Abstract
Normal anterior pituitary function is essential for fertility. Release from the gland of the reproductive hormones LH and FSH is regulated primarily by hypothalamically-derived gonadotropin releasing hormone (GnRH), although other releasing factors have been postulated to exist. Using a bioinformatic approach, we have identified a novel peptide, phoenixin, that regulates pituitary gonadotropin secretion by modulating expression of the GnRH receptor, an action with physiologically relevant consequences. Compromise of phoenixin in vivo using siRNA resulted in the delayed appearance of oestrus and a reduction in GnRH receptor expression in the pituitary. Phoenixin may represent a new class of hypothalamically-derived pituitary priming factors (PFs) that sensitise the pituitary to the action of other RFs, rather than directly stimulating the fusion of secretory vesicles to pituitary membranes.
Keywords: gonadotrophins, GnRH receptor, siRNA, bioinformatics, pituitary, reproduction
INTRODUCTION
Anterior pituitary hormone secretion is controlled by releasing and inhibiting factors largely of hypothalamic origin that reach the gland through the hypophyseal portal vessels (1,2). These releasing and inhibiting factors may act synergistically to alter the secretion of one or more pituitary hormones (3), and may enhance pituitary hormone release by initiating transcription of the hormone, fusion of secretory vesicles containing the hormone, or both (4). Gonadotropin releasing hormone (GnRH) is produced by neurones in the rostral and mediobasal hypothalamus, and is the primary regulator of gonadotropic hormone secretion [i.e., luteinizing hormone (LH) and follicle stimulating hormone (FSH)] (5,6). GnRH facilitates LH and FSH release by promoting transcription (7,8) and opening L-type calcium channels in pituitary gonadotrophs, leading to vesicular fusion and release (4). While several hormones have been identified that modulate the expression of GnRH [e.g., kisspeptin (9)] or directly alter pituitary gonadotroph secretions [e.g., peripherally-derived hormones such as gonadal steroids and proteins (10,11)], GnRH to date is thought to be the primary, hypothalamically-derived gonadotropin releasing factor, although additional factors have been postulated to exist (1).
We have developed a bioinformatic algorithm that utilises information provided by the Human Genome Project to predict previously unidentified, secreted, highly conserved peptide hormones, such as the recently described neuropeptide, neuronostatin (12). This algorithm utilizes several bioinformatic databases to exclude or include potential peptides. First, potential proteins that contain a transmembrane domain (i.e., receptors) are excluded (SMART database) and potential peptides that have a signal peptide are included (SignalP database). Next, we eliminate all sequences that encode a known peptide or protein, and include potential peptide sequences that contain dibasic cleavage sites flanking a mature region (BioRegEx database). Finally, we use NCBI BLAST to identify peptide sequences that are highly conserved across species. Using this algorithm, we have identified another novel peptide that we named phoenixin. Here we describe the characterization of the actions of phoenixin, and provide evidence for a possible mechanism by which phoenixin may exert its activities and for the potential physiological relevance of those actions.
MATERIALS AND METHODS
RT-PCR
RNA from primary, dispersed anterior pituitary cells or alphaT3-1 cells (13) was collected using an RNeasy Kit (Qiagen) according to the manufacturer’s instructions. First-strand cDNA synthesis was accomplished using oligo d(T) (Invitrogen) and MML-V reverse transcriptase (Promega). Real Time PCR reactions were conducted using iQ SybrGreen Master Mix (Bio-Rad) and a Bio-Rad 96CFX Real Time System. Primers (see Supplemental Methods) were designed using PrimerQuest software and purchased from Integrated DNA Technologies (Coralville, IA). Specificity of primers was confirmed using PrimerBLAST (NCBI). Changes in expression were calculated using the delta delta Ct method (14). Data were normalized to the housekeeping genes HPRT-1 [(NM_012583.2) for experiments evaluating primary rat tissues, except in the experiments evaluating FSHbeta and LHbeta expression, in Supplemental Figure 1], actin [(NM_031144.2) for experiments in Supplemental Figure 1], or GAPDH [(NM_008084.2) for alphaT3-1 experiments]. Primer efficiencies were calculated to be >85%.
Immunohistochemistry
Sprague-Dawley male and female rats, 7–8 weeks old, weighing 250–275 gm (Ace Animals Inc., Boyertown, PA) were used in immunohistochemical studies. Animal protocols were reviewed and approved by the Temple University Institutional Animal Care and Use Committee. All efforts were made to minimise the number of animals used. Rats anaesthetised with urethane (1.2 g/kg, i.p.) were intracardially perfused with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde/0.2% picric acid in PBS. Brains were removed, postfixed for 2 hr, and stored in 30% sucrose/PBS solution overnight. Tissues were processed for phoenixin immunoreactivity (irPNX) by the avidin-biotin complex procedure (15). The brain was embedded in agar and coronal sections of 40 μm were prepared with the use of a vibratome. Tissues were first treated with 3% H2O2 to quench endogenous peroxidase, washed several times, blocked with 10% normal goat serum, and incubated in PNX antiserum (1:750 dilution), a rabbit polyclonal raised against a conserved region of the human phoenixin (Asp-Val-Gln-Pro-Pro-Gly-Leu-Lys-Val-Trp-Ser-Asp-Pro-Phe-NH2; Phoenix Pharmaceuticals, Inc., Burlingame, CA). The PNX antiserum exhibits 100% cross-reactivity with human phoenixin 14 amide and rat phoenixin-20 amide in radioimmunoassay (Phoenix Pharmaceuticals, Inc). After thorough rinsing, sections were incubated in biotinylated anti-rabbit IgG (1:200 dilution, Vector Laboratories, Burlingame, CA) for 2 hr, and rinsed with PBS and incubated in avidin-biotin complex solution for 1.5 hr (1:100 dilution, Vector Laboratories). Following several washes in Tris-buffered saline, sections were developed in 0.05% diaminobenzidine/0.001% H2O2 solution and washed for at least 2 hr with Tris-buffered saline. Sections were mounted on slides with 0.25% gel alcohol, air-dried, dehydrated with absolute alcohol followed by xylene, and coverslipped with Permount.
Pituitary Cell Dispersal and Culture
Anterior pituitary glands from adult, random cycle female and intact male rats (Sprague Dawley, Harlan) were removed after decapitation and dispersed in the presence of 0.1% trypsin as previously described (16, 17). Cells were cultured for 48 hr in either 12×75 mm polystyrene tubes (static incubations) or 15 mm tissue culture dishes (dynamic incubations) in Minimum Essential Medium containing 10% horse serum and 1.0% antibiotic antimycotic (all Gibco Invitrogen, Grand Island, NY) at 37°C. Twenty-four hours before testing, additional medium alone or medium containing various fragments of the phoenixin prohormone (Phoenix Pharmaceuticals, Burlingame, CA) were added to the cultures.
Static incubations
On the day of testing, culture medium was removed following centrifugation (23°C, 600×g, 10 min) and replaced with test medium [Medium 199 containing 0.1% bovine serum albumin, 2×10−5 M bacitracin, 20 mM HEPES buffer (all Sigma-Aldrich, St. Louis, MO), and 1% penicillin-streptomycin (Gibco)] with or without the same peptide pretreatments as before. Cells were then incubated (37° C) for 30 or 60 min in the absence or presence of gonadotropin releasing hormone (GnRH, Phoenix Pharmaceuticals) and incubations terminated by centrifugation (23°C, 600×g, 10 min).
Dynamic incubations
Twenty-four hours before experimentation, medium alone or medium containing the 20 amino acid, amidated peptide was added to the culture dishes. On the day of testing, cells were harvested from the culture dishes and resuspended as described for static incubations. Cells (circa 2 million) were then loaded onto Bio-Gel P2 columns (0.4×1.5 cm, Bio-Rad, Hercules, CA) and columns perfused at a flow rate of 0.05 ml/min (17). After a stabilization period of at least 30 minutes, automated collection of perfusate fractions (5 min intervals) began. Cells were exposed then to the perfusate medium (with or without peptides) for the entire testing period. After collection of the first 10 fractions, a stimulatory concentration of GnRH (10 nM) was added to the perfusate medium for 50 minutes and then medium alone (with or without phoenixin but no GnRH) perfused for an additional 50 minutes. Then cells were exposed to a 10 minute exposure to 60 mM KCl, followed by an additional 40 minute exposure to medium alone.
Determination of medium LH content
Medium collected was stored frozen (−20 C) until determination of LH content by radioimmunoassay (18) using the kit provided by Dr. A.F. Parlow (National Hormone and Peptide Program, Torrance, CA). Results are expressed in terms of the RP-3 standards and values presented are means and standard errors of the mean.
Compromise of Endogenous Phoenixin using siRNA
All procedures have been approved by the Saint Louis University Animal Care and Use Committee. Cycling female Sprague-Dawley rats (225–250g)(Harlan Laboratories) received an indwelling stainless steel cannula (stainless steel, 23 gauge) implanted into the right lateral cerebroventricle as previously described (12). Oestrous cyclicity was monitored for at least one full cycle using vaginal cytology. On Dioestrus 1 and 2, rats were injected with either saline vehicle (2 ul), siRNA directed against phoenixin (2 ug in 2 ul sterile saline), or a control siRNA directed against enhanced green fluorescent protein (eGFP) (2 ug in 2 ul sterile saline). Each group consisted of at least 6 animals. Oestrous cyclicity was again monitored by vaginal cytology until the appearance of the next estrus, and then animals were sacrificed on the following day (dioestrus 1) by rapid decapitation for phoenixin protein evaluation. In a separate study, animal received 2 injections of either vehicle or siRNA on Diestrus 1 and 2, and were sacrificed the following day to confirm phoenixin protein knockdown by radioimmunoassay. Constructs were designed with the assistance of and purchased from Integrated DNA Technologies (Coralville, IA). Sequences of siRNA constructs are listed in the Supplemental Methods.
Enzyme-linked ImmunoAssay (EIA)
Tissues were homogenized in lysis buffer and large proteins were excluded using C18 Sep Pak columns (Millipore), and eluents were lyophilized. Samples were prepared according to the manufacturer’s instructions (Phoenix Pharmaceuticals, Burlingame, CA). The minimum detectable concentration was found to be 300 pg/ml, and the intra-assay coefficient of variability was 5–7%. Antibodies used in the EIA cross-reacted 100% with phoenixin-14 and phoenixin-20, and 0% with non-amidated forms of the peptide or other peptides containing C-terminal proline-phenylalanine residues (i.e. angiotensin II).
Purification of Endogenous Phoenixin
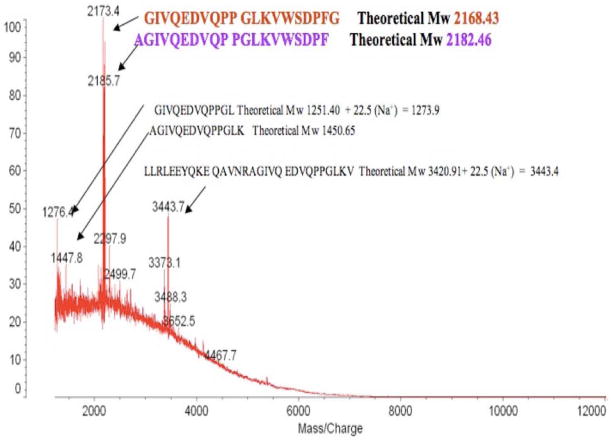
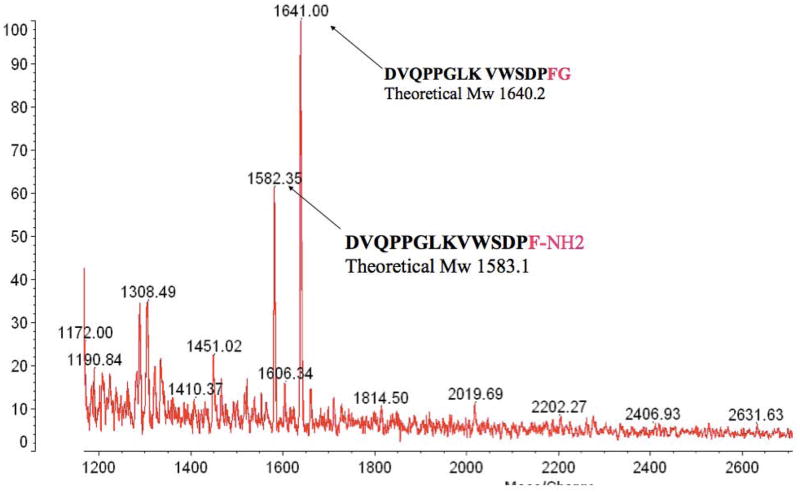
400 rat hypothalami (purchased from Pel-Freeze) were homogenised in 5 % acetic acid to obtain supernatant fraction and extract with C18 cartridges according to the method described by Koda et. al. (19). The rat heart homogenate 20 ml (2.73 g/ml) was purchased from Pel-Freez. Both C18 cartridges eluants of hypothalamii and heart supernatants were lyophilised, reconstituted in 1M acetic acid, and applied to a gel filtration column [BioGel fine, P-6 column (BioRad), 2.6 × 98 cm]. Fractions were collected at a flow rate 50 ml per hour and 10 ml for each fraction tube. Fractions 43–80 were lyophilised, and aliquots from each fraction were reconstituted in EIA assay buffer. Phoenixin immunoreactivity was assessed using an EIA (developed by Phoenix Pharmaceuticals). Immunoreactive fractions were pooled and phoenixin was affinity purified using MagnaBind beads (Pierce/Thermo scientific) conjugated with anti-phoenixin antibody (produced by Phoenix Pharmaceuticals). Eluents were lyophilised, reconstituted in 0.1% TFA, and proteins were further separated by reverse-phase HPLC (RP-HPLC). Immunoreactivity of RP-HPLC fractions was assessed using an EIA, and immunoreactive fractions were subjected to MALDI-TOF for the identification of Phoenixins. In the final stage of verification of purified phoenixins, the predicted phoenixin peptides that had been synthesized were processed under the same RP-HPLC separation conditions. A comparable chromatography profile from RP-HPLC and the molecular mass from MALDI-TOF confirmed the same properties of native phoenixins and synthetic phoenixins.
Radioligand Binding Displacement Studies
Rat pituitary adenoma cells, RC-4B/C (CRL-1903; ATCC, Manassas, VA, USA), were cultured in Dulbecco’s Modified Eagle’s Medium and Minimum Essential Alpha Medium (Invitrogen, CA, USA) supplemented with 0.01mM non-essential amino acids, 15 mM HEPES, 2.5 ng/ml epidermal growth factor (EGF), and dialyzed, heat-inactivated fetal bovine serum (FBS) at 37 °C in a humidified cell incubator containing 5% carbon dioxide (CO2). The RC-4B/C cells were plated in 24-well tissue culture plates at two days before the assay for the radioligand binding. At the day of binding displacement, cell were incubated for 30 min in serum- and EGF-free medium 30 min but with 50 pM I125-phoenixin-20 in the absence or presence of increasing concentrations of un-labelled phoenixin-20 or phoenixin-14 amide. Nonspecific binding was defined as total binding in the presence of 1 μM unlabelled phoenixin-20 or phoenixin-14 amide. After termination of the binding reaction by washing the cells with 1 ml of cold PBS, cells were solubilized with 0.5 ml 1% SDS, and the radioactivity of such a solubilized liquid from each well was detected in gamma counter. The value for non-specific binding was 13%. Results are displayed as % of original specific binding after the application of increasing concentration of unlabelled phoenixin-20 or phoenixin-14 amide.
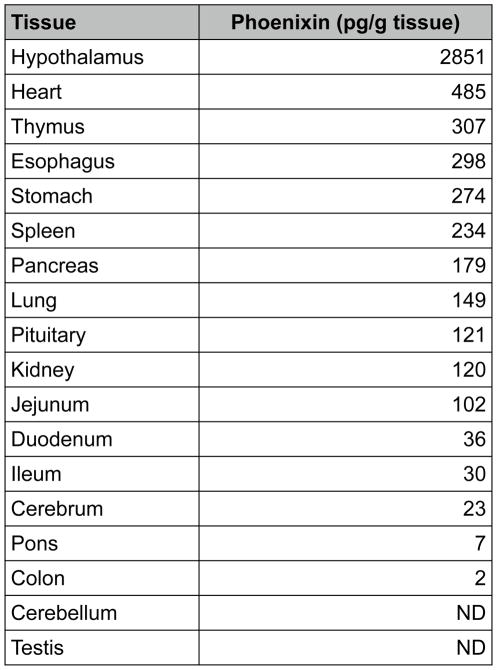
RESULTS
Using a bioinformatic algorithm (12), we identified a novel predicted peptide that was found to be highly conserved across multiple species, from human to fugu (Figure 1A). Only one amino acid difference was observed between the mature regions of human and rodent sequences (position 20 to 45). This sequence contained a C-terminal glycine residue (potential site for amidation) and several conserved dibasic residues, indicating potential cleavage sites, which could yield peptides of varying amino acid lengths. This sequence was synthesized, and antibodies were generated and utilised in the development of an enzyme-linked immunoassay to detect the peptide in rat tissues (Figure 1B). The peptide was detected in various peripheral tissues, including heart, thymus, stomach, and spleen; however, the tissue with the highest level of immunoreactivity was the hypothalamus. Expression of phoenixin mRNA was detected in similar tissues (Supplemental Figure 3). Because of the relative abundance of the peptide in hypothalamus and heart, the antibody then was used to purify the endogenous peptide from rat hypothalamus (Figure 1C) and bovine heart (Figure 1D). Tissues were homogenised, and large proteins were excluded using C18 chromatography. Eluants were separated further by a BioGel P6 column, followed by affinity purification and HPLC. The major products, identified by MALDI-TOF, were found to be amidated peptides of 20 and 14 amino acids in the hypothalamus and heart, respectively. We named this peptide phoenixin (PNX).
Figure 1. Isolation of endogenous phoenixin.
(A) Peptide sequence of phoenixin. Potential cleavage sites are indicated by red lettering on the consensus (top) sequence. Phoenixin is highly conserved across species.
(B) Phoenixin immunoreactivity can be detected in various tissues in adult rats, as indicated by EIA. Mass spectrometry analysis of endogenous phoenixin isolated from rat hypothalamus (C) and bovine heart (D) revealed peptides of 20 and 14 amino acids, respectively.
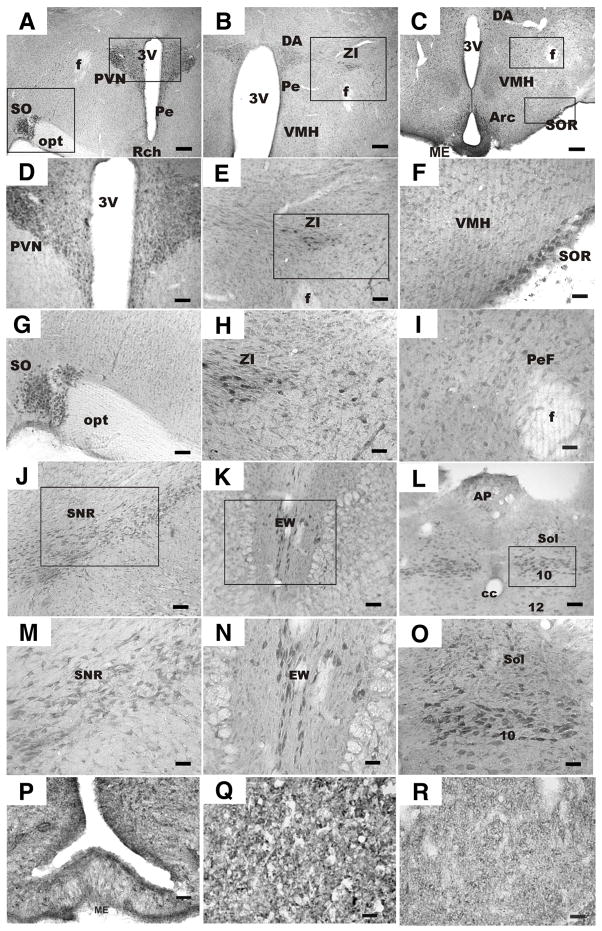
Because of the relatively high levels of phoenixin in hypothalamus, we reasoned that the peptide would exert actions in brain or pituitary gland. We therefore evaluated the expression pattern of phoenixin by immunohistochemistry using an antibody generated against C-terminally amidated phoenixin. Microscopic examination of brain sections from 7 male and 4 female rats revealed that irPNX cell bodies were conspicuously present in following areas: hypothalamus, substantia nigra recticulata, Edinger-Westphal nucleus, and nucleus of the solitary tract/dorsal motor nucleus of the vagus. This pattern of expression was similar in male and female brains. With respect to the hypothalamus, numerous irPNX cells were distributed to the magnocellular and parvocellular paraventricular (PVN) and in the supraoptic (SO) nucleus; fewer cells were noted in the periventricular nucleus (Pe) (Figures 2A, D, and G). Positively labelled cells were also detected in the dorsal hypothalamus (DA), zona incerta (ZI), ventromedial hypothalamus (VMH), lateral hypothalamus, and perifornical area (PeF) (Figures 2B, E, and H). In the region of the tuber cinereum, irPNX cells were present in the arcuate nucleus (Arc), VMH, PeF and supraoptic retrochasmatic nucleus (SOR) (Figures 2C, F, and I). Labelled cells in all regions of the hypothalamus, with the exception of SOR, were generally small diameter, ranging from 10–15 μm; irPNX neurones in the SOR were larger, 20–25 μm in diameter. Within the brainstem, irPNX cells were detected in several discrete areas including the substantia nigra reticulata (SNR), Edinger-Westphal nucleus (EW), nucleus of the solitary tract (NTS) and dorsal motor nucleus of the vagus (Figure 2J–O). Lightly to moderately labelled cells were seen in the area postrema (Figure 2L). Positive labeling was observed in the median eminence (Figure 2P), although clearly labeled individual fibers could not be discerned. Phoenixin immunoreactivity was observed also in anterior pituitary (Figure 2Q) and in posterior pituitary (Figure 2R). No phoenixin staining was noted when primary antibody was first preabsorbed with phoenixin peptide (Supplemental Figure 6).
Figure 2. Localization of phoenixin in brain.
Male and female Sprague-Dawley rats were anaesthetised with urethane and perfused intracardially. Brains were removed and coronal sections of 40 um were prepared using a vibratome. Tissues were blocked with 10% goat serum, and then incubated in PNX antiserum (1:750 dilution). Sections were developed using DAB (see Supplemental Methods). PNX immunoreactivity was observed in both hypothalamus (A–I) and brainstem (J–O). (A) Staining was observed in both PVN and SON. Enlargements of these areas are presented in (D) and (G), respectively. (B) Staining was detected also in the ZI (enlargements presented in E and H). 3V, third ventricle; AP, area postrema; Arc, arcuate nucleus; cc, central canal; DA, dorsal hypothalamus; EW, Edinger-Westphal nucleus; ME, median eminence; opt, optic chiasm; SO, supraoptic nucleus; Pe, periventricular nucleus; PeF, perifornical area; PVN, paraventricular nucleus; Sol, nucleus tractus solitarius; VMH, ventromedial hypothalamus; ZI, zona incerta. (P) A hypothalamic section through the median eminence (ME) where numerous small irPNX cells are seen in the arculate nucleus (Arc). (Q) a section of anterior pituitary lobe, where small irPNX cells are detected. (R) a section of posterior pituitary lobe, where irPNX cell processes are noted. Abbreviations: f, fornix; opt, optic tract, 3V, 3rd ventricle. Scale bar: A–I, 100 μm; J–O, 250 μm; P–R, 50 μm.
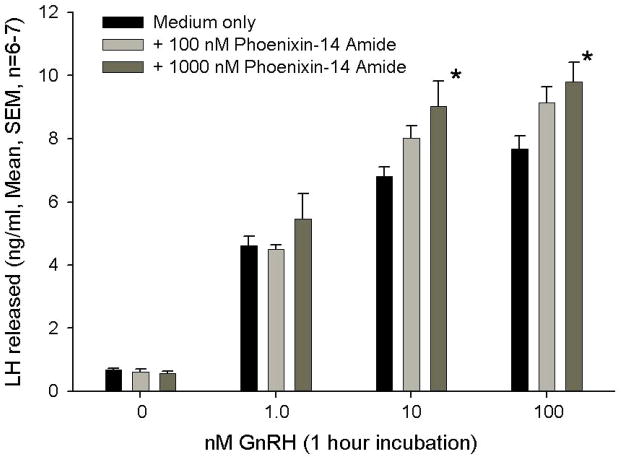
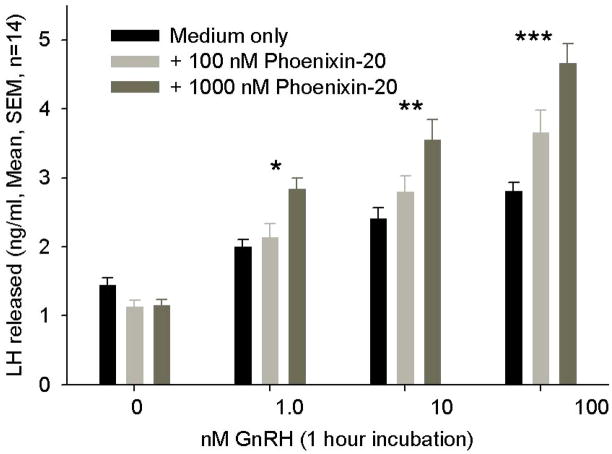
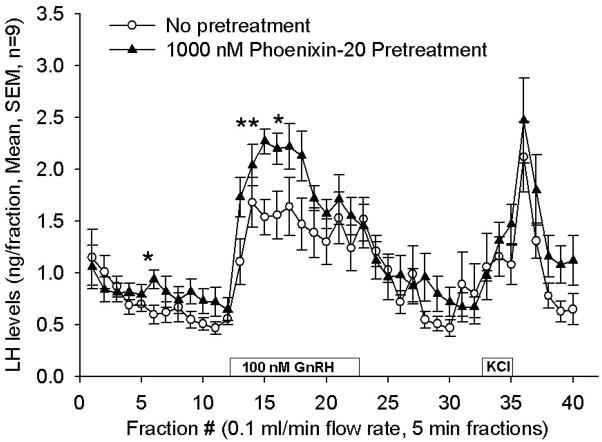
Based upon peptide localization in the hypothalamus and particularly in the region of the median eminence, a potential, primary site of action of phoenixin appeared to be the anterior pituitary gland. Therefore we tested the ability of the peptide to affect hormone secretion from dispersed, primary rat pituitary cultures. Treatment with phoenixin did not alter the basal release of LH, ACTH, prolactin, growth hormone, or TSH (data not shown). However, 24-hour pretreatment with either the 14- or 20-amino acid amidated forms potentiated GnRH-stimulated LH release from female pituitary cultures (Figure 3A and B). Phoenixin appeared to potentiate the upregulation of FSH mRNA induced by GnRH (Supplemental Figure 1); however, no significant differences were observed between vehicle- and phoenixin-pretreated cultures. In an additional protocol dispersed pituitary cells from random cycle, female donors were cultured overnight in medium alone or medium containing amidated phoenixin-20 and then loaded onto perifusion columns. Perifusion with phoenixin alone did not alter basal LH release, but significantly enhanced LH release when GnRH was included in the perifusate (Figure 3C). This appeared to be a gender-specific effect, as no differences in LH release were observed when the 20 amino acid, amidated peptide was included compared to medium alone in similarly prepared male pituitary cells (Supplemental Figure 2).
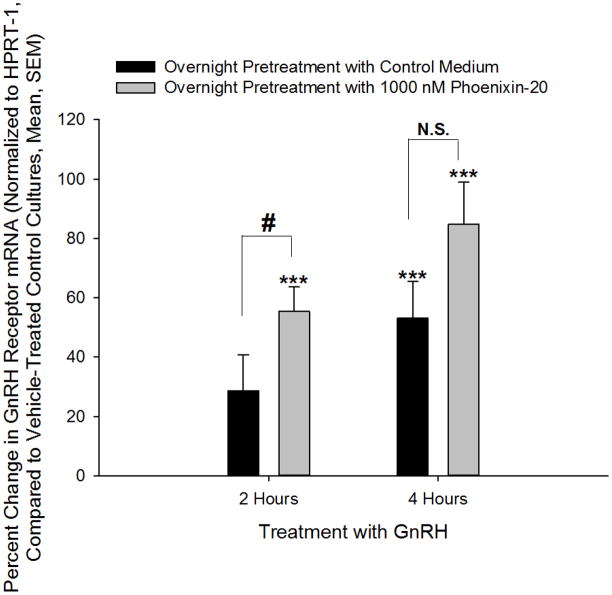
Figure 3. Phoenixin regulates pituitary gonadotropin secretion by modulating expression of the GnRH receptor.
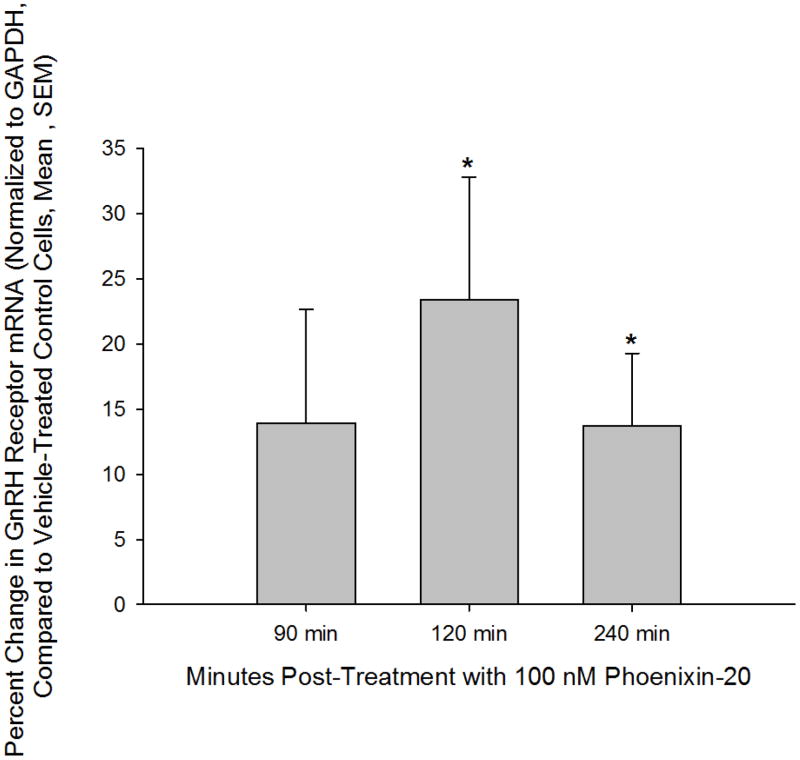
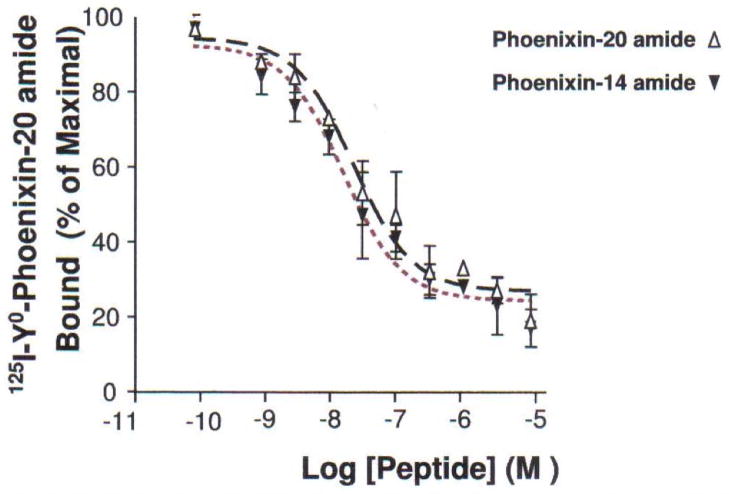
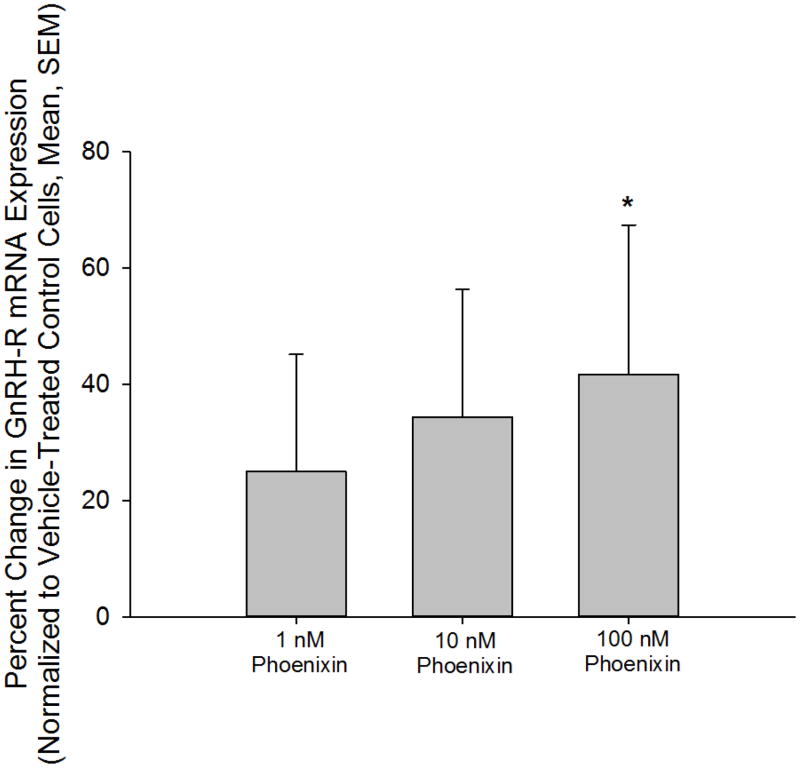
Anterior pituitary cell cultures from random cycle female rats were incubated overnight with either phoenixin-14 (A) or phoenixin-20 (B), and then exposed to GnRH for one hour. Both phoenixin-14 and -20 potentiated GnRH-stimulated LH release (A and B). To evaluate the time course of the effect of phoenixin on LH release, female anterior pituitary cells were incubated with either vehicle or 1000nM phoenixin-20 for 24 hours prior to loading onto BioGel P-2 columns and exposure to the perifusate media alone (Fractions 0–10), media containing 10 nM GnRH (Fractions 11–25), and media containing 60 mM KCl (Fractions 30–40) (C). Pretreatment with phoenixin enhanced GnRH-stimulated LH release. Overnight pretreatment with phoenixin-20 also potentiated the ability of GnRH to upregulate the expression GnRH receptor in dispersed male anterior pituitary cell cultures (D). In the immortalised mouse pituitary gonadotroph cell line, alphaT3-1, treatment with phoenixin-20 increased GnRH receptor mRNA (E), as determined by RT-PCR. Phoenixin-20 and phoenixin-14 bound with similar affinity to pituitary adenoma cell membrane preparations (F). In primary pituitary cell cultures collected from female donors, phoenixin, in the absence of GnRH, significantly increased GnRH receptor expression (G). *p<0.05, **p<0.01, ***p<0.001 versus non-pretreated control cells (A and B) or vehicle treated cells (C, D, E, and G). #p<0.05 vs. control-pretreated, GnRH-treated cultures (D). For A and B, a one-way ANOVA was utilised, and data in C, D, E, and G was analyzed using a t test.
GnRH is known to modulate the expression of its own receptor, leading to enhanced sensitivity of the pituitary gonadotroph to GnRH (20, 21). The mechanism of action by which phoenixin enhances GnRH-stimulated LH release could be through upregulation of transcription of the GnRH receptor. We therefore pretreated dispersed pituitary cultures with phoenixin prior to incubation with either medium alone or medium containing GnRH, and found that the peptide increased GnRH receptor messenger RNA and potentiated GnRH-stimulated upregulation of the GnRH receptor, as determined by RT-PCR (Figure 3D). Treatment with phoenixin also led to an increase in GnRH receptor expression in female primary pituitary cell cultures in the absence of GnRH (Figure 3G). To investigate this effect of the peptide in a homogenous population of pituitary gonadotrophs, we utilised the immortalised gonadotroph cell line, alphaT3-1 (13). AlphaT3-1 cells that were treated with the amidated 20 amino acid form exhibited an apparent increase in cFos message, although this effect failed to attain significance (not shown). However, exposure of alphaT3-1 cells to phoenixin-20 led to an increase in GnRH receptor message (Figure 3E).
In preliminary experiments, we observed that phoenixin-20 bound specifically to pituitary and ovary cell membranes using tissues from female rat donors, and this binding was not displaced by either gonadotropin-releasing hormone or urocortin (data not shown). Because 14 and 20 amino acid peptides were recovered from rat tissues, we tested the binding of the phoenixin-14 and phoenixin-20 to rat pituitary adenoma cell membranes. Both peptides bound with similar affinity (Figure 3F).
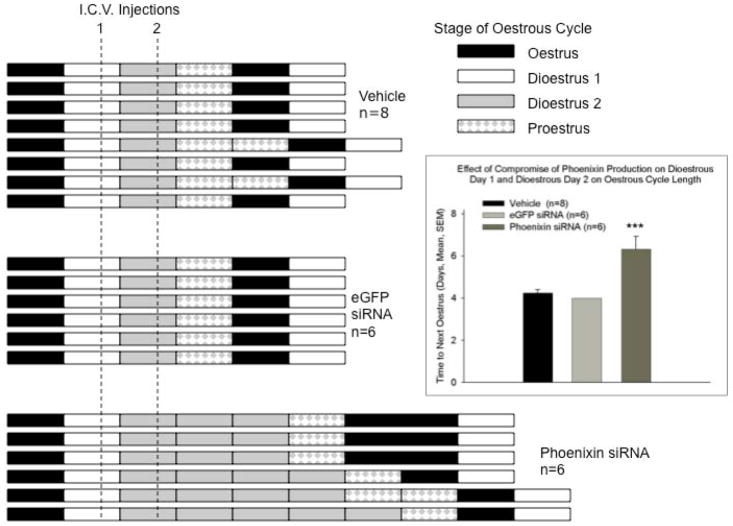
To determine whether the pharmacologic effects of phoenixin have physiologic relevance, we assessed the effect of compromised production of endogenous phoenixin in vivo on reproductive function. Female rats were treated for two consecutive days with saline vehicle, or with siRNA (2 micrograms in 2 microlitres sterile saline per day) directed against phoenixin, or with control siRNA against green fluorescent protein, injected into the right lateral cerebroventricle. As expected, treatment with siRNA directed against phoenixin resulted in a reduction of phoenixin levels in the hypothalamus (Figure 4B), compared to saline- or eGFP siRNA-injected control animals, as determined by radioimmunoassay. The oestrous cycle of these rats was followed using vaginal cytology. Injection with either saline vehicle or eGFP siRNA did not disrupt the oestrous cyclicity of those animals (Figures 4A). However, loss of endogenous phoenixin postponed the appearance of the next oestrus by an average of 2.3 days (Figure 4A), corresponding to the resolution of the effect of the siRNA (hypothalamic content of phoenixin, as measured by radioimmunoassay, the morning following the reappearance of the oestrous smear: Vehicle (n=8) 110.1 +/− 10.0. eGFP siRNA (n=6) 96.1 +/− 7.1, Phoenixin siRNA (n=6) 118.9+/− 8.5 ng/mg protein, no significant differences were observed using ANOVA). In preliminary experiments, the loss of endogenous phoenixin in hypothalamus paralleled a reduction in GnRH receptor expression in the anterior pituitary (Supplemental Figure 4), further indicating that phoenixin acts by modulating the expression of the GnRH receptor. Animals treated with siRNA did not exhibit any significant changes in body weight during treatment (Figure 4C), nor were any alterations in behaivour observed. Treatment with siRNA also did not significantly alter vasopressin content [(Median eminence: phoenixin siRNA 1.38 +/− 0.36 ng AVP/mg total protein; eGFP siRNA 1.34 +/− 0.16; p = 0.92) (Hypothalamus: phoenixin siRNA 2.18 +/− 0.13; eGFP siRNA 2.42 +/− 0.30; p = 0.51)], or significantly inhibit the expression of 3 potential unintended targets identified using NCBI BLAST (Supplemental Figure 5).
Figure 4. Compromise of endogenous hypothalamic phoenixin delays the appearance of the subsequent oestrus in cycling female rats.
Following verification of a four-day oestrous cycle in rats bearing an indwelling cerebroventricular cannula, test substances [vehicle (2 ul sterile saline), or eGFP siRNA or phoenxin siRNA (2 ug in 2 ul sterile saline)] were administered i.c.v. on the afternoon of dioestrous day 1 and again on dioestrous day 2. Vaginal cytology was employed to monitor oestrous cycle progression and detect the next day of oestrus. (A) The bars indicate stage of the oestrous cycle for individual animals. Animals were then sacrificed on the first day of dioestrus following the verified appearance of oestrus and brains harvested for determination of phoenixin mRNA levels. The inset demonstrates the significant interruption of the four-day oestrous cycle in phoenixin siRNA treated animals, due mainly to the extension of the time in dioestrus. *** p < 0.001 versus vehicle and eGFP siRNA treated animals (ANOVA). In cycling female rats that were treated with siRNA directed against phoenixin on dioestrus days 1 and 2, and sacrificed the following day, hypothalamic content of phoenixin was significantly (p<0.01 vs. eGFP siRNA-injected controls, t test) reduced, as determined by radioimmunoassay (B). However, treatment with siRNA did not alter body weight (C).
DISCUSSION
Phoenixin is a novel peptide that is produced in the hypothalamus and appears to exert direct effects on pituitary gonadotrophs. Additional biologic actions may exist, such as in reproductive tissues like the ovary. Alternatively, phoenixin may exert additional, yet to be determined, reproductive and non-reproductive actions in brain since peptide localization was not limited to the medial basal hypothalamus. Identification of the cognate receptor(s) for phoenixin or discrete areas of specific labelled phoenixin binding will facilitate discovery of those potential, additional biological actions. Just as important will be studies identifying possible co-localization of phoenixin with other peptides or amines known to be important in the control of gonadotroph function.
The pituitary action of phoenixin may be important for normal ovarian cyclicity in females, as compromise of endogenous phoenixin production in female rats delayed the onset of oestrus and resulted in a reduction of GnRH receptor mRNA in the anterior pituitary. On the other hand, treatment of either primary pituitary cells or immortalised pituitary gonadotrophs with phoenixin led to an increase in GnRH receptor expression, indicating that phoenixin may influence ovarian cyclicity through modulation of the GnRH receptor. Phoenixin may therefore represent a novel class of hypothalamically-derived pituitary priming factor that sensitises the pituitary to the action of GnRH. However, these studies do not establish hypophysiotropism, and future experiments must assess the presence of phoenixin immunoreactive fibers in the median eminence and of phoenixin peptide in the hypopheseal portal blood.
While we have demonstrated that phoenixin can modulate pituitary GnRH receptor expression, the mechanisms controlling the expression of phoenixin itself remain unknown. We have identified a potential oestrogen response element (ERE) upstream of phoenixin (NCBI Ref Seq NM_001145432); however, binding studies are necessary to confirm that this region is a true ERE. Certainly one could envision that the increase in oestrogen during proestrus could stimulate the transcription of phoenixin in hypothalamus, which could then sensitise the pituitary gonadotroph to the action of GnRH, and contribute to the preovulatory surge in LH and FSH. If this is the case, then our data suggest that this upregulation of phoenixin expression is necessary for the progression of the oestrous cycle. Future studies should also examine the potential interaction of phoenixin with kisspeptin, not only in the setting of normal oestrous cyclicity, but also for its potential participation in the hypothalamic mechanisms controlling the onset of puberty.
Localization in the hypothalamic paraventricular and supraoptic nuclei and in cells of the median eminence, when coupled with detection of phoenixin-like immunoreactivity in the posterior lobe of the pituitary gland strongly suggest secretion of the peptide into the general circulation and therefore sites of action in the periphery. Similarly, while our siRNA results suggest that phoenixin of hypothalamic origin is important in the control of oestrous cyclicity, we have observed phoenixin-like immunoreactivity in the anterior lobe of the pituitary gland. This could reflect uptake (i.e. scavenging) of peptide delivered by the hypophyseal portal vessels or local production in the gland (Supplemental Figure 3), and thus a potential paracrine action on the gonadotroph. Finally, phoenixin-like immunoreactivity was detected in brain sites not traditionally associated with reproduction such as the Edinger-Westphal nucleus, the dorsal motor nucleus of the vagus, and the nucleus of the tractus solitarius, instead sites related to autonomic function, suggesting actions related to cardiovascular or gastrointestinal function as well.
In summary, we have detailed here the discovery, based upon a bioinformatics approach, of a previously unidentified, endogenous peptide produced in neuroendocrine hypothalamus. That discovery allowed us to identify a potentially novel mechanism by which the hypothalamus controls reproductive hormone secretion. The physiological relevance of this mechanism is suggested by preliminary data describing our use of an siRNA approach to interrupt normal oestrous cyclicity in rats. If these initial studies can be extended to establish a physiologically relevant mechanism by which the responsiveness of the gonadotroph to GnRH may be increased, alternative strategies for enhancing reproductive capacity might be envisioned or, on the other hand, the development of antagonists for non-steroid based contraceptive approaches might be considered.
Supplementary Material
Acknowledgments
The cell line alphaT3-1 was a gift from Pam Mellon. This work was supported in part by NIH HL66023 to W.K.S. and HL51314 to N.D.
Footnotes
Disclosures: The authors have nothing to disclose. R.M.L. and J.K.C. are employees of Phoenix Pharmaceuticals.
AUTHOR INFORMATION
The authors declare no competing financial interests. R.M.L. and J.K.C. are employees of Phoenix Pharmaceuticals.
References
- 1.McCann SM, Lumpkin MD, Mizunuma H, Khorram O, Samson WK. Brain peptides in control of anterior pituitary hormone secretion. Peptides. 1984;5:3–7. doi: 10.1016/0196-9781(84)90259-6. [DOI] [PubMed] [Google Scholar]
- 2.Daniel PM. The blood supply of the hypothalamus and pituitary gland. Br Med Bull. 1966;22:202–208. doi: 10.1093/oxfordjournals.bmb.a070474. [DOI] [PubMed] [Google Scholar]
- 3.Gahete MD, Duran-Rado M, Luque RM, Martinez-Fuentes AJ, Quintero A, Gutierrez-Pascual E, Cordoba-Chacon J, Malagon MM, Gracia-Navarro F, Castano JP. Understanding the multifactorial control of growth hormone release by somatotropes: Lessons from comparative endocrinology. Ann NY Acad Sci. 2009;1163:137–153. doi: 10.1111/j.1749-6632.2008.03660.x. [DOI] [PubMed] [Google Scholar]
- 4.Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endo. 2004;33:559–584. doi: 10.1677/jme.1.01600. [DOI] [PubMed] [Google Scholar]
- 5.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 6.Levine JE, Ramirez VD. Luteinizing hormone-releasing hormone release during the rat oestrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology. 1982;111:1439–1448. doi: 10.1210/endo-111-5-1439. [DOI] [PubMed] [Google Scholar]
- 7.Vasilyev VV, Lawson MA, Dipaolo D, Webster NJ, Mellon PL. Different signaling pathways control acute induction versus long-term repression of LH beta transcription by GnRH. Endocrinology. 2002;143:3414–3426. doi: 10.1210/en.2001-211215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasilyev VV, Pernasetti F, Rosenberg SB, Barsoum MJ, Austin DA, Webster NJG, Mellon PL. Transcriptional activation of the ovine FSH beta gene by GnRH involves multiple signal transduction pathways. Endocrinology. 2002;143:1651–1659. doi: 10.1210/endo.143.5.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;311:126–134. doi: 10.1016/j.mce.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brann DW, O’Conner JL, Wade MF, Zamorano PL, Mahesh VB. Regulation of anterior pituitary gonadotropin subunit mRNA levels during the preovulatory gonadotropin surge: a physiological role of progesterone in regulating LH-beta and FSH-beta mRNA levels. J Steroid Biochem Mol Bio. 1993;46:427–437. doi: 10.1016/0960-0760(93)90097-g. [DOI] [PubMed] [Google Scholar]
- 11.Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12:295–305. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GL, Klein C, Lyu R, Wang YX, Chen XQ, Yang J, Price CJ, Hoyda TD, Ferguson AV, Yuan XB, Change JK, Hsueh AJ. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem. 2008;283:31949–31959. doi: 10.1074/jbc.M804784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Windle JJ, Weiner RI, Mellon PL. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4:597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]
- 14.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 15.Dun SL, Brailoiu GC, Tica AA, Yang J, Chang JK, Brailoiu E, Dun NJ. Neuronostatin is co-expressed with somatostatin and mobilizes calcium in cultured rat hypothalamic neurons. Neuroscience. 2010;166:455–463. doi: 10.1016/j.neuroscience.2009.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samson WK, Said SI, Snyder GD, McCann SM. In vitro stimulation of prolactin release by vasoactive intestinal polypeptide. Peptides. 1980;1:325–329. doi: 10.1016/0196-9781(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 17.Samson WK, Martin L, Mogg RJ, Fulton RJ. A nonoxytocinergic prolactin releasing factor and a nondopaminergic prolactin inhibiting factor in bovine neurointermediate lobe extracts: in vitro and in vivo studies. Endocrinology. 1990;126:1610–1617. doi: 10.1210/endo-126-3-1610. [DOI] [PubMed] [Google Scholar]
- 18.Samson WK, McCann SM. Effects of lesions in the organum vasculosum lamina terminalis on the hypothalamic distribution of luteinizing hormone releasing hormone and gonadotrophin secretion in the ovariectomized rat. Endocrinology. 1979;105:939–946. doi: 10.1210/endo-105-4-939. [DOI] [PubMed] [Google Scholar]
- 19.Koda A, Ukana T, Teranishi H, Ohta S, Yamamoto K, Kikuyama S, Tsutsui K. A novel amphibian hypothalamic neuropeptide: isolation, characterization, and biological activity. Endocrinology. 2002;143:411–419. doi: 10.1210/endo.143.2.8630. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993;133:931–934. doi: 10.1210/endo.133.2.8393779. [DOI] [PubMed] [Google Scholar]
- 21.Lerrant Y, Kottler ML, Bergametti F, Moumni M, Blumberg-Tick J, Counis R. Expression of gonadotropin-releasing hormone (GnRH) receptor gene is altered by GnRH agonist desensitization in a manner similar to that of gonadotropin beta-subunit genes in normal and castrated rat pituitary. Endocrinology. 1995;136:2803–2808. doi: 10.1210/endo.136.7.7789305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.