Abstract
The present study was designed to determine the effects of melatonin and its receptor agonists on SNP-released nitric oxide (NO) and cGMP production in aqueous humor producing cells of the ciliary body because these effects may play a role in melatonin receptor-mediated regulation of intraocular pressure (IOP). NO release protocols were carried out using human non-pigmented ciliary epithelial (hNPCE) cells treated in dye free DMEM containing L-arginine (10−3 M). The cGMP experimental protocols were performed using dye free DMEM containing 3-isobutyl-1-methylxanthine (IBMX, 10−4 M). The effects of varying concentrations (10−13, 10−11, 10−9, 10−7, and 10−5 M) of melatonin, 5-MCA-NAT (putative MT3 agonist), N-butanoyl-2-(2-methoxy-6H-isoindolo[2, 1-a]indol-11-yl)ethanamine (IIK7; selective MT2 agonist) or S-27633-1 (selective MT1 agonist) on sodium nitroprusside (SNP)-released NO or cGMP production were determined in separate experiments. NO and cGMP levels were measured using a colorimetric assay or enzyme immunoassay (EIA), respectively. Melatonin receptor selectivity was evaluated using luzindole (LUZ; nonselective MT1/MT2 antagonist) or 4-phenyl-2-propionamidotetralin (4P-PDOT; selective MT2 antagonist). Melatonin, 5-MCA-NAT, and IIK7 all caused concentration-dependent reduction of SNP-released NO and cGMP production. The inhibitory actions of melatonin, 5-MCA-NAT and IIK7 were either completely blocked at 10−13, 10−11, and 10−9 M concentrations of the agonists or partially at 10−7 and 10−5 M in the presence of luzindole or 4P-PDOT. Results from this study suggest that melatonin and its analogues, 5-MCA-NAT and IIK7 inhibit SNP-released NO and cGMP production via activation of MT2 receptors in human NPCE cells. These actions may play a role in melatonin agonist-induced regulation of aqueous humor secretion and IOP.
Keywords: MT2 receptors, melatonin, nitric oxide, cGMP, non-pigmented ciliary epithelial cells
1. Introduction
In the eye, melatonin is synthesized by the photoreceptors with high levels of melatonin at night and lower levels during the day (Tosini et al., 2007). Several lines of evidence support the possibility that melatonin may act as a protective agent in ocular conditions such as photokeratitis, cataract, retinopathy of prematurity, ischemia/reperfusion injury, and glaucoma (Rosenstein et al., 2010; Siu et al., 2006). Melatonin has also been shown to have protective effects on photoreceptors (Baba et al., 2009). Recent studies have implicated melatonin in the pathogenesis of age-related macular degeneration and have shown that it delays photoreceptor degeneration in the rds/rds mouse maintained on a cyclic photoperiod (12:12h light:dark, lights on 06.00 h (Liang et al., 2001). Some studies have also reported that melatonin receptors are present in the ciliary body (Osborne and Chidlow, 1994; Pintor et al., 2001; Pintor et al., 2003; Wiechmann and Wirsig-Wiechmann, 2001). The expression of melatonin receptors in the ciliary body processes has led to the hypothesis that melatonin may be involved in the regulation of IOP and indeed, several studies have shown that melatonin can modulate the IOP in various species (Pintor et al., 2001; Samples et al., 1988; Serle et al., 2004; Wiechmann and Wirsig-Wiechmann, 2001). These studies have demonstrated the IOP reducing effect of melatonin and its analogues both in nonhuman primates (Serle et al., 2004) and humans (Samples et al., 1988).
The specific functions of melatonin are mediated through cell membrane associated MT1, and MT2 receptors (Dubocovich, 1995) and a third MT3 receptor that has been identified as the enzyme quinone reductase 2 (QR2) in some mammalian species (Nosjean et al., 2000). Initially, the melatonin analogue 5-MCA-NAT was thought to produce its ocular hypotensive effect via MT3 (QR2 enzyme) receptors (Pintor et al., 2001). However, data now support the idea that the in vivo effect of 5-MCA-NAT on IOP is not mediated by the enzyme QR2, but is more likely to be mediated by some other melatonin receptor, possibly MT1 or MT2 (Alarma-Estrany et al., 2009). These conflicting data reveal the necessity for studies which could generate important information regarding melatonergic signaling in the anterior segment of the eye.
Compelling new evidence that melatonin is involved in the regulation of IOP (Alcantara-Contreras et al., 2011) since removal of the MT1 receptor increases IOP during the night. Because the increase in IOP occurred during the dark phase, it suggests that melatonin would be more useful in humans at night. Other studies also suggest that an increase in IOP at night may represent a significant but unappreciated risk factor in the development of glaucoma in humans (Liu et al., 2003). These data highlight the importance of melatonin receptors in glaucoma development and progression.
We conducted the present study using the well-established hNPCE cell line (Coca-Prados and Wax, 1986). Since the ciliary epithelium is the site of aqueous humor production, hNPCE cells are often used to determine mechanisms of action of drugs that affect aqueous humor formation. These transformed hNPCE cells were also shown to contain NO-activated heterodimeric soluble guanylyl cyclase (Danziger et al., 1993) and the human ciliary epithelium was demonstrated to express nitric oxide synthase (NOS) isozymes (Wu et al., 2007; Wu and Ma, 2012). Data presented in this paper provide evidence that melatonin acting via MT2 receptors, suppresses NO/cGMP signaling in aqueous humor producing hNPCE cells. These data suggest that MT2 receptors may play a role in regulating aqueous humor production in the human ciliary epithelium.
2. Materials and Methods
2.1 Drugs
4P-PDOT, 5-MCA-NAT, luzindole – Tocris Bioscience, Ellisville, MO
SNP, L-arginine, IIK7, melatonin – Sigma-Aldrich, St. Louis, MO
S-27633-1 – ADIR, Courbevoie Cedex, France
2.2 Cell culture
NPCE cells seeded in 12 (NO) or 6-well (cGMP) plates were grown to approximately 90% confluence before drug treatment. Experimental protocols were carried out on cells incubated at 37 °C in dye-free DMEM growth media containing the amino acid L-arginine. L-arginine was included in the media of control and treated cells to stimulate NO production in vitro. NOS catalyze the oxidation of the terminal guanidino nitrogen of L-arginine to produce NO and L-citrulline (Palmer et al., 1988).
2.3 Drug Treatments for NO and cGMP Determination
For these studies we decided to examine the effect of the melatonin receptor agonists on SNP released NO and cGMP production because the basal levels of NO were very low and we often would generate negative values that had no consistent pattern of inhibition. At times the levels would in fact be out of the range of the standard curve. Therefore, we used the NO donor SNP which consistently elevated NO and cGMP levels in the NPCE cells.
The effect of MEL, 5-MCA-NAT or IIK7 (10−13, 10−11, 10−9, 10−7 or 10−5 M) on SNP-released NO and cGMP production was determined in the absence or presence of luzindole (nonselective MT1/MT2 receptor antagonist) or 4P-PDOT (selective MT2 receptor antagonist). Cells were pretreated with antagonists for 20 minutes followed by treatment with agonist for 15 min before addition of SNP. Once all drugs were added, cells were incubated for 4 hr for the NO experiments or 15 min for cGMP. Following drug treatments, media samples and cell lysates were assayed for NO or cGMP, respectively. Cell lysates were analyzed for protein using the BioRad protein assay.
2.4 Nitrite measurements
NO is a very unstable molecule in solution with a half-life of only a few seconds. Therefore, in these studies NO was measured as its stable metabolites, nitrate and nitrite. In the cell, NO undergoes a series of reactions with several molecules present in biological fluids and is eventually metabolized to nitrite (NO2−) and nitrate (NO3−). The incubation medium surrounding the cells was assayed for NO (nitrates + nitrites) levels using a microplate assay from Active Motif, Carlsbad, California. The principle of the NO quantitation kit is that nitrate in the sample is converted to nitrite in the presence of nitrate reductase and cofactors. Then, nitrate and nitrite levels are assayed using Griess Reagent. Each experiment was performed in triplicate and repeated at least four times.
2.5 cGMP measurements
cGMP levels were measured using a competitive EIA from Cayman Chemical Company, Ann Arbor, Michigan. This assay is based on the competition between free cGMP and a cGMP acetylcholinesterase conjugate for a limited amount of sites. Plates are precoated with mouse monoclonal anti-rabbit IgG and blocked with a proprietary formulation of proteins. In the first step, plates are incubated with tracer, antiserum, and either standard or sample. Next, the plate is washed to remove all unbound reagents. Finally, the plate is developed with Ellman’s Reagent. Each experiment was performed in triplicate and repeated at least four times.
2.6 Statistical Analyses
Data were analyzed for differences using one-way analysis of variance followed by the Bonferroni method for multiple comparisons. Results are expressed as mean values ± SEM and were considered significant when P < 0.05.
3. Results
3.1 Effect of Melatonin on SNP-released NO and cGMP Production
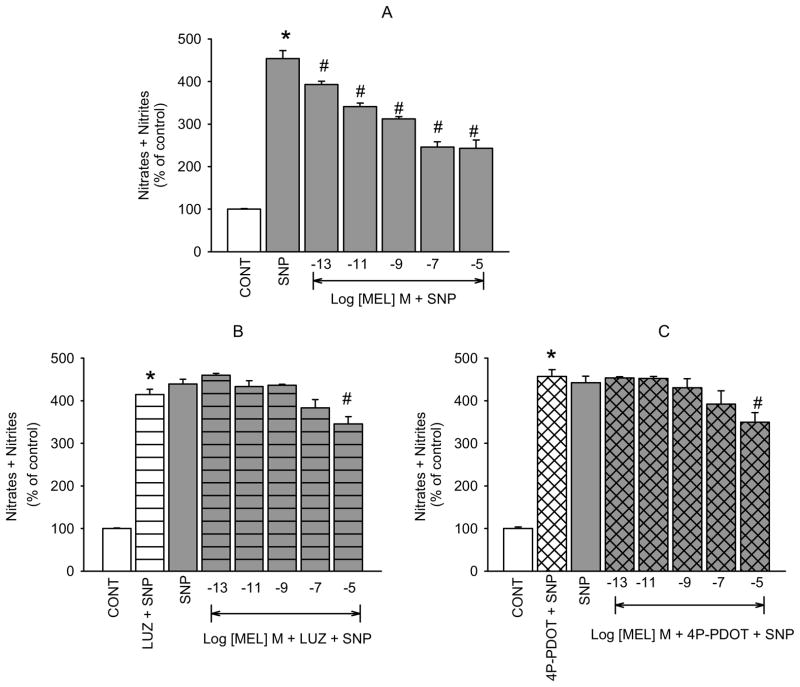
Melatonin caused a concentration-dependent reduction of SNP-released NO in hNPCE cells (figure 1A). The effects of the lower concentrations of melatonin (10−13, 10−11,10−9, and 10−7 M) were completely inhibited by the nonselective MT1/MT2 receptor antagonist, luzindole (10−5 M; figure 1B), whereas that of the highest concentration (10−5 M) was partially but not significantly inhibited. Because MT2 receptor mRNA has been shown to be present in the iris-ciliary body of several species (Saenz et al., 2002; Siu et al., 2004; Stumpf et al., 2009 Tunstall et al., 2011) where the NPCE cells are located, we hypothesized that the effect of melatonin on NO activity may be mediated by activation of the MT2 receptor subtype in the hNPCE cell type used in these studies. Therefore, we used the highly selective MT2 receptor antagonist, 4P-PDOT to confirm this hypothesis. Pretreatment with 4P-PDOT (10−7 M), resulted in the same pattern of inhibition of melatonin-induced reduction of SNP-released NO (figure 1C) as demonstrated with luzindole. When used alone, neither luzindole nor 4P-PDOT produced significant changes in SNP elevated NO.
Fig. 1.
Inhibition of melatonin-induced reduction of SNP-released NO by luzindole and 4P-PDOT in hNPCE cells. CONT (1% DMSO); SNP (10−4 M); LUZ (10−5 M); 4P-PDOT (10−7 M). Data are mean ± SEM of 5 – 8 experiments in triplicate. *P < 0.05 compared to control; #P < 0.05 compared to SNP.
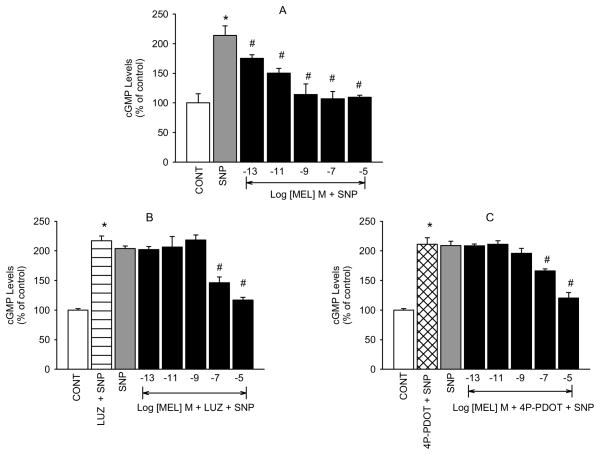
Because NO is known to be linked to the cGMP signaling cascade, we decided to also determine the effect of melatonin on stimulated levels of cGMP. As expected, melatonin also produced concentration-dependent inhibition of SNP stimulated cGMP production (figure 2A). Because the antagonists, at the concentrations utilized in the NO experiments, produced inhibitory effects on SNP-elevated cGMP levels (data not shown), we used lower concentrations of luzindole (10−7 M) and 4P-PDOT (10−8 M) for the cGMP experiments. Neither agent produced significant changes in SNP-elevated cGMP at these concentrations but did, however, produce complete inhibition of all but the two highest concentrations of melatonin (figures 2B and C). We postulate that the inhibitory actions of the antagonists at concentrations higher than 10−7 (luzindole) or 10−8 M (4P-PDOT) are due to their producing an agonist effect on cGMP production in the hNPCE cells. For example, 4P-PDOT was shown to have partial antagonist/agonist efficacy on leukocyte rolling (Lotufo et al., 2001).
Fig. 2.
Inhibition of melatonin-induced reduction of SNP-stimulated cGMP production by luzindole and 4P-PDOT in hNPCE cells. CONT (1% DMSO); SNP (10−3 M); LUZ (10−7 M); 4P-PDOT (10−8 M). Data are mean ± SEM of 4 – 5 experiments in triplicate. *P < 0.05 compared to control; #P < 0.05 compared to SNP.
3.2 5-MCA-NAT Inhibits SNP-released NO and cGMP Production via Activation of MT2 Receptors
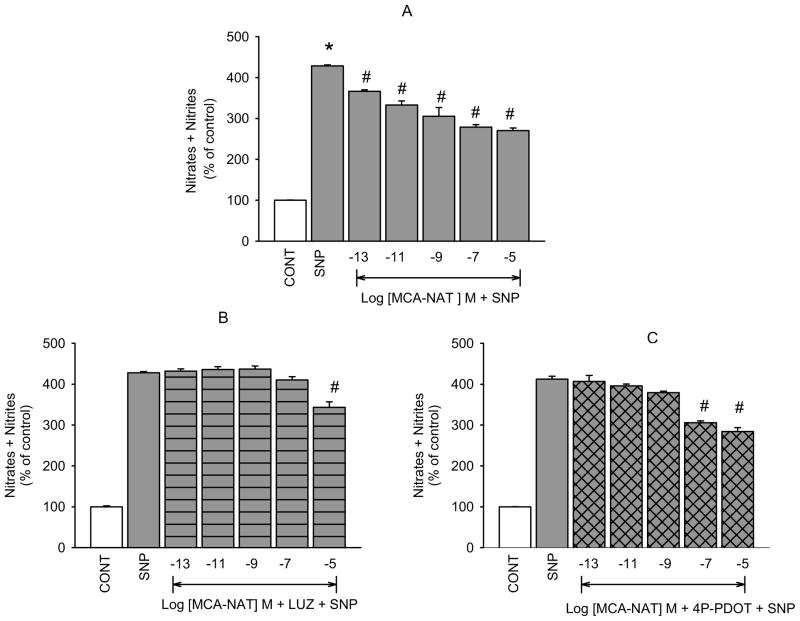
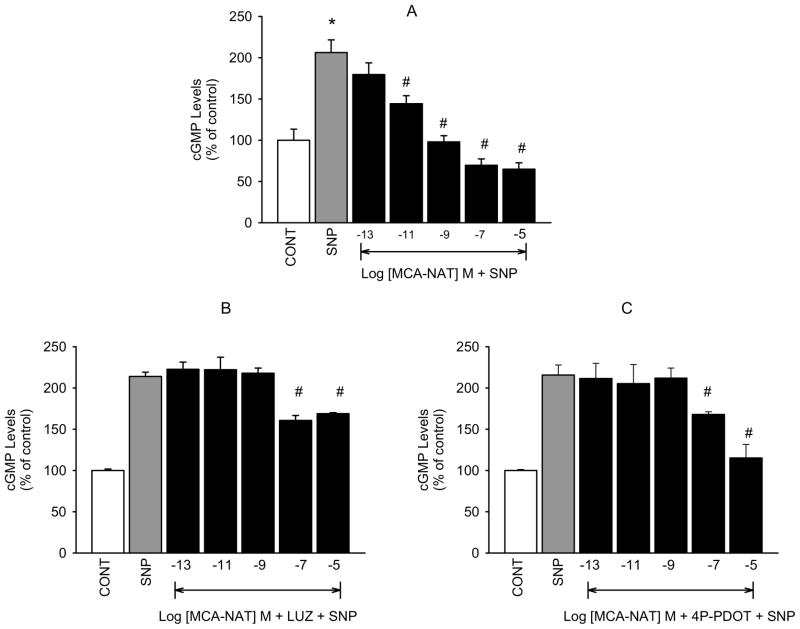
5-MCA-NAT caused concentration-dependent inhibition of SNP-released NO in isolated hNPCE cells (figure 3A) that was significantly inhibited in the presence of luzindole (10−5 M; figure 3B) at all but 10−5 M 5-MCA-NAT. As with melatonin, we thought that the inhibitory actions of 5-MCA-NAT on NO release would be due to activation of the MT2 melatonin receptor subtype. Pretreatment with 4P-PDOT (10−7 M) completely inhibited 5-MCA-NAT induced reduction of SNP elevated NO produced by the low concentrations but not the highest concentration of the melatonin receptor agonist (figure 3C). Cyclic GMP formation was similarly affected by 5-MCA-NAT which produced concentration-dependent inhibition of SNP-elevated cGMP (Figure 4A) that was inhibited in the presence of luzindole (10−7 M, 4B) and 4P-PDOT (10−8 M, 4C). Inhibition was significant at all but the 10−7 and 10−5 M concentrations of the agonist.
Fig. 3.
Inhibition of 5-MCA-NAT-induced reduction of SNP-released NO by luzindole and 4P-PDOT in NPCE cells. CONT (1% DMSO); SNP (10−4 M); LUZ (10−5 M); 4P-PDOT (10−7 M). Data are mean ± SEM of 4 – 5 experiments in triplicate. *P < 0.05 compared to control; #P < 0.05 compared to SNP.
Fig. 4.
Inhibition of 5-MCA-NAT-induced reduction of SNP-stimulated cGMP production by luzindole and 4P-PDOT in hNPCE cells. CONT (1% DMSO); SNP (10−3 M); LUZ (10−7 M); 4P-PDOT (10−8 M). Data are mean ± SEM of 6 – 9 experiments in triplicate. *P < 0.05 compared to control; #P < 0.05 compared to SNP.
3.3 Effects of Selective MT1 and MT2 Receptor Agonists on SNP-released NO and cGMP Production
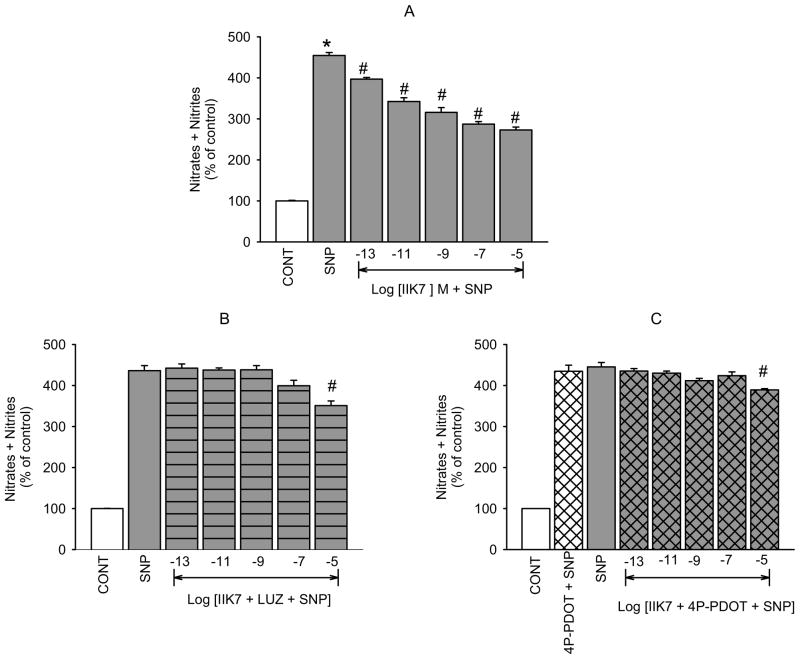
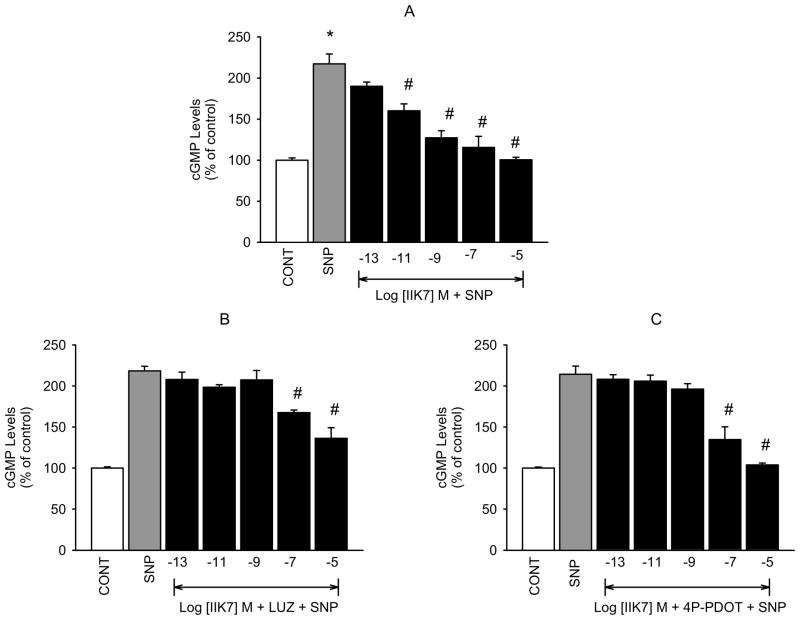
To further confirm that the inhibitory action of melatonin agonists on NO release and cGMP production that we have demonstrated is mediated by activation of MT2 receptors, we determined the effects of the selective MT2 receptor agonist IIK7 and the MT1 selective agonist S-27633-1 on the levels of the second messengers in our hNPCE cell type. Similar to melatonin and 5-MCA-NAT, IIK7 produced concentration-dependent decreases in SNP-released NO (figure 5A) and cGMP formation (figure 6A). However, the reported MT1 receptor agonist, S-27633-1, had no significant effect on SNP-released NO or cGMP production at concentrations below 10−5 M (figures 7A and B). Luzindole (10−5 M) significantly inhibited IIK7’s effect on NO levels produced by all but the 10−5 M concentration of the agonist (figure 5B). Because the effects of IIK7 on NO levels were not significantly inhibited in the presence of 10−7 M 4P-PDOT (data not shown), we increased the concentration to 10−5 M, which completely blocked the inhibitory effects of all but the 10−5 M concentration of IIK7 on NO release (figure 5C). 4P-PDOT (10−5 M) alone did not produce a significant change in SNP elevated NO levels. IIK7’s (10−13, 10−11, 10−9 M) inhibitory effects on cGMP production were significantly blocked in the presence of luzindole (10−7 M; figure 6B) and 4P-PDOT (10−8 M; figure 6C). The two highest concentrations of IIK7, however, were not significantly inhibited by either antagonist at the concentrations utilized.
Fig. 5.
Inhibition of IIK7-induced reduction of SNP-elevated NO by luzindole and 4P-PDOT in hNPCE cells. CONT (1% DMSO); SNP (10−4 M); LUZ (10−5 M); 4P-PDOT (10−5 M). Data are mean ± SEM of 4 – 5 experiments in triplicate. *P < 0.05 compared to control; #P < 0.05 compared to SNP.
Fig. 6.
Inhibition of IIK7-induced reduction of SNP-stimulated cGMP production by luzindole and 4P-PDOT in hNPCE cells. CONT (1% DMSO); SNP (10−3 M); LUZ (10−7 M); 4P-PDOT (10−8 M). Data are mean ± SEM of 4 experiments in triplicate. *P < 0.05 compared to control; #P < 0.05 compared to SNP.
Fig. 7.
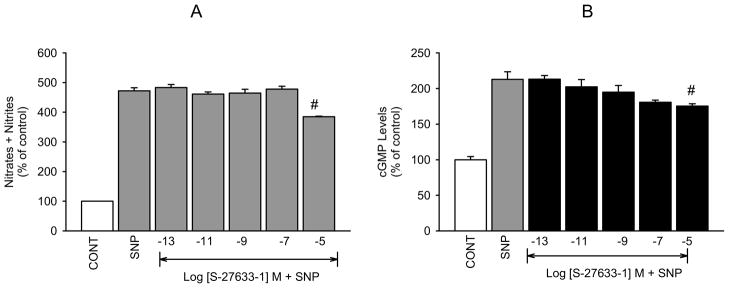
Effect of S-27633-1 on SNP-released NO and cGMP in hNPCE cells. CONT (1% DMSO); SNP (10−3 M). Data are mean ± SEM of 4 experiments in triplicate. #P < 0.05 compared to SNP.
4. Discussion
Besides its well-known regulatory role on circadian rhythm, the pineal gland hormone melatonin has other biological functions and a distinct metabolism in various cell types and peripheral tissues. For example melatonin modulates many important functions within the eye by interacting with a family of G-protein-coupled receptors MT1 and MT2 (JOCKERS et al., 2008; Reppert, 1997) as well as the enzyme quinone reductase 2, putative MT3 receptor (Calamini et al., 2008). In addition to its well known effects on retinal function (Baba et al., 2009; Rufiange et al., 2002; Tosini et al., 2012), melatonin is also known for its intraocular pressure (IOP) lowering effects in animals (Alarma-Estrany, 2009; Pintor et al., 2001; Serle et al., 2004) and humans (Samples et al., 1988). In rabbits, MT2 receptors have been shown to be present and the ocular hypotensive action of this receptor subtype has been demonstrated to be modulated by the sympathetic nervous system (Alarma-Estrany et al., 2008) Also, orally administered melatonin has been shown to lower IOP in humans (Samples et al., 1988). Contradictory to its effect on IOP in humans, it has been reported that endogenous concentrations of melatonin in plasma and during the day do not suppress aqueous humor flow in humans (Viggiano et al., 1994). The primary difference between the two studies is that one (Samples et al., 1988) showed significant decreases in IOP at night after oral administration, while the other (Viggiano et al, 1994) was conducted during the day. These findings reveal the fact that melatonin’s effect on IOP is dependent, at least in part, on the time of day that the measurements are taken (Alcantara-Contreras et al., 2011). In cat eyes, intracameral infusion of melatonin caused aqueous humor synthesis to decrease but caused a greater decrease in aqueous humor outflow facility, leading to a significant increase in IOP (Rohde et al., 1985). These contradictory effects may very well be species dependent.
Intraocular pressure is in a constant state of flux, varying with the cardiac and respiratory cycles and being influenced by factors such as posture and diet. That there is also a diurnal variation in human IOP was recognized a century ago. Subsequently, a number of studies have addressed this issue in “normal” and in glaucomatous eyes (Sihota et al., 2005; Wilensky; 1991 Worthen, 1978) using a variety of methodologies and with differing interpretations of “diurnal”. The majority of investigators found a rhythmic pressure variation during the day: a close approximation of diurnal pattern to a cosine curve has been claimed (Kitazawa and Horie, 1975). In many studies, the morning has often been the time when relatively higher values have been recorded, with the range of diurnal variation being of the order 3–5 mmHg.
Diurnal rhythms in IOP have been documented in many species, including humans (Drance, 1960; Henkind et al., 1973; Frampton et al., 1987) rabbits (Rowland et al., 1981; Liu and Shieh, 1995; Schnell et al., 1996), cats (DelSole et al., 2007) rats (Krishna et al., 1995), chicks (Papastergiou et al., 1998) and mice (Savinova et al., 2001; Alcantara-Contreras et al., 2011), although the phase and amplitude of the rhythm in IOP differs among species. Some of the discrepancies in the pattern of IOP may be due to species differences, or to differences in sleep/wakefulness and/or body position (Frampton et al., 1987). However, when IOP is measured in the normal physiologic positions of sitting while awake (day) and supine when asleep (night), investigators have shown that IOP was significantly higher during the nocturnal/sleep period than the diurnal/wake period (Liu et al., 1998; Liu et al., 1999; Liu et al., 2003; Hara et al., 2006; Sit et al., 2006).
Since melatonin levels are highest during the dark period, just prior to the early morning when IOP begins to decline, it has been hypothesized that melatonin may influence IOP (Rhode et al., 1985; Samples et al., 1988; Pintor et al., 2001). In humans, melatonin levels rise at night but IOP is higher at night than during the day (Krag et al., 1999). Also, the rate of aqueous humor secretion in humans falls 50–60% during sleep (Brubaker, 1991). These data are in contrast to endogenous melatonin lowering IOP. This may suggest, however, that endogenous melatonin contributes to elevation of IOP at night. Furthermore, this may mean that there is a difference between physiologic and pharmacologic concentrations of melatonin and the resulting IOP reading.
Levels of melatonin in the aqueous humor demonstrate a circadian rhythmicity, with peak levels occurring during the dark period (Yu et al., 1990; Liu and Dacus, 1991), which is in parallel with plasma melatonin levels. Melatonin has been demonstrated to be synthesized by the ciliary epithelium (Martin et al, 1992). AANAT, the rate-limiting enzyme in the synthesis of melatonin, has been shown to have higher activity at night than in the morning in the iris root-ciliary body complex (Chiou et al., 1985). This local synthesis of melatonin may account for the lack of effect of pinealectomy on melatonin levels in the aqueous humor or on IOP (Rohde et al., 1985).
Studies investigating the effect of melatonin administration on IOP have had conflicting results; topical administration of melatonin or its agonist, 5-MCA-NAT, causes dose-dependent lowering of IOP in rabbits (Pintor et al., 2001) and in humans (Samples et al., 1988). Moreover, administration of the competitive melatonin receptor antagonist luzindole blocks the reduction of IOP mediated by melatonin agonists (Pintor et al., 2001). In contrast, increased IOP has been reported following injection of melatonin into a superior vortex vein of rabbits (Rohde et al., 1993), and intracameral infusion of melatonin in cats (Rohde et al., 1985) and rats (Chiou et al., 1985). The conflicting results of the effects of melatonin on IOP may be due to differences in species and strains, as well as methods of administration and measurement (Osborne, 1994; Pintor et al., 2001).
The above results demonstrate that fluctuation of IOP in humans is well established, and the relationship between melatonin and IOP has been explored in view of the involvement of pineal melatonin in the regulation of many circadian rhythms (Lewy et al., 1999; Moore, 1997). Despite contradictory findings, the most widely held conclusion is that decreased IOP correlates positively with increased intraocular melatonin levels (Komaromy et al., 1998; Pointer, 1997; Rohde et al., 1985). However, pharmacological characterization of melatonin receptors and the relationship between individual receptor subtypes and IOP has not been evaluated comprehensively. The putative selective MT3 receptor agonist 5-MCA-NAT has been shown to be a potent ocular hypotensive agent (Pintor et al., 2001; Serle et al., 2004; Wiechmann and Wirsig-Wiechmann, 2001) and the selective MT2 receptor agonist IIK7 also markedly decreases the IOP and was inhibited by selective MT2 receptor antagonists (Alarma-Estrany et al., 2008). Both 5-MCA-NAT and IIK7 offer themselves as starting points for new classes of drugs to lower IOP and for treating ocular hypertension and glaucoma.
Knowing that the ciliary body is the site of aqueous humor formation (To et al., 2002) and even appears to be capable of melatonin synthesis (Martin et al., 1992), it results as a good candidate to be the tissue that binds melatonin and analogues to the melatonin receptors. The melatonin of origin within the ciliary body would likely be produced for a local function and would not contribute to the circulating levels similar to what occurs in the retina (Vaughan and Reiter, 1986). On the other hand, by their location on the basolateral surface of the non-pigmented ciliary epithelium, the melatonin receptors are positioned to be in the path of melatonin that is released either from the circulation or the ciliary body (Wiechmann and Wirsig-Wiechmann, 2001). With this information in mind and because all current therapeutic measures for glaucoma work by lowering IOP, either by increasing aqueous humor outflow or reducing its secretion (Shahidullah, 2006), we examined the effects of melatonin, 5-MCA-NAT and IIK7 on NO/cGMP release/production in isolated hNPCE cells. These studies were conducted in order to determine the possible role of NO/cGMP signaling in the ocular hypotensive effects of melatonin agonists. In addition, we determined the melatonin receptor selectivity of the compounds by utilizing available nonselective and selective antagonists.
For the present study, we performed a comparative analysis of the effects of melatonin, IIK7 (selective MT2 receptor agonist), 5-MCA-NAT (putative MT3 receptor agonist) and S-27633-1 (MT1 receptor agonist) on SNP-released NO and cGMP production to determine the receptor subtype that could be involved in melatonin agonist-induced regulation of aqueous secretion and IOP. S-27633 is reported to be a pure MT1 receptor agonist with a Kb of 46 nM in functional tests with GTP gamma S and affinity for MT1 being 2.4 nM (Ki). No functional activity of S-27633 has been detected at MT2 receptors and is why this compound was used to determine activity at MT1 receptors. Because the MT2 receptor subtype has been detected in the ciliary body and has also been shown to inhibit NO/cGMP signaling in other tissues and cells within and outside the eye (Saenz et al., 2002; Siu et al., 2004; Stumpf et al., 2009; Tunstall et al., 2011), we hypothesized that in our hNPCE cell line the MT2 melatonin receptor subtype is likely to be the one that modulates NO release and cGMP production in the NPCE cell type. Furthermore, it has been demonstrated that in New Zealand albino rabbits the melatonin agonist IIK7 decreased IOP by acting on MT2 receptors (Alarma-Estrany et al., 2008). This action was presumed to be by decreasing aqueous humor formation.
The literature is inconsistent regarding the role on the NO/cGMP pathway in aqueous humor dynamics. Some authors report that topical and intraocular NO donors decrease IOP (Behar-Cohen et al., 1996; Nathanson and McKee, 1995 ;), possibly by increasing conventional outflow facility (Schuman, et al., 1994). By contrast, other studies find that topical NO donors increase IOP without affecting aqueous production (Larson et al., 1995) or tonographic outflow facility (Krupin et al., 1977). Activation of the NO/cGMP pathway was first reported (Krupin et al., 1977) to elevate IOP as a result of an increase in the rate of aqueous flow. This was determined after topical application of sodium azide or SNP to the eyes of anaesthetized male albino rabbits. These results suggest that inhibition of the pathway would decrease aqueous production and consequently reduce IOP. Interestingly, there are also reports that systemic NOS inhibition has no effect on IOP in conscious humans (Kiss et al., 1999) and rabbits (Taniguchi et al., 1998).
In vitro studies also indicate a complex role of NO in aqueous humor dynamics. In the isolated porcine ciliary process, NO is produced in response to stimulators or analogs of cAMP (Liu et al., 1999), and stimulation of the NO-cGMP pathway causes epithelial depolarization (Fleischhauer et al., 2000). These results suggest that NO plays a facilitative or direct role in aqueous production. In isolated ciliary muscle and trabecular meshwork, NO causes relaxation (Wiederholt et al., 1994; Goh et al., 1995). In vivo relaxation of ciliary muscle would tend to decrease aqueous trabecular outflow facility and increase uveoscleral outflow, while relaxation of the trabecular meshwork would increase trabecular outflow facility. Thus, the in vitro evidence indicates that the IOP effects of NO donors and NOS inhibitors should vary depending upon the site of action.
In addition to direct effects on aqueous dynamics, NO may play an indirect role in IOP homeostasis. In a study (Kiel 1999) systemic NOS inhibition caused significant choroidal vasoconstriction and ocular hypotension in anesthetized rabbits. Given L-NAME’s constricting effect on the choroid, the immediate decrease in IOP is most likely due to the loss of choroidal blood volume. However, the expulsion of choroidal blood volume does not explain the sustained decrease in IOP since aqueous dynamics would compensate for the lost blood volume under normal circumstances. This implies that NOS inhibition decreases aqueous production or increases aqueous outflow, consistent with the previously mentioned in vitro studies. However, because L-NAME is such a potent choroidal constrictor, we hypothesize that it has a similar effect on the ciliary circulation, perhaps sufficient to cause a blood flow-dependent decrease in aqueous production.
Results from the present experimental work have demonstrated that melatonin, 5-MCA-NAT and IIK7 all reduce SNP-released NO and cGMP production in a concentration-dependent manner in our hNPCE cell type. Each agonist used demonstrated similar efficacies based on their maximum % inhibition of NO (46.47, 36.93 or 36.94; MEL, IIK7 and 5-MCA-NAT, respectively) and cGMP (68.52, 53.83, and 48.77; 5-MCA-NAT, IIK7 or MEL, respectively). The agonists were also of somewhat equal potencies with melatonin being slightly less potent than 5-MCA-NAT and IIK7 in its effect on NO but slightly more potent than the two analogues in its effect on cGMP production based on the EC50 values calculated for the agonists effects on NO (MEL 0.94 pM; 5-MCA-NAT 1.00 pM; IIK7 0.98 pM) and cGMP (MEL 1.02 pM; 5-MCA-NAT 0.88 pM; IIK7 0.92 pM). Although these results are not entirely consistent with more recent literature, in which it has been shown that IIK7 is more potent and efficacious than melatonin and 5-MCA-NAT in reducing IOP in rabbits, the discrepancy could be due to species differences or differences between in vitro versus in vivo techniques. Because this is the first in vitro study performed using an hNPCE cell line, no further comparisons can be made at this time.
The inhibitory effects of the lower concentrations (10−13, 10−11, 10−9) of all three agonists were completely inhibited in the presence of luzindole or 4P-PDOT, thereby, suggesting that these effects occur via activation of a G-protein linked melatonin receptor that is likely the MT2 subtype. At times, the inhibitory effects of 10−7 and 10−5 M concentrations of the agonists were not significantly inhibited by luzindole or 4P-PDOT, thus suggesting that those effects are nonspecific or that the interaction between agonist and antagonist is competitive and requires larger or even equimolar concentrations of the agonist to overcome the antagonist effect. Evidence of receptors in the iris-ciliary body of animals (Alarma-Estrany et al., 2008) and in anterior the presence of MT2 chamber uveal melanocytes of humans Roberts et al., 2000) has been generated. The presence of MT2 receptors in hNPCE cells of the ciliary body has, however, not been determined making this study the first to show their functional presence in the NPCE cell type of a mammalian species. Previous studies with the reported MT3 receptor agonist 5-MCA-NAT demonstrated that it produced its ocular hypotensive effect by activation of MT3 receptors (Pintor et al., 2001; Pintor et al., 2003). More recent evidence including that produced in the present study, suggests that it does not act through MT3 but rather MT1 or MT2 (Alarma-Estrany et al., 2009) with the present study pointing to MT2.
As we have previously mentioned, a recent study has reported that genetic ablation of the MT1 receptors in mice induced a significant increase in the mean values of nocturnal IOP, whereas, the removal of MT2 did not produce any changes on the regulation of the daily rhythm in the IOP (Alcantara-Contreras et al., 2011). Nevertheless administration of exogenous melatonin (1mg/kg) suppressed IOPs in wild-type, but not in MT1 and MT2 receptors knock-out mice. Such a result indicates that also in the mouse activation of MT2 receptors may lead to IOP reduction and suggest that MT1 and MT2 receptors signaling may act on different structures and or cells in the anterior part of the eye to regulate IOP. MT1 receptors may actually be located in the pigmented ciliary epithelium and therefore may affect aqueous humor secretion and IOP via activation of this receptor subtype in the pigmented ciliary epithelial cells.
In conclusion, results from this study suggest that melatonin, 5-MCA-NAT and IIK7 all produce their effects on NO release and cGMP production via activation of MT2 receptors in hNPCE cells. Also, results with the putative MT1 selective agonist suggest that the MT1 receptor subtype does not play a role in melatonin agonist-induced effects on NO or cGMP levels in the hNPCE cell type used in this study. MT1 receptors may be present in this cell type but linked to another signaling mechanism such as the AC/cAMP or PLC/IP3. Preliminary experiments, however, did not show an effect of melatonin on forskolin elevated cAMP. Other experiments are being performed to determine if melatonin affects basal cAMP production in the hNPCE cells.
MT1 receptors could also be found in some other cell type involved in IOP regulation such as the ciliary muscle cells or trabecular meshwork. We are presently conducting studies examining the effects of melatonin on cAMP production in human trabecular meshwork (hTM) cells. If we find that melatonin reduces cAMP formation in this particular cell type, it could explain why at times melatonin causes elevation of IOP rather than reduction. Lowering cAMP levels in hTM cells causes them to contract, an action that decreases outflow facility thereby increasing IOP. If this action occurs in vivo and IOP is lowered, it could suggest that the net effect of melatonin on IOP in this case is reduction.
Our hypothesis regarding the sequence of events that lead to melatonin agonist-induced reduction of IOP begins with the activation of MT2 receptors in the NPCE cells. This effect is followed by a reduction in NO released from the non-pigmented ciliary epithelium or, in our case, released from SNP. Next, in either an autocrine or a paracrine manner, reduced levels of NO leads to a reduction in the levels of cGMP in the ciliary epithelium or the ciliary muscle, respectively. We propose that either of these events may reduce IOP by decreasing aqueous humor secretion (autocrine) or, inadvertently contracting the ciliary muscle (paracrine) thereby increasing the outflow of aqueous humor. Reduction of IOP could also be the result of a combination of the two disparate mechanisms. Future studies are being designed to address the issue of whether the effects demonstrated in the present study are indeed associated with activation of MT2 receptors. In those studies, with the use of SiRNA, we plan to knockdown the MT1, MT2 and the putative MT3 receptors in hNPCE cells to provide convincing evidence that the effects of melatonin and its agonists used in this study are actually mediated by activation of MT2 receptors.
Highlights.
We demonstrate the functional presence of MT2 melatonin receptors in hNPCE cells
Melatonin and its congeners inhibit NO release and cGMP production in hNPCE cells
Possible role of melatonin receptors in the regulation of aqueous humor secretion
Acknowledgments
The authors gratefully acknowledge the financial support from the NIH (EY022216 and EY020821) and Morehouse School of Medicine RCMI (RR003034). The authors thank Dr. Miguel Coca Prados, Yale University School of Medicine and Dr. Mohammad Shahidulah for their kind gifts of hNPCE (ODM) cells and Dr. Danita Eatman, Pharmacology Department, Morehouse School of Medicine for her donation of nitric oxide assay kits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarma-Estrany P, Crooke A, Mediero A, Pelaez T, Pintor J. Sympathetic nervous system modulates the ocular hypotensive action of MT2-melatonin receptors in normotensive rabbits. J Pineal Res. 2008;45(4):468–475. doi: 10.1111/j.1600-079X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Alarma-Estrany P, Crooke A, Pintor J. 5-MCA-NAT does not act through NQO2 to reduce intraocular pressure in New-Zealand white rabbit. J Pineal Res. 2009;47(2):201–209. doi: 10.1111/j.1600-079X.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- Alcantara-Contreras S, Baba K, Tosini G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neurosci Lett. 2011;494(1):61–64. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM, Tosini G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci USA. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar-Cohen FF, Goureau O, D’Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996;37:1711–1715. [PubMed] [Google Scholar]
- Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture] Invest Ophthalmol Vis Sci. 1991;32:3145–3166. [PubMed] [Google Scholar]
- Calamini B, Santarsiero BD, Boutin JA, Mesecar AD. Kinetic, thermodynamic and X-ray structural insights into the interaction of melatonin and analogues with quinone reductase 2. Biochem J. 2008;413(1):81–91. doi: 10.1042/BJ20071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou GC, Aimoto T, Chiou LY. Melatonergic involvement in diurnal changes of intraocular pressure in rabbit eyes. Ophthalmic Res. 1985;17:373–378. doi: 10.1159/000265403. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Wax M. Transformation of human ciliary epithelial cells by simian virus 40: induction of cells proliferation and retention of B2-adrenergic receptors. Proc Natl Acad Sci USA. 1986;83:8754–8758. doi: 10.1073/pnas.83.22.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger RS, Star RA, Matsumoto S, Coca-Prados M, DeSantis L, Pang IH. Characterization of soluble guanylyl cyclase in transformed human non-pigmented epithelial cells. Biochem Biophys Res Commun. 1993;195:958–62. doi: 10.1006/bbrc.1993.2137. [DOI] [PubMed] [Google Scholar]
- Del Sole MJ, Sande PH, Bernades JM, Aba MA, Rosenstein RE. Circadian rhythm of intraocular pressure in cats. Vet Ophthalmol. 2007;10:155–161. doi: 10.1111/j.1463-5224.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- Drance SM. The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol. 1960;64:494–501. doi: 10.1001/archopht.1960.01840010496004. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin receptors: are there multiple subtypes? Trends Pharmacol Sci. 1995;16:50–56. doi: 10.1016/s0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- Fleischhauer JC, Beny J, Flammer J, Haefliger IO. NO/cGMP pathway activation and membrane potential depolarization in pig ciliary epithelium. Invest Ophthalmol Vis Sci. 2000;41:1759–1763. [PubMed] [Google Scholar]
- Frampton P, Da Rin D, Brown B. Diurnal variation of intraocular pressure and the overriding effects of sleep. Am J Optom Physiol Opt. 1987;64:54–61. doi: 10.1097/00006324-198701000-00010. [DOI] [PubMed] [Google Scholar]
- Goh Y, Hotehama Y, Mishima HK. Characterization of ciliary muscle relaxation induced by various agents in cats. Invest Ophthalmol Vis Sci. 1995;36:1188–1192. [PubMed] [Google Scholar]
- Hara T, Hara T, Tsuru T. Increase of peak intraocular pressure during sleep in reproduced diurnal changes by posture. Arch Ophthalmol. 2006;124:165–168. doi: 10.1001/archopht.124.2.165. [DOI] [PubMed] [Google Scholar]
- Henkind P, Leitman M, Weitzman E. The diurnal curve in man: new observations. Invest Ophthalmol. 1973;12:705–707. [PubMed] [Google Scholar]
- Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: What’s new? Br. J Pharmacol. 2008;154(6):1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel JW. Modulation of choroidal autoregulation in the rabbit. Exp Eye Res. 1999;69:413–429. doi: 10.1006/exer.1999.0717. [DOI] [PubMed] [Google Scholar]
- Kiss B, Dallinger S, Findl O, Rainer G, Eichler H, Schmetterer L. Acetazolamide-induced cerebral and ocular vasodilation in humans is independent of nitric oxide. Am J Physiol. 1999;276:R1661–R1667. doi: 10.1152/ajpregu.1999.276.6.R1661. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y, Horie T. Diurnal variation of intraocular pressure in primary open-angle glaucoma. Am J Ophthalmol. 1975;79:557–566. doi: 10.1016/0002-9394(75)90792-8. [DOI] [PubMed] [Google Scholar]
- Komaromy AM, Brooks DE, Kubilis PS, Dawson WW, Sapp HL, Jr, Nelson G, Collins BR, Sherwood MB. Diurnal intraocular pressure curves in healthy rhesus macaques (Macaca mulatta) and rhesus macaques with normotensive and hypertensive primary open-angle glaucoma. J Glaucoma. 1998;7(2):128–131. [PubMed] [Google Scholar]
- Krag S, Andersen HB, Sorensen T. Circadian intraocular pressure variation with beta-blockers. Acta Ophthalmol Scand. 1999;77:500–503. doi: 10.1034/j.1600-0420.1999.770502.x. [DOI] [PubMed] [Google Scholar]
- Krishna R, Mermoud A, Baerveldt G, Minckler DS. Circadian rhythm of intraocular pressure: a rat model. Ophthalmic Res. 1995;27:163–167. doi: 10.1159/000267660. [DOI] [PubMed] [Google Scholar]
- Krupin T, Weiss A, Becker B, Holmberg N, Fritz C. Increased intraocular pressure following topical azide or nitroprusside. Invest Ophthalmol Vis Sci. 1977;16:1002–1007. [PubMed] [Google Scholar]
- Larson LI, Maus TI, Brubaker RE, Nathanson JA. Topically applied hydralazine: effects on cardiovascular parameters, blood-aqueous barrier, and aqueous humor dynamics in normotensive humans. J Ocular Pharm Therpeut. 1995;11:145–156. doi: 10.1089/jop.1995.11.145. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14(3):227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Aleman TS, Zaixin Y, Cideciyan AV, Jacobson SG, Bennett J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport. 2001;12(5):1011–1014. doi: 10.1097/00001756-200104170-00029. [DOI] [PubMed] [Google Scholar]
- Liu JH, Dacus AC. Endogenous hormonal changes and circadian elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 1991;32:496–500. [PubMed] [Google Scholar]
- Liu JH, Shieh BE. Suprachiasmatic nucleus in the neural circuitry for the circadian elevation of intraocular pressure in rabbits. J Ocul Pharmacol Ther. 1995;11:379–388. doi: 10.1089/jop.1995.11.379. [DOI] [PubMed] [Google Scholar]
- Liu JH, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- Liu JH, Dripke DF, Twa MD, Hoffman RE, Mansberger SL, Rex KM, Girkin CA, Weinreb RN. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40(12):2912–2917. [PubMed] [Google Scholar]
- Liu R, Flammer J, Haefliger IO. Isoproterenol, forskolin, and cAMP-induced nitric oxide production in pig ciliary processes. Invest Ophthalmol Vis Sci. 1999;40:1833–1837. [PubMed] [Google Scholar]
- Liu JH, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003;44:4439–4442. doi: 10.1167/iovs.03-0349. [DOI] [PubMed] [Google Scholar]
- Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44(4):1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- Lotufo CMC, Lopes C, Dubocovich ML, Farsky SHP, Markus RP. Melatonin and N-acetylserotonin inhibit leukocyte rolling and adhesion to rat microcirculation. Eur J Pharmacol. 2001;430:351–357. doi: 10.1016/s0014-2999(01)01369-3. [DOI] [PubMed] [Google Scholar]
- Martin XD, Malina HZ, Brennan MC, Hendrickson PH, Lichter PR. The ciliary body--the third organ found to synthesize indoleamines in humans. Eur J Ophthalmol. 1992;2(2):67–72. doi: 10.1177/112067219200200203. [DOI] [PubMed] [Google Scholar]
- Moore RY. Circadian rhythms: basic neurobiology and clinical applications. Annu Rev Med. 1997;48:253–266. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995;36:1765–1773. [PubMed] [Google Scholar]
- Nosjean O, Ferro M, Beauverger P, Henlink JMK, Lefoulon F, Fauchere JL, Delagrange P, Canet E, Boutin JA. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- Osborne NN. Serotonin and melatonin in the iris/ciliary processes and their involvement in intraocular pressure. Acta Neurobiol Exp (Wars) 1994;54:57–64. [PubMed] [Google Scholar]
- Osborne NN, Chidlow G. The presence of functional melatonin receptors in the iris-ciliary processes of the rabbit eye. Exp Eye Res. 1994;59:3–9. doi: 10.1006/exer.1994.1076. [DOI] [PubMed] [Google Scholar]
- Palmer R, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- Peters JL, Cassone VM. Melatonin regulates circadian electroretinogram rhythms in a dose- and time-dependent fashion. J Pineal Res. 2005;38:209–215. doi: 10.1111/j.1600-079X.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- Pintor J, Martin L, Pelaez T, Hoyle CH, Peral A. Involvement of melatonin MT3 receptors in the regulation of intraocular pressure in rabbits. Eur J Pharmacol. 2001;416:251–254. doi: 10.1016/s0014-2999(01)00864-0. [DOI] [PubMed] [Google Scholar]
- Pintor J, Paleaz T, Hoyle CH, Peral A. Ocular hypotensive effects of melatonin agonists in the rabbit: further evidence for an MT3 receptor. Br J Pharmacol. 2003;138:831–836. doi: 10.1038/sj.bjp.0705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointer JS. The diurnal variation of intraocular pressure in non-glaucomatous subjects: relevance in a clinical context. Ophthalmic Physiol Opt. 1997;17(6):456–465. [PubMed] [Google Scholar]
- Reppert SM. Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J Biol Rhythms. 1997;12(6):528–531. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Wiechmann AE, Hu DN. Melatonin receptors in human uveal melanocytes and melanoma cells. J Pineal Res. 2000;28(3):165–171. doi: 10.1034/j.1600-079x.2001.280306.x. [DOI] [PubMed] [Google Scholar]
- Rohde BH, McLaughlin MA, Chiou LY. Existence and role of endogenous ocular melatonin. J Ocul Pharmacol. 1985;1(3):235–243. doi: 10.1089/jop.1985.1.235. [DOI] [PubMed] [Google Scholar]
- Rohde BH, Li BH, Chiou GC. Effects of melatonin and haloperidol given via vortex vein on the intraocular pressure. Ophthalmic Res. 1993;25:10–15. doi: 10.1159/000267215. [DOI] [PubMed] [Google Scholar]
- Rosenstein RE, Pandi-Perumal SR, Srinivasan V, Spence DW, Brown GM, Cardinali DP. Melatonin as a therapeutic tool in ophthalmology: implications for glaucoma and uveitis. J Pineal Res. 2010;49:1–13. doi: 10.1111/j.1600-079X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr Eye Res. 1981;1:169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- Rufiange M, Dumont M, Lachappelle P. Correlating retinal function with melatonin secretion in subject with an early or late circadian phase. Invest Ophthalmol Vis Sci. 2002;43:2491–2499. [PubMed] [Google Scholar]
- Saenz DA, Turjanski AG, Sacca GB, Marti M, Doctorovich F, Sarmiento MI, Estrin DA. Physiological concentrations of melatonin inhibit the nitridergic pathway in the Syrian hamster retina. J Pineal Res. 2002;33(1):31–36. doi: 10.1034/j.1600-079x.2002.01880.x. [DOI] [PubMed] [Google Scholar]
- Savinova OV, Sugiyama F, Martin JE, Tomarev SI, Paigen BJ, Smith RS, John SW. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2001;2(1):1471–2156. doi: 10.1186/1471-2156-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples JR, Krause G, Lewy AJ. Effect of melatonin on intraocular pressure. Curr Eye Res. 1988;7:649–653. doi: 10.3109/02713688809033192. [DOI] [PubMed] [Google Scholar]
- Schnell CR, Debon C, Percicot CL. Measurement of intraocular pressure by telemetry in conscious, unrestrained rabbits. Invest Ophthalmol Vis Sci. 1996;376:958–965. [PubMed] [Google Scholar]
- Serle JB, Wang RF, Peterson WM, Plourde R, Yerxa BR. Effect of 5-MCA-NAT, a putative melatonin MT3 receptor agonist, on intraocular pressure in glaucomatous monkey eyes. J Glaucoma. 2004;13:385–388. doi: 10.1097/01.ijg.0000133150.44686.0b. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;48(6):871–880. doi: 10.1038/sj.bjp.0706795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994;58:99–105. doi: 10.1006/exer.1994.1199. [DOI] [PubMed] [Google Scholar]
- Sihota R, Saxena R, Gogoi M, Sood A, Gulati V, Pandey RM. A comparison of the circadian rhythm of intraocular pressure in primary chronic angle closure glaucoma, primary open angle glaucoma and normal eyes. Indian J Ophthalmol. 2005;53:243–247. doi: 10.4103/0301-4738.18905. [DOI] [PubMed] [Google Scholar]
- Sit AJ, Weinreb RN, Crowston JG, Kripke DF, Liu JH. Sustained effect of travoprost on diurnal and nocturnal intraocular pressure. Am J Ophthalmol. 2006;141(6):1131–1133. doi: 10.1016/j.ajo.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Siu AW, Ortiz GG, Benitez-King G, To CH, Reiter RJ. Effects of melatonin on the nitric oxide treated retina. Br J Ophthalmol. 2004;88(8):1078–1081. doi: 10.1136/bjo.2003.037879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AW, Maldonado M, Sanchez-Hidalgo M, Tan DX, Reiter RJ. Protective effects of melatonin in experimental free radical-related ocular diseases. J Pineal Res. 2006;40:101–109. doi: 10.1111/j.1600-079X.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- Stumpf I, Bazwinsky I, Peschke E. Modulation of the cGMP signaling pathway by melatonin in pancreatic beta-cells. J Pineal Res. 2009;46(2):140–147. doi: 10.1111/j.1600-079X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Kawakami H, Sawada A, Iwaki M, Tsuji AM, Sugiyama K, Kitazawa Y. Effects of nitric oxide synthase inhibitor on intraocular pressure and ocular inflammation following laser irradiation in rabbits. Curr Eye Res. 1998;17:308–315. doi: 10.1076/ceyr.17.3.308.5225. [DOI] [PubMed] [Google Scholar]
- To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin Exp Optom. 2002;85(6):335–349. [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Baba K, Hwang CK, Iuvone MP. Melatonin: An underappreciated play in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall RR, Shukla P, Grazul-Bilska A, Sun C, O’Rourke ST. MT2 receptors mediate the inhibitory effects of melatonin on nitric oxide-induced relaxation of porcine isolated coronary arteries. J Pharmacol Exp Ther. 2011;336(1):127–133. doi: 10.1124/jpet.110.174482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan GM, Reiter RJ. Pineal dependence of the Syrian hamster’s nocturnal serum melatonin surge. J Pineal Res. 1986;3(1):9–14. doi: 10.1111/j.1600-079x.1986.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Viggiano SR, Koskela TK, Klee GG, Samples JR, Arnce R, Brubaker RF. The effect of melatonin on aqueous humor flow in human during the day. Ophthalmol. 1994;101(2):326–331. doi: 10.1016/s0161-6420(94)31332-7. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Wirsig-Wiechmann CR. Melatonin receptor mRNA and protein expression in Xenopus laevis nonpigmented ciliary epithelial cells. Exp Eye Res. 2001;73:617–623. doi: 10.1006/exer.2001.1073. [DOI] [PubMed] [Google Scholar]
- Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol VisSci. 1994;35:2515–2520. [PubMed] [Google Scholar]
- Wilensky JT. Diurnal variations in intraocular pressure. Trans Am Ophthalmol Soc. 1991;89:757–790. [PMC free article] [PubMed] [Google Scholar]
- Worthen DM. Intraocular pressure and its diurnal variation. In: Heilmann L, Richardson KT, editors. Glaucoma: Conceptions of a Disease. W.R. Saunders Company; Philadelphia, U.S.A: 1978. pp. 54–66. [Google Scholar]
- Wu R, Yin J, Yao K, Haefliger I. Inhibition by brimonidine of forskolin-induced nitric oxide synthase expression in human ciliary bodies in vitro. Mol Vis. 2007;13:493–496. [PMC free article] [PubMed] [Google Scholar]
- Wu RY, Ma N. Expression of nitric oxide synthase and guanlyate cyclase in the human ciliary body and trabecular meshwork. Chin Med J. 2012;125(1):129–133. [PubMed] [Google Scholar]
- Yu HS, Yee RW, Howes KA, Reiter RJ. Diurnal rhythms of immunoreactive melatonin in the aqueous humor and serum of male pigmented rabbits. Neuroci Lett. 1990;116:309–314. doi: 10.1016/0304-3940(90)90092-n. [DOI] [PubMed] [Google Scholar]