Abstract
Prorenin-induced intracellular signaling pathway is not fully elucidated. We investigated whether the (pro)renin receptor mediates epidermal growth factor (EGF) receptor transactivation through angiotensin (Ang) II-dependent and -independent pathways in human embryo kidney 293 cells. Prorenin (2 nmol/L) caused biphasic phosphorylation of EGF receptor (Tyr992) and extracellular signal-regulated kinase (ERK) 1/2, peaking at 5 minutes followed by a decrease and a second peak at 60–120 minutes, whereas EGF receptor (Tyr1068) and Src were phosphorylated at only 120 minutes. These prorenin-induced phosphorylation processes were inhibited by (pro)renin receptor siRNA. Similarly, Ang II type 1 (AT1) receptor blocker (ARB) or AT1 receptor siRNA completely inhibited prorenin-induced phosphorylation of EGF receptor (Tyr1068) and Src, as well as the late peaks of EGF receptor (Tyr992) and ERK 1/2. However, early peaks of EGF receptor (Tyr992) and ERK 1/2 at 5 minutes were not effectively blocked by ARB or AT1 receptor siRNA. Incubation with prorenin significantly increased Ang II levels of cell lysate. These data indicate that the (pro)renin receptor mediates EGF receptor transactivation in both Ang II-dependent and -independent pathways.
Keywords: Pro-Renin Receptor, Prorenin, Epidermal Growth Factor, EGF receptor, Angiotensin II, Ang II, Extracellular Signal-Regulated Kinase, ERK1/2
2. INTRODUCTION
Prorenin is generally known as an inactive precursor of mature renin; it is activated by proteolytic cleavage of a 43-amino acid prosegment. Binding of prorenin to the (pro)renin receptor promotes two distinctive mechanisms, the angiotensin (Ang) II-dependent and -independent pathways (1). (Pro)renin receptor-bound prorenin induces cleavage of angiotensinogen into Ang I without its proteolytic conversion to renin (nonproteolytic activation) (2–4). It has also been demonstrated that binding of prorenin to (pro)renin receptor directly triggers activation of intracellular signaling pathways, such as mitogen-activated protein (MAP) kinase, independent of Ang II generation (5–13).
We recently showed that prorenin–(pro)renin receptor binding directly activates extracellular signal-regulated kinase (ERK) 1/2 via epidermal growth factor (EGF) receptor-dependent signal transduction in rat vascular smooth muscle cells (VSMCs) (12). However, EGF receptor is reportedly not activated by prorenin in U937 monocytes (5). Thus, phosphorylation of EGF receptor by prorenin is controversial. In this regard, activation of the intracellular signaling pathway by prorenin-induced Ang II by nonproteolytic activation has also not yet been fully elucidated. Several tyrosine residues in EGF receptor play a regulatory role in mediating the intracellular signaling pathway (14). In VSMCs, Ang II phosphorylates at both Tyr992 and Tyr1068 sites of EGF receptor which activates c-Src and ERK 1/2 (15, 16). Different phosphorylation sites of EGF receptor may induce activation of different downstream signaling pathways.
Previous studies showed that Ang II levels in cell lysates were significantly increased by prorenin in cultured human podocytes and human embryo kidney (HEK) 293 cells (17, 18). However, our previous data showed that prorenin did not produce detectable Ang II in rat VSMCs (12). It is possible that the basal expression of (pro)renin receptor and other components of renin-angiotensin system (RAS) in rat VSMCs were insufficient to produce enough Ang II by prorenin to activate the downstream signals, such as EGF receptor.
In the present study, we hypothesized that prorenin activates intracellular signaling pathways via both Ang II-dependent and -independent mechanisms. To test this hypothesis, we evaluated the effects of prorenin on transactivation of EGF receptor in HEK293 cells which reportedly expressed all RAS components (17, 19–22). We also investigated the effects of prorenin on the downstream signaling pathway of EGF receptor, such as ERK 1/2 and Src phosphorylation.
3. MATERIALS AND METHODS
3.1. Materials
An active form of candesartan (CV11974), the Ang II type 1 (AT1) receptor blocker (ARB), was kindly provided by Takeda Pharmaceutical Co. Ltd. (Osaka, Japan). Recombinant human prorenin was prepared from Chinese hamster ovary cell lines harboring human prorenin cDNA (23).
3.2. Cell culture
HEK293 cells were grown in Dubecco’s modified Eagle’s medium (Sigma-Aldrich, St Louis, MO) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), penicillin (100 U/mL; Life Technologies, Carlsbad, CA), streptomycin (100 μg/mL; Life Technologies) and maintained at 37°C under 5% CO2/95% air in a humidified incubator.
3.3. Experimental protocols
Cells were treated with 2 nmol/L human recombinant prorenin or Ang II (100 mmol/L), and cells were harvested for analyses. Experiments were repeated in cells pretreated with candesartan (100 nmol/L), 60 minutes before prorenin treatment. In another series of experiments, we performed a transient transfection of HEK293 cells with siRNA of AT1 receptor or (pro)renin receptor (ATP6AP2). The Stealth Select RNAi pre-designed against AT1 receptor or (pro)renin receptor was synthesized by Life Technologies. HEK293 cells were transfected with Lipofectamine 2000 (Life Technologies) and 100 pmol of siRNA for 48 hours, according to the manufacturer’s protocol. As a negative control, a non-targeting scrambled siRNA (control siRNA) was purchased from Life Technologies. After 24 hours transfection, cells were washed and incubated with or without prorenin.
3.4. Western blot analysis
Following HEK293 cells incubation with prorenin or Ang II, protein was extracted as previously described (24). The protein concentration was determined by Bio-Rad Protein Assay using bovine serum albumin as the standard. Total protein extracts (30 μg) were electrophoretically separated using 10% SDS-polyacrylamide gels at 150 V for 90 minutes. Proteins were subsequently transferred electrophoretically onto a Hybond-ECL polyvinylidene difluoride membrane (GE Healthcare, Buckinghamshire, UK). Blots were blocked for 60 minutes at room temperature with 5% skimmed milk in PBS with Tween 20. The sample protein was immunoblotted with appropriate anti-phospho-EGF receptor (Tyr992 and Tyr1068), anti-phospho-Src (Tyr416), anti-phospho-ERK 1/2 (Cell Signaling Technology, Danvers, MA), anti-(pro)renin receptor or anti-AT1 receptor (N-10) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). To confirm equal protein loading, each membrane was reprobed with an antibody against β-actin (Sigma-Aldrich), anti-EGF receptor (Millipore, Temecula, CA), anti-Src and anti-ERK 1/2 (Cell Signaling Technology). The protein bands were visualized with an ECL plus system (GE Healthcare). Band intensities were quantified by densitometry of the immunoblots using NIH ImageJ software.
3.5. Gene expression of the RAS components
Total RNA was extracted by ISOGEN (Nippon Gene, Tokyo, Japan) from HEK293 cells. Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed applying a SYBR Green reaction mix, and was run on Applied Biosystems 7300 Real-Time PCR System (Life Technologies). The primers used for the RT-PCR analyses are summarized in Table 1. RT-PCR amplification was performed as follows: 35 cycles of denaturation for 2 minutes at 94°C, annealing for 1 minute at 58°C, and extension for 1 minute and 15 seconds at 72°C. The PCR products were size fractioned on 2% agarose gel, and visualized with ethidium bromide staining and ultraviolet (UV) transillumination. β-actin was used as a housekeeping gene control. Human proximal tubular (HPT) cells were applied to positive controls, because HPT cells are known to have all the RAS components (25–27).
Table 1.
The sequence of sense and antisense primers for RT-PCR of target gene mRNA
| Gene | Sense Primer sequence (5′-3′) | Antisense Primer sequence (5′-3′) | GenBank accession number |
|---|---|---|---|
| AGT | ACTGCACCTCCCGGCTGGAT | TGAACACGCCCACCACCGTG | NM000029 |
| Renin | CCACCCCAAACCTTCAAAGTCG | TGCCCACAACCCCATCAAACTC | NM000537 |
| ACE | GCAGCCCGGCAACTTTTCTGC | ATGTTGGTGTCGTGCGCCCA | NM00789 |
| AT1R | TGATGCCATCCCAGAAAGTCGGC | CCTCCTCTGAGCTGTGGAGCCTTT | NM031850 |
| β-actin | CACAGAGCCTCGCCTTTGCCGATC | ACGAGCGCGGCGATATCATCATC | NM001101 |
AGT: angiotensinogen, ACE: angiotensin-converting enzyme, AT1R: angiotensin II type 1 receptor
3.6. Ang II content of cell lysate
The cell lysate of HEK293 cells was collected from dishes with or without prorenin stimulation. After a treatment with human recombinant prorenin, culture medium was removed, and cells were washed twice with ice-cold PBS. Cells were extracted in 450 μL/well lysis buffer containing 20 mmol/L Tris-HCl, 10 mmol/L EDTA, 10 μmol/L captopril, 5 mmol/L EGTA, 5 mmol/L mercaptoethanol, 50 μg/mL phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin and 1 μg/mL pepstatin A. Each cell sample was scraped, collected into a microcentrifuge tube and centrifuged at 4°C, 13,000 rpm for 15 minutes. The supernatant was filtered on Amicon Ultra devises (10 kDa cut-off; Millipore). We measured Ang II concentration by radioimmunoassay using specific antibody for Ang II, monoiodinated 125I-labeled Ang II, and Ang II standard as previously described (15, 28). In brief, each lyophilized antiserum was diluted sufficiently to yield a specific binding rate after incubation with a 9,000 cpm label for 48 hours at 4°C. Bound and free Ang II were separated with dextran-coated charcoal (10 mg of charcoal and 1 mg of dextran in 1 mL of assay buffer/tube). After centrifugation, the supernatants were decanted and counted 3 minutes in an automated gamma counter.
3.7. Statistical analysis
All results are expressed as mean ± S.E. Statistical significance was assessed using one-way ANOVA and Bonferroni’s post-hoc test. A value of P<0.05 was considered to be statistically significant.
4. RESULTS
4.1. Effect of prorenin on EGF receptor, Src and ERK phosphorylation
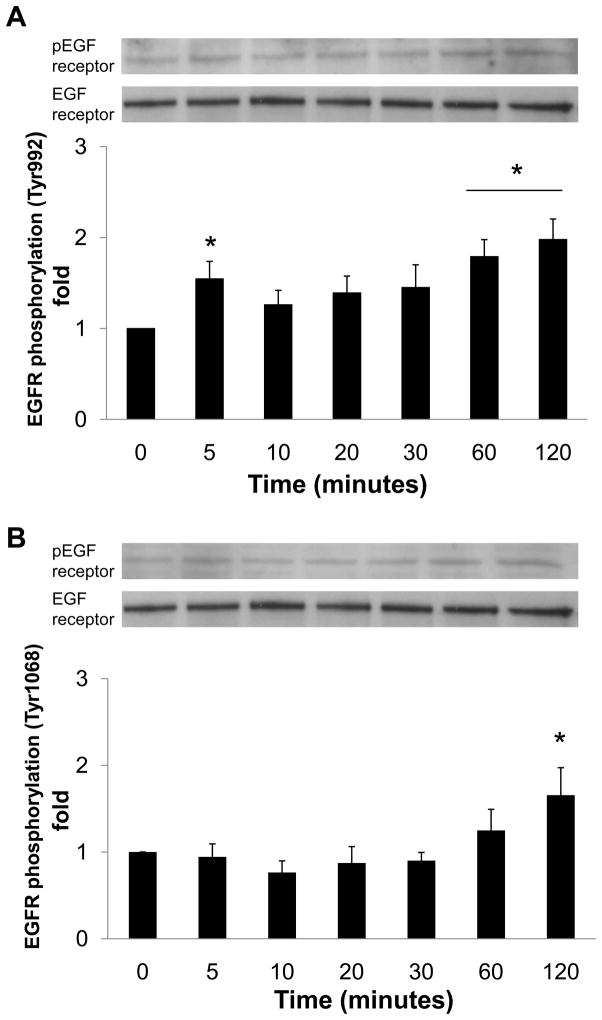
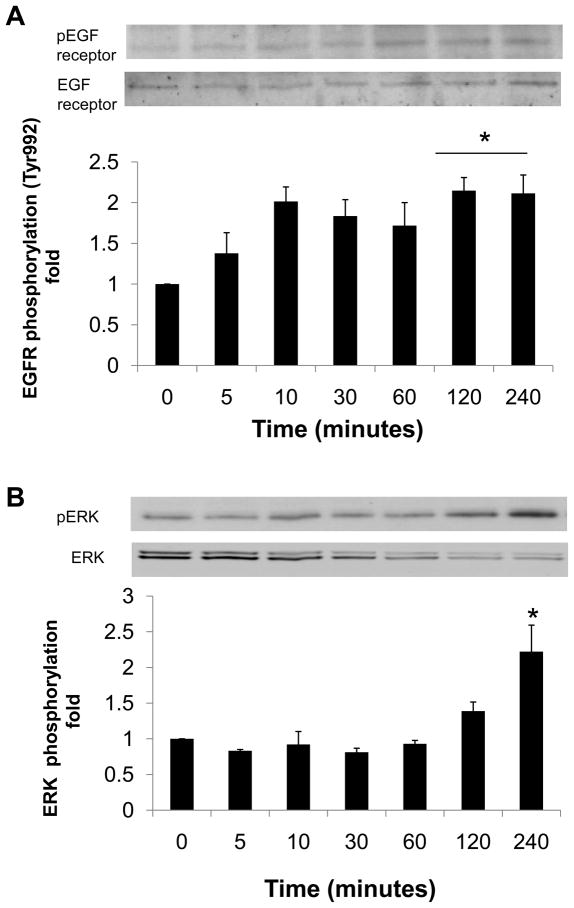
Recombinant human prorenin (2 nmol/L) significantly increased phosphorylation of two different tyrosine sites (Tyr992 and Tyr1068) of EGF receptor, as well as Src and ERK 1/2 in a time-dependent manner (Figure 1, n=4, P<0.05). Prorenin augmented EGF receptor phosphorylation at Tyr992 not only at 5 minutes, but also at 60 and 120 minutes (Figure 1A, n=4, P<0.05). On the other hand, EGF receptor phosphorylation at Tyr1068 was increased at 120 minutes (Figure 1B, n=4, P<0.05). Phosphorylation of Src was increased at 60 and 120 minutes (Figure 1C, n=4, P<0.05), whereas ERK 1/2 phosphorylation was biphasic, similarly to Tyr992 on EGF receptor (Figure 1D, n=4, P<0.05). Ang II (100 nmol/L) increased phosphorylations of both EGF receptor (Tyr992) at 120 and 240 minutes, and ERK 1/2 at 240 minutes (Figures 2A and 2B).
Figure 1.
Effect of prorenin on phosphorylation of EGF receptor at Tyr992 (A) and Tyr1068 (B), Src (C) and ERK 1/2 (D) in HEK293 cells. Bar graphs represent mean ± SE (n=4), expressed as fold change compared with vehicle-treated cells. *P<0.05 vs. vehicle-treated cells.
Figure 2.
Effect of Ang II on phosphorylation of EGF receptor at Tyr992 (A) and ERK 1/2 (B) in HEK293 cells. Bar graphs represent mean ± SE (n=4), expressed as fold change compared with vehicle-treated cells. *P<0.05 vs. vehicle-treated cells.
4.2. Role of (pro)renin receptor in EGF receptor transactivation
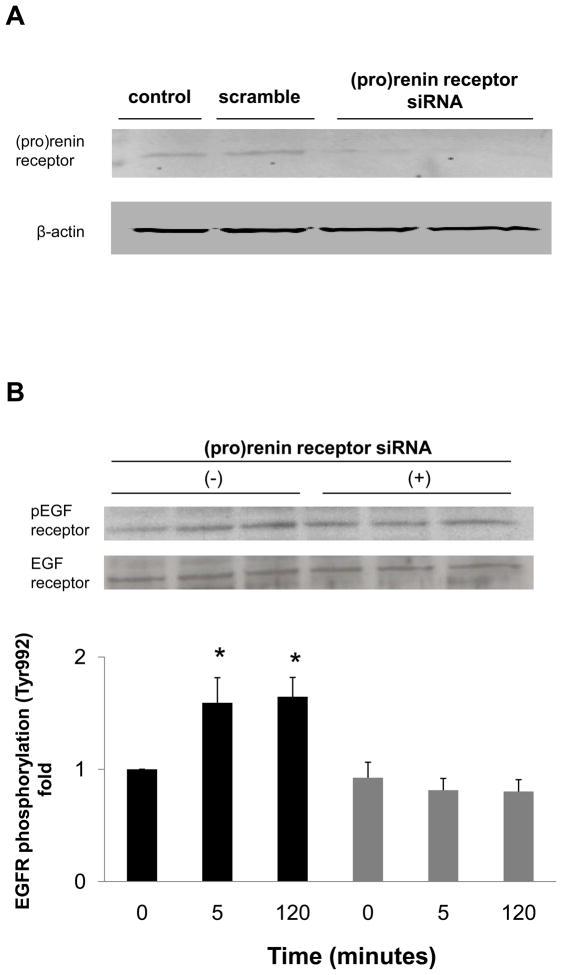
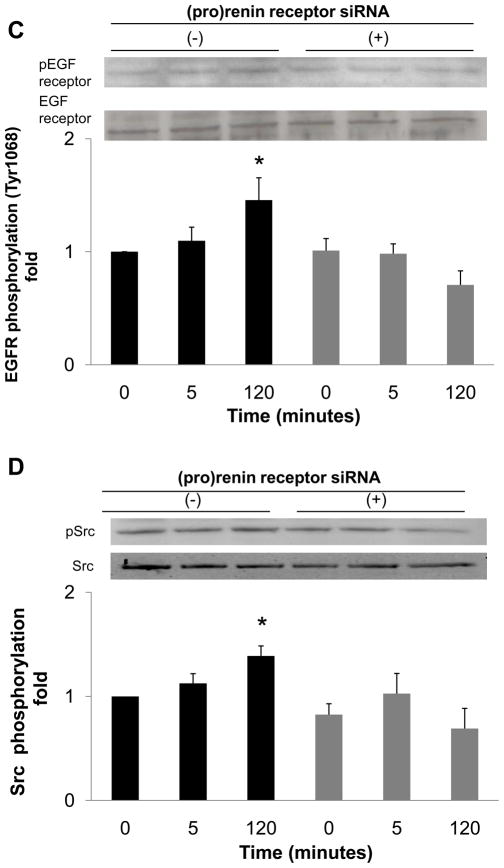
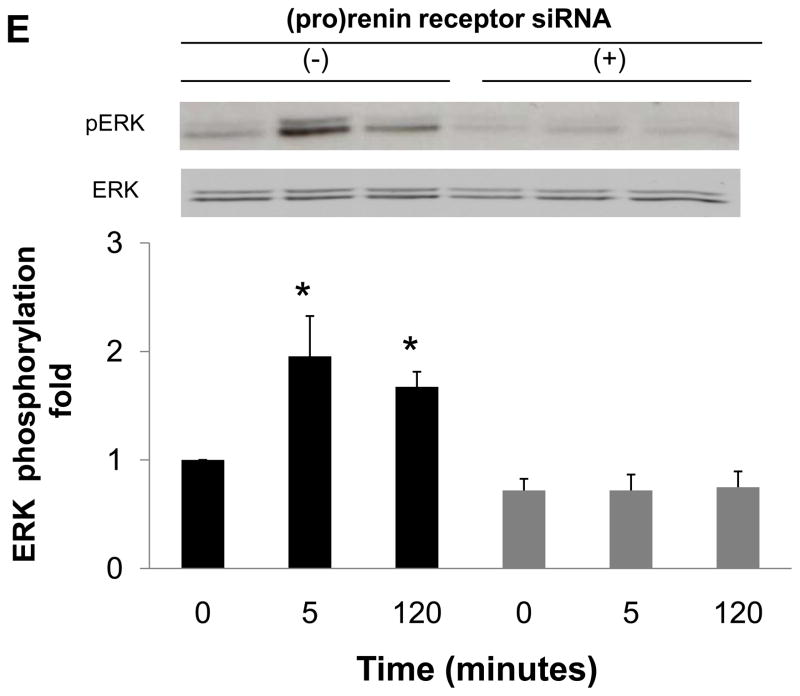
To determine whether EGF receptor transactivation was mediated by the (pro)renin receptor, we measured the effect of (pro)renin receptor knockdown using siRNA on EGF receptor phosphorylation. The transfection of siRNA targeting the (pro)renin receptor decreased protein expression of (pro)renin receptor (Figure 3A), while scrambled siRNA did not affect protein expression. The depletion of (pro)renin receptor significantly reduced prorenin-induced EGF receptor (Tyr992) phosphorylation at both 5 and 120 minutes (Figure 3B, n=4, P<0.05). Prorenin-induced increase in EGF receptor (Tyr1068) and Src phosphorylation were also blocked by siRNA of (pro)renin receptor (Figures 3C and 3D, n=4, P<0.05). Similarly, ERK 1/2 phosphorylation induced by prorenin was completely suppressed by (pro)renin receptor knockdown (Figure 3E, n=4, P<0.05).
Figure 3.
Effect of (pro)renin receptor siRNA on (pro)renin receptor protein expression (A), and prorenin-induced phosphorylation of EGF receptor at Tyr992 (B) and Tyr1068 (C), Src (D) and ERK 1/2 (E) in HEK293 cells. Bar graphs represent mean ± SE (n=4), expressed as fold change compared with vehicle-treated cells. *P<0.05 vs. vehicle-treated cells.
4.3. EGF receptor transactivation induced by Ang II generation
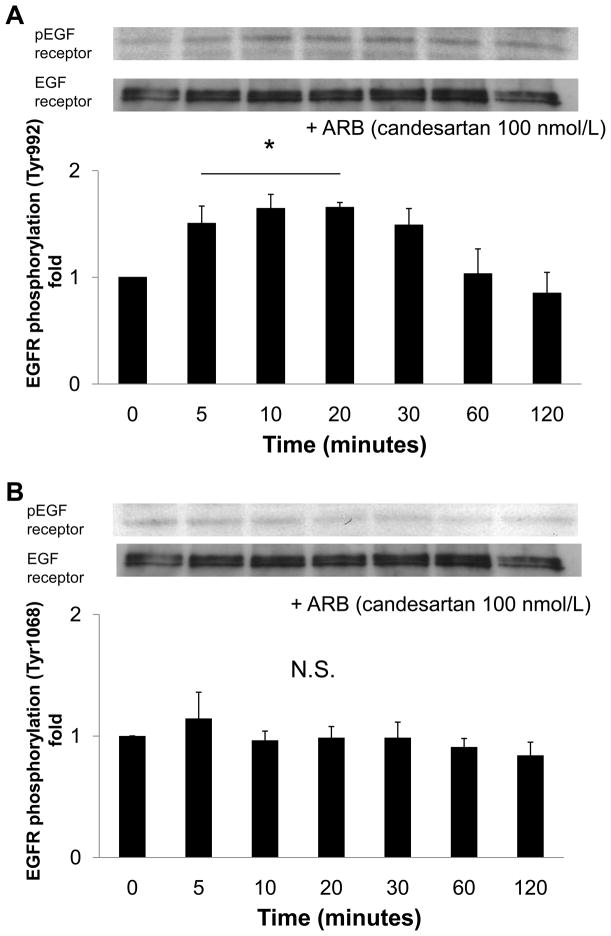
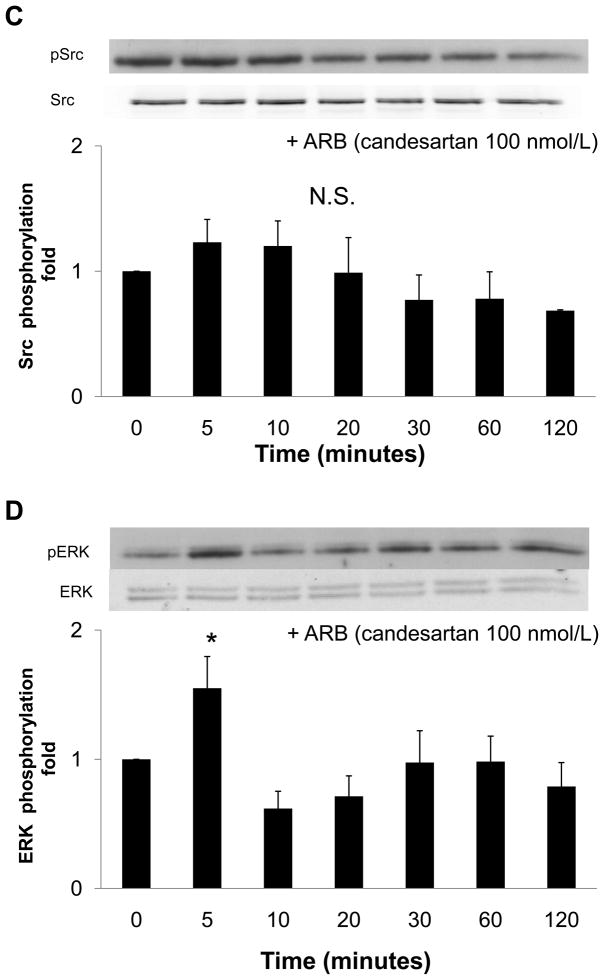
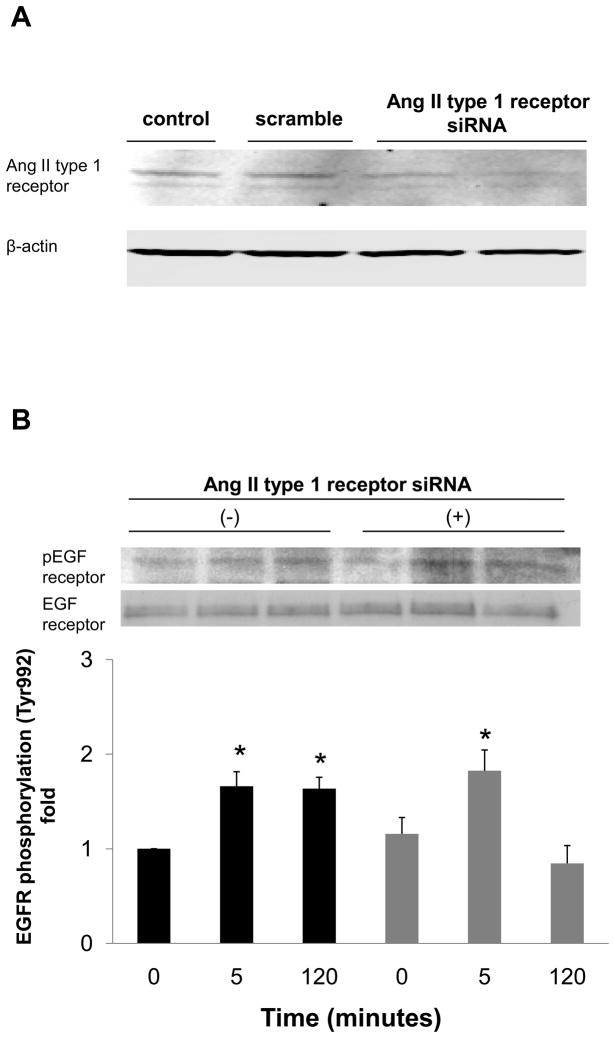
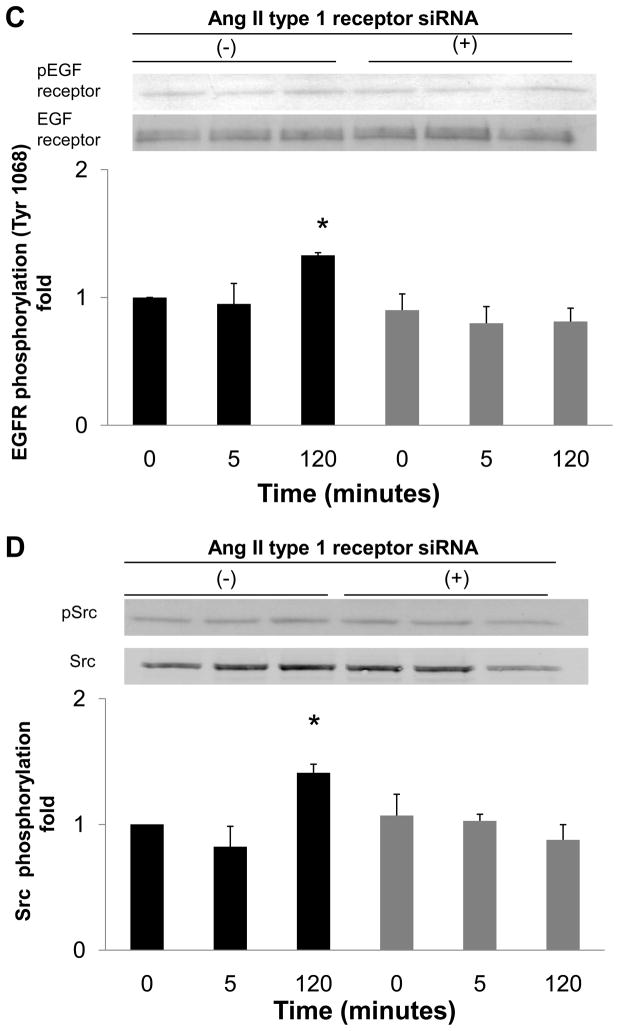
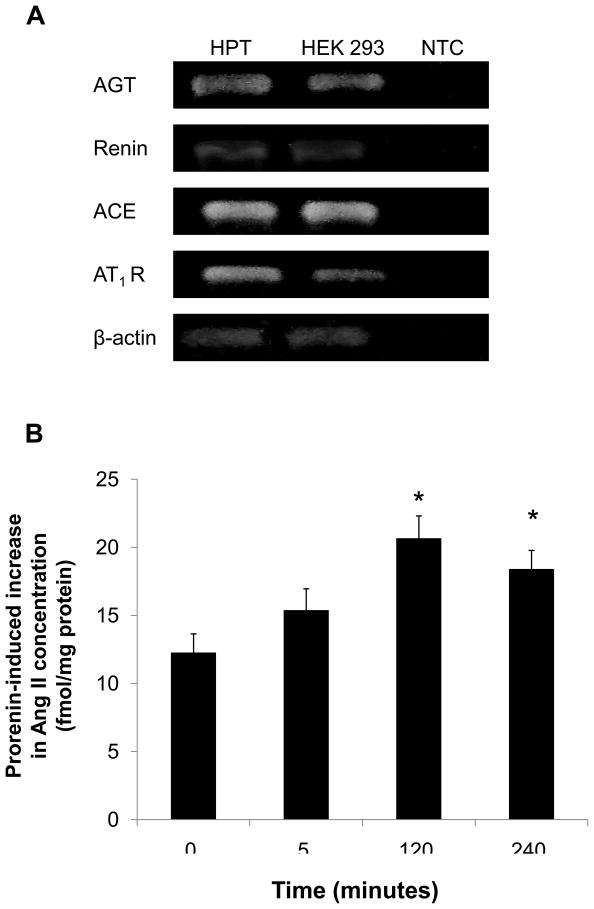
To examine whether Ang II generated by nonproteolytic activation of prorenin was involved in EGF receptor transactivation, we examined the effect of an ARB (candesartan; CV11974) and siRNA of AT1 receptor on EGF receptor transactivation. Pretreatment with candesartan (100 nmol/L) inhibited late-phase prorenin-induced phosphorylation of EGF receptor (Tyr992) (Figure 4A, n=4, P<0.05). Candesartan treatment did not affect EGF receptor phosphorylation at 5, 10 or 20 minutes. Prorenin-induced phosphorylation of EGF receptor (Tyr1068) and Src were completely suppressed by treatment with candesartan (Figures 4B and 4C, n=4). Candesartan did not affect ERK 1/2 at 5 minutes, but suppressed its phosphorylation at late phase (Figure 4D, n=4, P<0.05). The transfection of siRNA targeting AT1 receptor decreased AT1 receptor protein expression (Figure 5A). The AT1 receptor siRNA failed to inhibit EGF receptor (Tyr992) phosphorylation at 5 minutes, and also reduced prorenin-induced EGF receptor transactivation at 120 minutes (Figures 5B, 5C, 5D and 5E, n=4, P<0.05). The ERK 1/2 phosphorylation was increased, in the same manner as EGF receptor (Tyr992) (Figure 5E, n=4, p<0.05).
Figure 4.
Effect of an ARB on prorenin-induced phosphorylation of EGF receptor at Tyr992 (A) and Tyr1068 (B), Src (C) and ERK 1/2 (D) in HEK293 cells. Bar graphs represent mean ± SE (n=4), expressed as fold change compared with vehicle-treated cells. *P<0.05 vs. vehicle-treated cells
Figure 5.
Effect of AT1 receptor siRNA on AT1 receptor protein expression (A), and prorenin-induced phosphorylation of EGF receptor at Tyr992 (B) and Tyr1068 (C), Src (D) and ERK 1/2 (E) in HEK293 cells. Bar graphs represent mean ± SE (n=4), expressed as fold change compared with vehicle-treated cells. *P<0.05 vs. vehicle-treated cells.
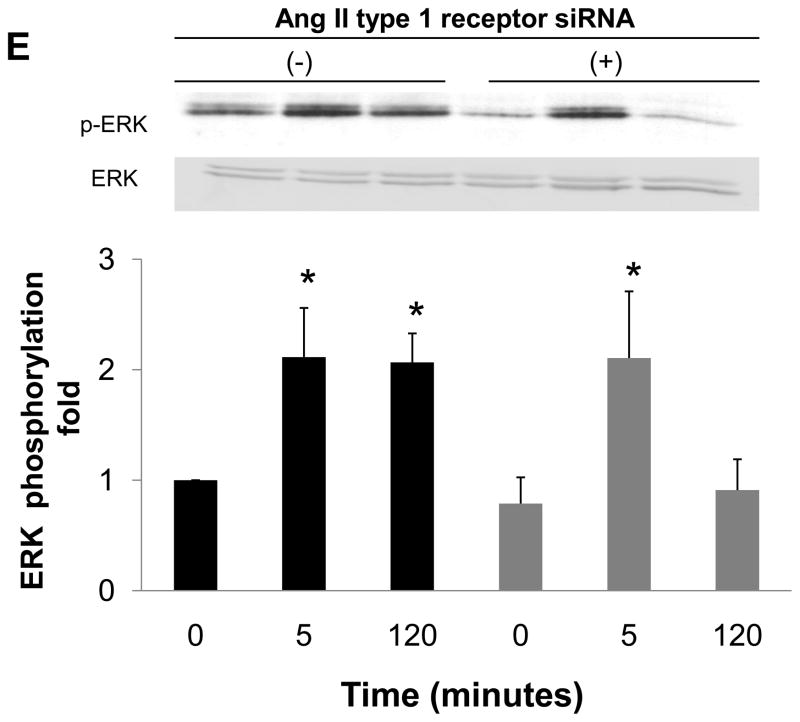
We measured Ang II concentration in cell lysate to investigate whether Ang II levels were increased by prorenin. First, we confirmed mRNA expression of all RAS components by RT-PCR in HEK293 cells (Figure 6A). Prorenin significantly increased Ang II concentrations in cell lysates (Figure 6B, n=6, p<0.05).
Figure 6.
RT-PCR expression analysis of all the RAS components. HPT and HEK293 cells were analyzed by RT-PCR. AGT: angiotensinogen; ACE: angiotensin-converting enzyme. β-actin served as positive control. A reaction without a non-template control served as negative control (NTC). Intracellular Ang II concentration in HEK293 cells (B). Bar graphs represent mean ± SE (n=6), expressed as fold change compared with vehicle-treated cells. *P<0.05 vs. vehicle-treated cells.
5. DISCUSSION
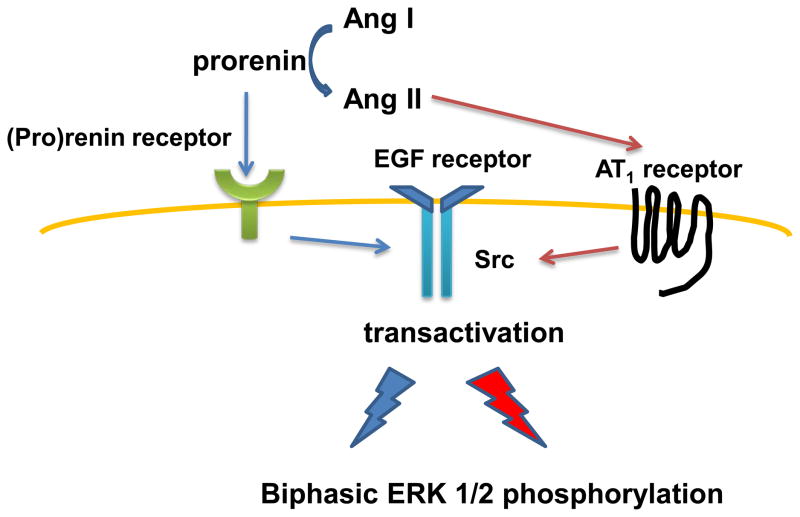
We confirmed that HEK293 cells express all RAS components in agreement with previous studies (17, 19–22). The present study also showed that human recombinant prorenin binds to the (pro)renin receptor and activates intracellular signaling pathways via both Ang II-dependent and -independent mechanisms in HEK293 cells (Figure 7). Prorenin triggers transactivation of two different EGF receptor tyrosine sites (Tyr992 and Tyr1068). Early-peak prorenin-induced ERK 1/2 phosphorylation is associated with EGF receptor (Tyr992) phosphorylation. Consistent with our previous results in VSMCs (12), we could not detect the early-peak EGF receptor (Tyr1068) phosphorylation in
Figure 7.
Proposed molecular mechanisms of prorenin-induced biphasic activation of intracellular signaling. See Discussion for details.
Thus, EGF receptor transactivation was induced differently by prorenin stimulation depending on which of its tyrosine sites were phosphorylated, as suggested by other investigators (5). The present study also showed that EGF receptor Tyr992 and Tyr1068 sites, as well as Src and ERK 1/2 phosphorylation, are significantly activated by prorenin at the late phase. Previous studies indicated that transactivations of EGF receptor Tyr992 and Tyr1068 play a role in the activation of ERK/MAPK cascade (29). In addition, EGF receptor (Tyr992) induces PKC-dependent ERK 1/2 phosphorylation via PLCγ (30); EGF receptor (Tyr1068) associates with the adaptor Grb2, which is the main step in EGF receptor-dependent induction of RAS/MAPK (30). Further studies with site-directed mutation of each EGF receptor tyrosine residue are needed to clarify which tyrosine residues are associated with phosphorylation of Src and ERK 1/2. Since (pro)renin receptor siRNA attenuated both early- and late-phase EGF receptor transactivation, both phases are mediated by (pro)renin receptor.
Another novel finding in the present study is that prorenin-induced EGF receptor, Src and ERK 1/2 phosphorylation at 120 minutes are blocked by an ARB or AT1 receptor siRNA, whereas early-peak EGF receptor (Tyr992) and ERK 1/2 phosphorylation are not inhibited. We also confirmed that Ang II levels of cell lysate were significantly increased after prorenin treatment. These results indicate that prorenin increases Ang II production, which induces EGF receptor transactivation and is associated with Src and ERK 1/2 phosphorylation. However, Ang II levels in cell medium were under detectable range. Although Ang II levels in cell lysate may reflect Ang II generation in cells, further evaluation of Ang II levels in cell medium will be needed. The siRNAs of both (pro)renin receptor and AT1 receptor inhibited prorenin-induced EGF receptor transactivation at 120 minutes. Therefore, Ang II generated by nonproteolytic activation of prorenin might induce EGF receptor transactivation via the activation of AT1 receptor. Previous studies showed that Ang II levels in the cell lysate are elevated in human podocytes and HEK293 cells by prorenin stimulation (17, 18). Furthermore, EGF receptor is transactivated via the activation of AT1 receptor induced by Ang II in VSMCs and HEK293 cells (15, 16, 31).
As shown in previous studies using mesangial cells (3, 32), VSMCs (9, 12), vascular endothelial cells (13) and U937 monocytes (5), the present results indicate that prorenin-induced early-peak EGF receptor (Tyr992) and ERK 1/2 phosphorylation is mediated through mechanisms that are independent of Ang II generation (12). On the other hand, our results also indicate that prorenin-induced late-phase EGF receptor, Src and ERK 1/2 phosphorylation are mediated by Ang II-dependent pathways. Previous studies failed to show Ang II-dependent EGF receptor and ERK 1/2 phosphorylation. A possible reason may be due to the observation time. In the present study, Ang II levels of cell lysate increased at 120 minutes after prorenin stimulation in HEK293 cells, while the period for prorenin stimulation was limited to less than 60 minutes in previous studies. Another possibility is the basal expression of RAS components in cells. We and others showed that HEK293 cells express all the RAS components (17, 19–22), which could generate endogenous Ang II, leading to activation of the intracellular signaling pathway in the end. However, it is unclear whether previously used cultured cells actually express all RAS components.
Basically, biphasic phosphorylation is an important issue, because there are two independent mechanisms of phosphorylation. Early phosphorylation occurred in an Ang II-independent manner, and later phosphorylation in an Ang II-dependent manner. Further experiments are needed to know the clinical implications of biphasic response, focusing on which phase of phosphorylation contributes to a proliferating signaling pathway. In addition, conventional RAS blockade such as ARB failed to inhibit early intracellular signaling activation, although ARB completely attenuated late-phase of activation. Therefore, only depletion of prorenin and renin, or inhibition of (pro)renin receptor bindings may attenuate Ang II-independent proliferative signaling pathways. These findings suggest that prorenin-(pro)renin receptor activation may be a new target to prevent proliferative changes in cardiovascular and renal disease. Further studies are required to elucidate the clinical benefits of inhibiting Ang II-independent activation of proliferative signaling pathway induced by (pro)renin receptor upon attenuating cardiovascular and renal pathogenesis.
In conclusion, the present study demonstrates that both binding of prorenin to (pro)renin receptor, and binding of Ang II generated by nonproteolytic activation to AT1 receptor, lead to activation of the intracellular signaling pathway, including EGF receptor, Src and ERK 1/2.
Acknowledgments
We are grateful to Takeda Pharmaceutical Co. Ltd for supplying CV11974. This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20590253 and 22790792).
References
- 1.Ichihara A, Sakoda M, Kurauchi-Mito A, Narita T, Kinouchi K, Bokuda K, Itoh H. New approaches to blockade of the renin-angiotensin-aldosterone system: characteristics and usefulness of the direct renin inhibitor aliskiren. J Pharmacol Sci. 2010;113(4):296–300. doi: 10.1254/jphs.10r04fm. [DOI] [PubMed] [Google Scholar]
- 2.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med. 2006;18(3):483–8. [PubMed] [Google Scholar]
- 3.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417–27. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satofuka S, Ichihara A, Nagai N, Yamashiro K, Koto T, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Suzuki F, Oike Y, Ishida S. Suppression of ocular inflammation in endotoxin-induced uveitis by inhibiting nonproteolytic activation of prorenin. Invest Ophthalmol Vis Sci. 2006;47(6):2686–92. doi: 10.1167/iovs.05-1458. [DOI] [PubMed] [Google Scholar]
- 5.Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A, Jan Danser AH, Bader M, Nguyen G, Luft FC, Muller DN. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension. 2008;51(3):682–8. doi: 10.1161/HYPERTENSIONAHA.107.101444. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Siragy HM. Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150(12):5557–65. doi: 10.1210/en.2009-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69(1):105–13. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G, Muller DN. The biology of the (pro)renin receptor. J Am Soc Nephrol. 2010;21(1):18–23. doi: 10.1681/ASN.2009030300. [DOI] [PubMed] [Google Scholar]
- 9.Sakoda M, Ichihara A, Kaneshiro Y, Takemitsu T, Nakazato Y, Nabi AH, Nakagawa T, Suzuki F, Inagami T, Itoh H. (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res. 2007;30(11):1139–46. doi: 10.1291/hypres.30.1139. [DOI] [PubMed] [Google Scholar]
- 10.Saris JJ, Hoen PAt, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan Danser AH. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension. 2006;48(4):564–71. doi: 10.1161/01.HYP.0000240064.19301.1b. [DOI] [PubMed] [Google Scholar]
- 11.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54(2):261–9. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Hitomi H, Hosomi N, Shibayama Y, Nakano D, Kiyomoto H, Ma H, Yamaji Y, Kohno M, Ichihara A, Itoh H, Nishiyama A. Prorenin induces vascular smooth muscle cell proliferation and hypertrophy via epidermal growth factor receptor-mediated extracellular signal-regulated kinase and Akt activation pathway. J Hypertens. 2011;29(4):696–705. doi: 10.1097/HJH.0b013e328343c62b. [DOI] [PubMed] [Google Scholar]
- 13.Uraoka M, Ikeda K, Nakagawa Y, Koide M, Akakabe Y, Nakano-Kurimoto R, Takahashi T, Matoba S, Yamada H, Okigaki M, Matsubara H. Prorenin induces ERK activation in endothelial cells to enhance neovascularization independently of the renin-angiotensin system. Biochem Biophys Res Commun. 2009;390(4):1202–7. doi: 10.1016/j.bbrc.2009.10.121. [DOI] [PubMed] [Google Scholar]
- 14.Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984;311(5985):483–5. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, Hitomi H, Hosomi N, Lei B, Pelisch N, Nakano D, Kiyomoto H, Ma H, Nishiyama A. Mechanical stretch potentiates angiotensin II-induced proliferation in spontaneously hypertensive rat vascular smooth muscle cells. Hypertens Res. 2010;33(12):1250–7. doi: 10.1038/hr.2010.187. [DOI] [PubMed] [Google Scholar]
- 16.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21(4):489–95. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 17.Clavreul N, Sansilvestri-Morel P, Magard D, Verbeuren TJ, Rupin A. (Pro)renin promotes fibrosis gene expression in HEK cells through a Nox4-dependent mechanism. Am J Physiol Renal Physiol. 2011;300(6):F1310–8. doi: 10.1152/ajprenal.00119.2010. [DOI] [PubMed] [Google Scholar]
- 18.Sakoda M, Ichihara A, Kurauchi-Mito A, Narita T, Kinouchi K, Murohashi-Bokuda K, Saleem MA, Nishiyama A, Suzuki F, Itoh H. Aliskiren inhibits intracellular angiotensin II levels without affecting (pro)renin receptor signals in human podocytes. Am J Hypertens. 2010;23(5):575–80. doi: 10.1038/ajh.2009.273. [DOI] [PubMed] [Google Scholar]
- 19.Mundell SJ, Benovic JL. Selective regulation of endogenous G protein-coupled receptors by arrestins in HEK293 cells. J Biol Chem. 2000;275(17):12900–8. doi: 10.1074/jbc.275.17.12900. [DOI] [PubMed] [Google Scholar]
- 20.Obeid D, Nguyen J, Lesavre P, Bauvois B. Differential regulation of tumor necrosis factor-alpha-converting enzyme and angiotensin-converting enzyme by type I and II interferons in human normal and leukemic myeloid cells. Oncogene. 2007;26(1):102–10. doi: 10.1038/sj.onc.1209779. [DOI] [PubMed] [Google Scholar]
- 21.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99(12):1355–66. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 22.Steege A, Fahling M, Paliege A, Bondke A, Kirschner KM, Martinka P, Kaps C, Patzak A, Persson PB, Thiele BJ, Scholz H, Mrowka R. Wilms’ tumor protein (-KTS) modulates renin gene transcription. Kidney Int. 2008;74(4):458–66. doi: 10.1038/ki.2008.194. [DOI] [PubMed] [Google Scholar]
- 23.Poorman RA, Palermo DP, Post LE, Murakami K, Kinner JH, Smith CW, Reardon I, Heinrikson RL. Isolation and characterization of native human renin derived from Chinese hamster ovary cells. Proteins. 1986;1(2):139–45. doi: 10.1002/prot.340010206. [DOI] [PubMed] [Google Scholar]
- 24.Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287(2):C494–9. doi: 10.1152/ajpcell.00439.2003. [DOI] [PubMed] [Google Scholar]
- 25.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91(3):774–9. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sibony M, Gasc JM, Soubrier F, Alhenc-Gelas F, Corvol P. Gene expression and tissue localization of the two isoforms of angiotensin I converting enzyme. Hypertension. 1993;21(6 Pt 1):827–35. doi: 10.1161/01.hyp.21.6.827. [DOI] [PubMed] [Google Scholar]
- 27.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43(6):1251–9. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39(1):129–34. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 29.Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4(8):651–7. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- 30.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 31.Turner NA, Ball SG, Balmforth AJ. The mechanism of angiotensin II-induced extracellular signal-regulated kinase-1/2 activation is independent of angiotensin AT(1A) receptor internalisation. Cell Signal. 2001;13(4):269–77. doi: 10.1016/s0898-6568(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72(1):45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]