination of 0.3 mg/kg scopolamine and PWZ-029 (2, 5 and 10 mg/kg) on the rats’ performance in the object recognition task after a 1-h delay: (a) total exploration time, (b) time exploring familiar and novel objects and (c) discrimination index. Data are represented as mean + S.E.M. Significant differences from control (SAL + VEH) are indicated with asterisks (*p<0.05 for one-way ANOVA on total exploration time with post-hoc SNK, and for paired-samples t-test for the familiar vs. novel exploration times); +p < 0.05 significant difference from SOL + SCOP 0.3. A significant difference from zero is indicated with hashes (one sample t-test, #p<0.05). Number of animals per each treatment group was 12-15. SAL = saline, VEH = vehicle.
3.2. Morris water maze
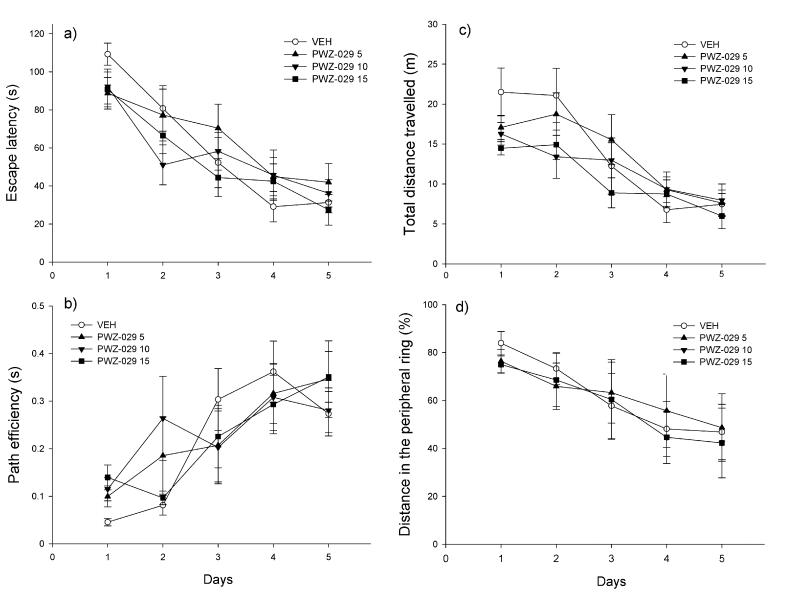
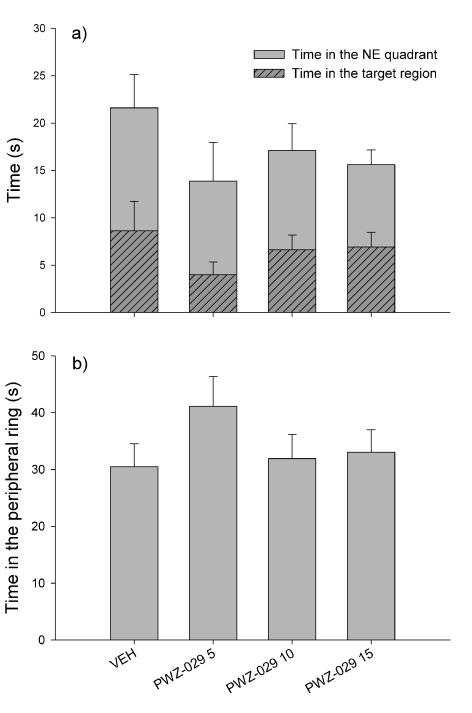
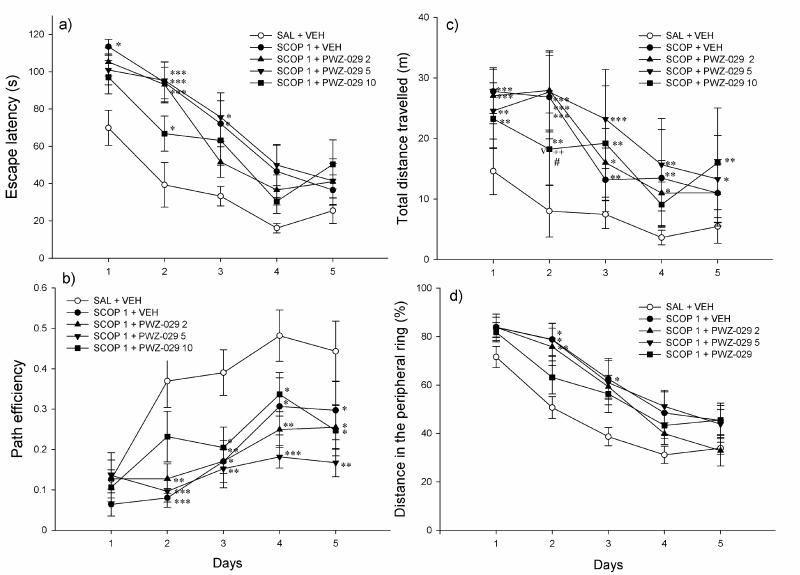
The application of PWZ-029 during acquisition days did not significantly influence swim patterns in the Morris water maze (Table 1). None of the doses tested (5, 10 and 15 mg/kg) significantly influenced any of the four indices of memory during training days: escape latency (Fig. 3a), path efficiency (Fig. 3b), total distance travelled (Fig. 3c) and percent of the distance in the peripheral ring (Fig. 3d). This reflected in performance during probe trial, where no significant differences were found among treatments in the time spent in the NE region (Fig. 4a), time in the target zone (Fig. 4a) and time in the peripheral ring (Fig. 4b).
Table 1.
The influence of factors treatment and day on different parameters of MWM peformance during acquisition days, and respective F and p values from the two-way ANOVAs with repeated measures.
| Experiment | VEH, PWZ-029 (5, 10 and 15 mg/kg) | SAL+VEH; SCOP 1 + VEH; SCOP 1 + PWZ-029 (2, 5 and 10 mg/kg) |
||||
|---|---|---|---|---|---|---|
| Parameter | Treatment | Day | Interaction | Treatment | Day | Interaction |
| Escape latency (s) |
F(3,24)=0.733 p=0.542 |
F(4,96)=26.289 p<0.001 |
F(12, 96)=1.064 p=0.399 |
F(4, 35)=4.324 p=0.006 |
F(4, 140)=62.947 p<0.001 |
F(16, 140)=1.553 p=0.090 |
| Path efficiency |
F(3,24)=0.121 p=0.947 |
F(4,96)=11.296 p<0.001 |
F(12, 96)=1.00 p=0.455 |
F(4, 35)=8.355 p<0.001 |
F(4, 140)=14.637 p<0.001 |
F(16, 140)=1.49 p=0.112 |
| Total distance (m) |
F(3,24)=1.799 p=0.174 |
F(4,96)=19.234 p<0.001 |
F(12, 96)=1.076 p=0.389 |
(F(4,35)=5.355 p=0.002 |
F(4,140)=35.616 p<0.001 |
F(16, 140)=2.392 p=0.003 |
| Distance in the peripheral ring (%) |
F(3,24)=1.365 p=0.277 |
F(4,96)=26.638 p<0.001 |
F(12, 96)=0.516 p=0.900 |
F(4, 35)=2.744 p=0.044 |
F(4, 140)=76.725 p<0.001 |
F(16, 140)=1.122 p=0.341 |
Figure 3.
The influence of PWZ-029 (5, 10 and 15 mg/kg) on parameters related to learning in the 2-trial per day version of Morris water maze test: (a) escape latency, (b) path efficiency, (c) total distance travelled and (d) % of distance in the peripheral ring (mean ± SEM). Number of animals per each treatment group was 7. VEH = vehicle.
Figure 4.
Parameters of memory retention assessed during the drug-free probe trial, in animals previously treated with 5, 10 and 15 mg/kg PWZ-029: (a) time in the NE quadrant and time in the target region (stripped bars) and (b) time in the peripheral ring. Data are represented as mean + S.E.M. Number of animals per treatment group was 7. VEH = vehicle.
In the second experiment, there were significant effects of factor treatment and factor day regarding all the parameters observed during acquisition (Table 1). After the post hoc analysis was performed, it appeared that all groups treated with scopolamine (scopolamine in combination with vehicle or 2, 5 and 10 mg/kg PWZ-029) increased escape latency compared to control (respective p values from SNK test: 0.009, 0.006, 0.019 and 0.019), and had lower path efficiency (respective p values: <0.001, <0.001, <0.001 and p=0.002). The respective significant differences among treatments during days are presented in Figures 5a and 5b. Scopolamine pretreatment was also a source of significant variation among groups regarding the total distance travelled (Fig. 5c) and % of distance in the peripheral ring (Fig. 5d). Scopolamine increased total distance compared to control (p=0.005), and addition of PWZ-029 did not influence this score since the groups treated with combination of scopolamine and PWZ-029 (2, 5 and 10 mg/kg) still had significantly longer swim path than control group (respective p values: 0.007, 0.002 and 0.005). Additionally, all scopolamine-treated groups swam significantly faster during acquisition days (data not shown).
Figure 5.
The effects of 1 mg/kg scopolamine (SCOP 1+VEH) and combination of 1 mg/kg scopolamine and 2, 5 and 10 mg/kg PWZ-029 (SCOP 1+PWZ-029 2, SCOP 1+PWZ-029 5 and SCOP 1+PWZ-029 10) on: (a) escape latency, (b) path efficiency, (c) total distance travelled and (d) % of distance in the peripheral ring during 5-day acquisition trials in the water maze. * p<0.05, ** p<0.01, *** p<0.001, compared to control (SAL+VEH); ++p<0.01 compared to SCOP 1 + VEH, #p<0.05 compared to SCOP 1 + PWZ-029 2, Vp<0.05 compared to SCOP 1 + PWZ-029 5. Number of animals per treatment was 8. SAL= saline, VEH=vehicle.
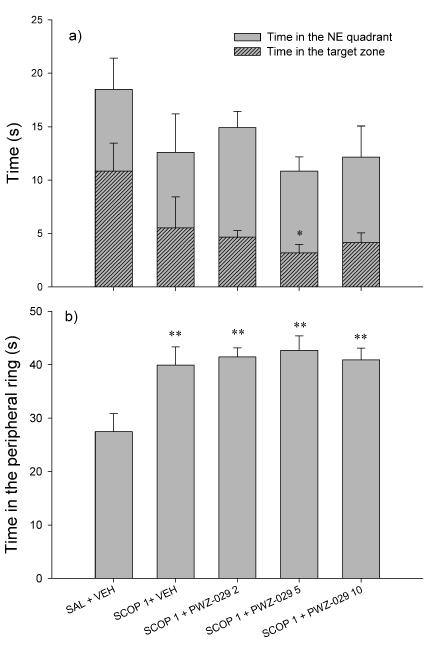
In Fig. 6a, the time that rats spent in the platform quadrant (NE) during probe trial is presented alongside the respective time in the portion of NE quadrant lying in the platform annulus of the maze (‘the target region’). There were no significant differences among groups regarding time spent in the NE quadrant. However, there was a significant effect of treatment for the time spent in the target region (F(4, 35)=2.682, p=0.047). Post hoc test revealed that rats treated with combination of scopolamine and 5 mg/kg PWZ-029 spent significantly less time in the target region (p=0.043). During probe trial, groups also spent significantly different times in the peripheral ring (F(4,35)=5.137, p=0.002). Rats treated with scopolamine and rats treated with combination of scopolamine and PWZ-029 (2, 5 and 10 mg/kg) spent more time searching in the peripheral ring compared to control. The respective differences (p=0.003, p=0.005, p=0.004 and p=0.004) are presented in Fig. 6b.
Figure 6.
The representative parameters of water-maze performance in the probe trial: (a) time spent in the NE quadrant and time spent in the target zone (stripped bars), and (b) time in the peripheral ring. Data are represented as mean + S.E.M. *p < 0.05, **p < 0.01, compared to control (SAL+VEH). Number of animals per treatment group was 8. SAL= saline, VEH= vehicle.
4. Discussion
A substantial amount of preclinical research has focused on identifying compounds that may improve different aspects of cognition. Successful translation from promising in vitro properties into a clinically efficacious and safe therapy has long been recognized as a major bottle-neck in this field, which necessitates screening of newer ligands and thorough assessment of their effects in a diversity of the behavioral tasks in normal and experimentally impaired laboratory animals [31, 40].
PWZ-029 is one of the several inverse agonists whose selective affinities and/or efficacies at α5 GABAA receptor population were set as a basic premise for their protective or therapeutic action in various cognitive impairments, and whose positive results in preclinical studies have already been published [41]. To date, memory enhancing effects of PWZ-029 were demonstrated in the passive avoidance task in rats and in Pavlovian fear conditioning test in mice. At the same time, PWZ-029 proved to be devoid of serious adverse effects such are anxiogenic and convulsive activity [32,33]. In the present study, PWZ-029 was tested in normal and scopolamine-impaired rats using two paradigms indicative of different forms of memory, the novel object recognition test and Morris water maze.
Object recognition test examines capability of animals to spontaneously discriminate between familiar and novel objects after the prescribed retention interval. Normal rats accurately discriminate between the novel and the familiar object after a 1-h but usually not after a 24-h delay [42]. The delay-dependent decline in memory recognition is believed to be a result of failure of the perirhinal cortex to maintain information about the object during longer retention intervals [43,44]. In the present experiment, PWZ-029 was able to improve object recognition after a 24-h delay, which is shown by the difference between exploration time of novel and old objects as well as discrimination indices. The result is complementary to findings that both systemic and central (in the perirhinal cortex) administration of benzodiazepines produced impairment in object recognition task, indicating involvement of GABAergic neurotransmission in regulation of this form of learning [45,46,47]. Since the cited studies used nonselective agonists of the benzodiazepine binding site, it remained unclear whether the impairment occurred due to activity at widespread α1 subunit-containing receptors or at α5 GABA receptors, whose distribution is limited to the hippocampus and certain cortical structures [48]. Results from our study showed for the first time that memory enhancement in the object recognition task can be achieved by systemic administration of an α5 GABA selective inverse agonist to unimpaired rats, suggesting the significance of α5 GABA receptor population in this type of memory. However, in order to confirm the present results, additional experiments using full agonists and antagonists selective for the α5 subunit would be worthwhile.
Scopolamine-induced cognitive impairment is frequently used to model the memory, attention, and executive function-related deficits that are seen in aging and dementia [49]. In the NORT, rats treated with scopolamine do not discriminate between the familiar and the novel object after a 1-h delay. The hypothesis that negative modulation of GABAA receptors could facilitate cholinergic transmission and thus improve cognitive functions is based on the fact that cholinergic pathways in certain brain regions involved in learning/memory processes are under the strong inhibitory influence of GABA neurons [50,51]. Results from our study supported this hypothesis as PWZ-029 successfully reversed the scopolamine-induced deficit in recognition memory after a 1-h delay. However, only the lowest dose of PWZ-029 (2 mg/kg) was capable of shifting the difference in time exploring novel vs. familiar objects and discrimination index to the control levels. The inefficacy of the two higher PWZ-029 doses (5 and 10 mg/kg) in this model could be discussed from the point of possible nonspecific behavioral effects of the combined treatment. For example, combination of scopolamine and 5 mg/kg PWZ-029, but neither of these treatments alone, resulted in a significant decrease of the total exploration time, which may have confounded the results.
Unlike NORT which measures cognitive capability, MWM is a paradigm which tests cognitive efficiency, i.e. it tests whether treatment groups significantly differ from control in efficiency to find a hidden platform (cf. Ref. 31). In a delayed matching-to-position version of MWM task, which tests spatial working memory (WM) when the platform position changes every day, this difference is revealed through the saving times between the first and the second trial on each of the testing days. In this version of the task, mice lacking the α5 GABAA receptor subunit [14], as well as rats treated with inverse agonists at α5 GABAA receptors [27], have been reported to have greater saving times than control. In the protocol that tests reference memory (RM), however, position of the platform remains the same throughout the days, and latency, distance and other related parameters are calculated. In RM protocol, McNamara and Skelton [39] reported improvement with the nonselective GABAA inverse agonist β-CCM. Nonetheless, rats treated with PWZ-029 remained at the control level in the same protocol. The lack of efficacy of an α5 GABAA receptor inverse agonist in the current experiment could be considered in the light of the relative importance of the α5 and α1 modulation in the RM protocol. Our previous study [17] suggested a greater contribution of the α1 than the α5 subunit-containing GABAA receptors to the diazepam-induced impairment in RM protocol, which clearly reduced probability of detecting putative facilitating effects exerted via inverse agonism at the α5 subtype. Furthermore, although both WM and RM protocols measure spatial memory, they reflect distinct mnemonic processes, which probably have distinct neurobiological mechanisms and thus are susceptible to modulation to a different degree [52,53]. Since there are no other reports on the use of a selective α5 GABAA receptor inverse agonist in unimpaired animals in RM protocol, the suggested explanation needs to be confirmed by further research.
There are numerous examples where administration of a cognition-enhancing drug exerted no measurable effects in normal animals, whereas improved the measured cognitive parameters in subjects with experimentally induced deficits (reviewed in Ref. 40). In our study, however, this was not the case, since PWZ-029 failed to reverse the scopolamine-induced impairment in MWM. Hence, besides the previously shown lack of activity of PWZ-029 on its own in RM protocol, an overall inefficiency of PWZ-029 in this experimental model may be postulated. In a similar experimental setup, it was shown that both, S8510 and CGS8216, an inverse agonist and an antagonist at GABAA receptors, reversed the scopolamine-induced deficit in MWM [54]. However, the other nonselective inverse agonist, FG7142, that had been previously shown to improve short-term memory in unimpaired rats [55], was ineffective in this manner [54]. Taken together, these examples with MWM results imply a limited and substance- and task-dependent potential of efficacy of inverse agonists at GABAA receptors. In conclusion, our study showed for the first time that an inverse agonist at α5 GABAA receptors, PWZ-029, may be efficacious in improving performance in the novel object recognition test in unimpaired rats, and also ameliorates deficits in memory and learning function induced by a muscarinic antagonist in this test. On the other hand, PWZ-029 proved to be inefficacious in Morris water maze, either alone or in reversing the scopolamine-induced impairment. These results indicate that behavioral screening of the putative cognition-enhancing compounds should include a variety of tests, models and experimental conditions in order to increase the predictability of the sometimes erratic positive preclinical results.
Research Highlights.
 PWZ-029 is an α5 GABAA selective inverse agonist.
PWZ-029 is an α5 GABAA selective inverse agonist.
 We tested PWZ-029, on its own and with scopolamine, in two memory paradigms.
We tested PWZ-029, on its own and with scopolamine, in two memory paradigms.
 It improved object recognition in the novel object recognition test.
It improved object recognition in the novel object recognition test.
 PWZ-029 was ineffective in the Morris water maze test.
PWZ-029 was ineffective in the Morris water maze test.
 Testing of putative procognitive compounds needs use of a variety of models.
Testing of putative procognitive compounds needs use of a variety of models.
Acknowledgements
This work was supported in part by The Ministry of Education, Science and Technological Development, R. Serbia – Grant No. 175076 (MMS) and by NIMH 46851 (JMC). We acknowledge the support of this work by the Research Growth Initiative of the University of Wisconsin-Milwaukee and the Lynde and Harry Bradley Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brandt AL, Oakes FD. Preanesthesia medication: double-blind study of a new drug, diazepam. Anesth Analg. 1965;44:125–9. [PubMed] [Google Scholar]
- [2].Haslett WH, Dundee JW. Studies of drugs given before anaesthesia. XIV. Two benzodiazepine derivatives–chlordiazepoxide and diazepam. Br J Anaesth. 1968;40:250–8. doi: 10.1093/bja/40.4.250. [DOI] [PubMed] [Google Scholar]
- [3].Jensen LH, Stephens DN, Sarter M, Petersen EN. Bidirectional effects of beta-carbolines and benzodiazepines on cognitive processes. Brain Res Bull. 1987;19:359–64. doi: 10.1016/0361-9230(87)90104-3. [DOI] [PubMed] [Google Scholar]
- [4].Haefely WE, Martin JR, Richards JG, Schoch P. The multiplicity of actions of benzodiazepine receptor ligands. Can J Psychiatry. 1993;38:S102–8. [PubMed] [Google Scholar]
- [5].Venault P, Chapouthier G, de Carvalho LP, Simiand J, Morre M, Dodd RH, et al. Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature. 1986;321:864–6. doi: 10.1038/321864a0. [DOI] [PubMed] [Google Scholar]
- [6].Duka T, Edelmann V, Schütt B, Dorow R. Beta-carbolines as tools in memory research: human data with the beta-carboline ZK 93426. Psychopharmacol Ser. 1988;6:246–60. doi: 10.1007/978-3-642-73288-1_18. [DOI] [PubMed] [Google Scholar]
- [7].Dorow R, Horowski R, Paschelke G, Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983;2:98–9. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- [8].Petersen EN. DMCM: a potent convulsive benzodiazepine receptor ligand. Eur J Pharmacol. 1983;94:117–24. doi: 10.1016/0014-2999(83)90448-x. [DOI] [PubMed] [Google Scholar]
- [9].Rudolph U, Möhler H. Analysis of GABA(A) receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–98. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- [10].Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- [11].Rudolph U, Crestani F, Möhler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–94. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- [12].Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- [13].McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;6:587–92. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- [14].Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–80. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, et al. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–5. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Savić MM, Huang S, Furtmüller R, Clayton T, Huck S, Obradović DI, et al. Are GABA(A) receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008;33:332–9. doi: 10.1038/sj.npp.1301403. [DOI] [PubMed] [Google Scholar]
- [17].Savić MM, Milinković MM, Rallapalli S, Clayton T, Sr, Joksimović S, Van Linn M, et al. The differential role of alpha1- and alpha5-containing GABA(A) receptors in mediating diazepam effects on spontaneous locomotor activity and water-maze learning and memory in rats. Int J Neuropsychopharmacol. 2009;12:1179–93. doi: 10.1017/S1461145709000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Evans AK, Lowry CA. Pharmacology of the beta-carboline FG-7142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA(A) receptor: neurochemical, neurophysiological, and behavioral effects. CNS Drug Rev. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Atack JR, Bayley PJ, Fletcher SR, McKernan RM, Wafford KA, Dawson GR. The proconvulsant effects of the GABA(A) alpha5 subtype-selective compound RY-080 may not be alpha5-mediated. Eur J Pharmacol. 2006;548:77–82. doi: 10.1016/j.ejphar.2006.02.055. [DOI] [PubMed] [Google Scholar]
- [20].Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, et al. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABA(A) alpha5 receptors with cognition enhancing properties. J Med Chem. 2004;47:5829–32. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- [21].Liu R, Hu RJ, Zhang P, Skolnick P, Cook JM. Synthesis and pharmacological properties of novel 8-substituted imidazobenzodiazepines: high-affinity, selective probes for alpha 5-containing GABA(A) receptors. J Med Chem. 1996;39:1928–34. doi: 10.1021/jm950887n. [DOI] [PubMed] [Google Scholar]
- [22].Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, et al. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABA(A) receptors which contain the alpha 5 subunit. Neuropharmacology. 1996;35:1331–5. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- [23].Savić MM, Clayton T, Furtmüller R, Gavrilović I, Samardzić J, Savić S, et al. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res. 2008;1208:150–9. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sternfeld F, Carling RW, Jelley RA, Ladduwahetty T, Merchant KJ, Moore KW, et al. Selective, orally active gamma-aminobutyric acidA alpha5 receptor inverse agonists as cognition enhancers. J Med Chem. 2004;47:2176–9. doi: 10.1021/jm031076j. [DOI] [PubMed] [Google Scholar]
- [25].Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABA(A) receptors. Neuropharmacology. 2006;51:1023–9. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- [26].Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, et al. RO4938581, a novel cognitive enhancer acting at GABA(A) alpha5 subunit-containing receptors. Psychopharmacology. 2009;202:207–23. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- [27].Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. An inverse agonist selective for alpha5 subunit-containing GABA(A) receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology. 2006;188:619–28. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- [28].Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, et al. An inverse agonist selective for alpha5 subunit-containing GABA(A) receptors enhances cognition. J Pharmacol Exp Ther. 2006;316:1335–45. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- [29].Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, et al. Identification of a novel, selective GABA(A) alpha5 receptor inverse agonist which enhances cognition. J Med Chem. 2003;46:2227–40. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- [30].Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR. Blockade of alcohol’s amnestic activity in humans by an alpha5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology. 2007;53:810–20. doi: 10.1016/j.neuropharm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [31].Lynch G, Palmer LC, Gall CM. The likelihood of cognitive enhancement. Pharmacol Biochem Behav. 2011;99:116–29. doi: 10.1016/j.pbb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Savić MM, Clayton T, Furtmüller R, Gavrilović I, Samardzić J, Savić S, et al. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res. 2008;1208:150–9. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Harris D, Clayton T, Cook J, Sahbaie P, Halliwell RF, Furtmüller R, et al. Selective influence on contextual memory: physiochemical properties associated with selectivity of benzodiazepine ligands at GABAA receptors containing the alpha5 subunit. J Med Chem. 2008;51:3788–803. doi: 10.1021/jm701433b. [DOI] [PubMed] [Google Scholar]
- [34].Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- [36].Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–11. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- [37].Lieben CK, Blokland A, Sik A, Sung E, van Nieuwenhuizen P, Schreiber R. The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsychopharmacology. 2005;30:2169–79. doi: 10.1038/sj.npp.1300777. [DOI] [PubMed] [Google Scholar]
- [38].Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McNamara RK, Skelton RW. Benzodiazepine receptor antagonists flumazenil and CGS 8216 and inverse agonist β-CCM enhance spatial learning in the rat: Dissociation from anxiogenic actions. Psychobiology. 1993;21:101–108. [Google Scholar]
- [40].Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology. 2011;36:227–50. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wallace TL, Ballard TM, Pouzet B, Riedel WJ, Wettstein JG. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99:130–45. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- [42].Ennaceur A, Meliani K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav Brain Res. 1992;51:83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- [43].Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11:188–93. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- [44].Reger ML, Hovda DA, Giza CC. Ontogeny of Rat Recognition Memory measured by the novel object recognition task. Dev Psychobiol. 2009;51:672–8. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Longone P, Impagnatiello F, Guidotti A, Costa E. Reversible modification of GABA(A) receptor subunit mRNA expression during tolerance to diazepam-induced cognition dysfunction. Neuropharmacology. 1996;35:1465–73. doi: 10.1016/s0028-3908(96)00071-8. [DOI] [PubMed] [Google Scholar]
- [46].Wan H, Warburton EC, Zhu XO, Koder TJ, Park Y, Aggleton JP, et al. Benzodiazepine impairment of perirhinal cortical plasticity and recognition memory. Eur J Neurosci. 2004;20:2214–24. doi: 10.1111/j.1460-9568.2004.03688.x. [DOI] [PubMed] [Google Scholar]
- [47].Bertaina-Anglade V, Enjuanes E, Morillon D, Drieu la Rochelle C. The object recognition task in rats and mice: a simple and rapid model in safety pharmacology to detect amnesic properties of a new chemical entity. J Pharmacol Toxicol Methods. 2006;54:99–105. doi: 10.1016/j.vascn.2006.04.001. [DOI] [PubMed] [Google Scholar]
- [48].Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- [49].Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–50. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- [50].Sarter M, Schneider HH. High density of benzodiazepine binding sites in the substantia innominata of the rat. Pharmacol Biochem Behav. 1988;30:679–82. doi: 10.1016/0091-3057(88)90083-4. [DOI] [PubMed] [Google Scholar]
- [51].Imperato A, Dazzi L, Serra M, Gessa GL, Biggio G. Differential effects of abecarnil on basal release of acetylcholine and dopamine in the rat brain. Eur J Pharmacol. 1994;261:205–8. doi: 10.1016/0014-2999(94)90320-4. [DOI] [PubMed] [Google Scholar]
- [52].Lindner MD, Balch AH, VanderMaelen CP. Short forms of the “reference-” and “working-memory” Morris water maze for assessing age-related deficits. Behav Neural Biol. 1992;58:94–102. doi: 10.1016/0163-1047(92)90303-l. [DOI] [PubMed] [Google Scholar]
- [53].Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–60. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- [54].Kawasaki K, Eigyo M, Ikeda M, Kihara T, Koike K, Matsushita A, et al. A novel benzodiazepine inverse agonist, S-8510, as a cognitive enhancer. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1413–25. doi: 10.1016/s0278-5846(96)00136-4. [DOI] [PubMed] [Google Scholar]
- [55].Cole BJ, Hillmann M. Effects of benzodiazepine receptor ligands on the performance of an operant delayed matching to position task in rats: opposite effects of FG 7142 and lorazepam. Psychopharmacology. 1994;115:350–7. doi: 10.1007/BF02245076. [DOI] [PubMed] [Google Scholar]